INTRODUCTION
Breast cancer is the most common cancer in women and, after lung cancer, is the second leading cause of cancer-related death. Data from the Centers for Disease Control and Prevention estimate 232,670 breast cancer diagnoses and 40,000 related deaths in 2014. The commonly reported statistic is that 1 in 8 women will develop breast cancer in their lifetime.
As providers of primary health care to women in all phases of their life, the obstetrician-gynecologist can be instrumental in the management of breast health concerns. The American Congress of Obstetricians and Gynecologists (ACOG) advocates for the obstetrician-gynecologist to have a working knowledge of the diagnosis of breast disease and cancer, to advise patients to perform self-breast examination, and to refer patients for screening mammography. These recommendations have resulted in increased ability to detect breast diseases and cancer. In the event of a malignant diagnosis, the obstetrician-gynecologist should be able to counsel the patient concerning the general treatment of the disease and facilitate a smooth transition to a breast cancer specialist. The Society of Gynecologic Oncology recently has recognized the expanding role of their specialty in the management of breast cancer. Several fellowship programs now include one additional year of training in the management of breast cancer and allied diseases. The American Board of Obstetrics and Gynecology Guide to Learning in Gynecologic Oncology indicates fellows are expected to have a working knowledge of breast diseases and be able to advise patients with regard to incidence of breast cancer, high-risk lesions, staging of the disease, and the role of multimodal therapy in the management of the disease process.
This chapter discusses basic strategies for the evaluation, diagnosis, and treatment of both benign and malignant breast diseases. The multidisciplinary approach for the evaluation of breast cancer is addressed, including surgical, radiotherapeutic, and adjuvant medical management.
EMBRYOLOGY
The mammary glands are specialized skin derivatives of ectodermal origin. During the 4th week of gestation, a pair of ectodermal thickenings—also known as the mammary ridges or milk line—form on the ventral surface of the embryo and extend in a curvilinear fashion from the axilla to the medial thigh. During the 5th week of gestation, an ingrowth of the ridge in the pectoral region at the fourth intercostal space occurs and forms a primary tissue bud. The remaining milk line regresses. Failure of regression of the milk line can lead to accessory nipples (polythelia) or breasts (polymastia)
(Fig. 45.1). Subsequently, over the course of weeks, 15 to 20 secondary buds develop and become the breast lobules.
Lactiferous ducts form at approximately the 20th week as a result of luminae that develop within the buds. At term, there are 15 to 20 breast lobes and associated lactiferous ducts. The ducts drain into ampullae located beneath the developing nipple-areolar complex. The nipple forms as a result of mesenchymal proliferation and the formation of circular and longitudinally oriented smooth muscle fibers.
In both the male and female neonates, the breast contains radially arranged breast lobes that drain into the nipple via the lactiferous ducts. The female breast undergoes rapid changes, known as thelarche, during puberty. Exposure to rising levels of estrogen and progesterone induces changes in the ductal epithelium and breast acini. Other circulating agents, including growth hormone, cortisol, insulin, thyroxine, and prolactin, help the breast achieve complete functional status. To gauge pubertal development of the breast, Tanner staging can be assessed.
Table 45.1 lists staging criteria. Failure to progress in accordance with established criteria can help identify hormone deficiencies and lead to early evaluation and treatment.
ANATOMY
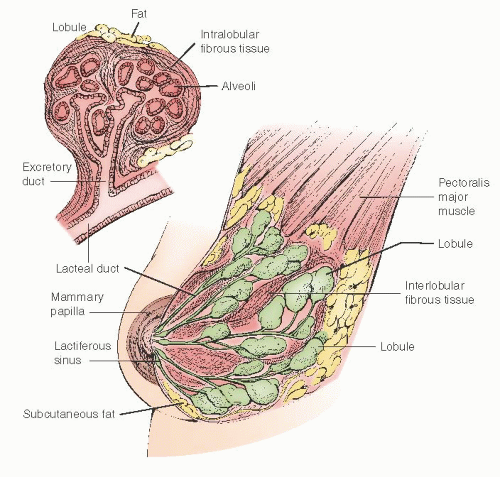
The mammary gland is a modified sweat gland and is an accessory to reproduction in the female. The breast is located between the second rib and the sixth intercostal space in the vertical axis and extends horizontally from the sternum to beyond the anterior axillary fold. A portion of the breast—the axillary tail of Spence—extends into the axillary basin. It has a dome shape with a conical contour in a nulliparous female and may become pendulous in the mature or parous individual. The gland is composed of skin, subcutaneous tissue, epithelial glandular structures, and supporting stroma (
Fig. 45.2).
The breast is composed of 15 to 20 lobes with each lobe being an irregular, flattened pyramid of glandular tissue with the apex directed toward the nipple-areolar complex. From each lobe, a single lactiferous duct extends to the nipple. Within the lobes, these ducts have multiple divisions beginning with branches to 20 to 40 lobules and subsequent branching to 10 to 100 alveoli units. The ducts, referred to as the lactiferous sinuses, are 1 to 2 mm in diameter but dilate to 4 to 5 mm beneath the nipple-areolar complex. The lactiferous sinuses drain to collecting ducts that have openings within the nipple (
Fig. 45.3). Nomenclature for the epithelial system is highlighted in
Table 45.2.
The stroma consists of connective tissue, adipose tissue, blood vessels, nerves, and lymphatics. The superficial pectoral fascia envelops the entire gland. The superficial pectoral fascia is in continuity with superficial fascia of the abdominal wall (Camper fascia). The posterior surface of the breast abuts the deep pectoralis fascia.
The nipple and areola are pigmented, covered with keratinized squamous epithelium, and contain smooth muscle fibers, which causes wrinkling of the skin and associated with nipple erection. Within the nipple are two receptor-type nerve endings, Ruffini-like bodies and bulb of Krause, that detect tactile stretch and pressure. The areola has sebaceous and apocrine sweat glands and accessory areolar glands also known as Montgomery glands. In the latter, these glands open onto the areola as small elevations known as Morgagni tubercles.
The major muscles proximate to the breast and axilla include the pectoralis major and minor, serratus anterior, and the latissimus dorsi. The pectoralis major has two parts: the clavicular portion and the sternocostal portion. This muscle forms the fullness of the upper chest and helps flex and adduct the arm. Innervation is from the medial (C8, T1) and lateral pectoral nerves (C5, C6, and C7) that course from the brachial plexus and are named based on their cord of origin rather than anatomic location. The pectoralis minor arises from the third, fourth, and fifth rib and inserts into the coracoid process of the scapula. Motor function is from the medial pectoral nerve (C8, T1). The serratus anterior muscle arises from the first eight ribs and inserts into the vertebral border of the scapula on its costal surface. It is innervated by the long thoracic nerve of Bell
that originates from the posterior roots of C5, C6, C7, and C8 of the brachial plexus. This nerve traverses the posterior aspect of the axilla and courses along the superficial aspect of the fascia of the serratus anterior. Injury to the nerve leads to the clinical presentation of a “winged” scapula and impairment of the shoulder.
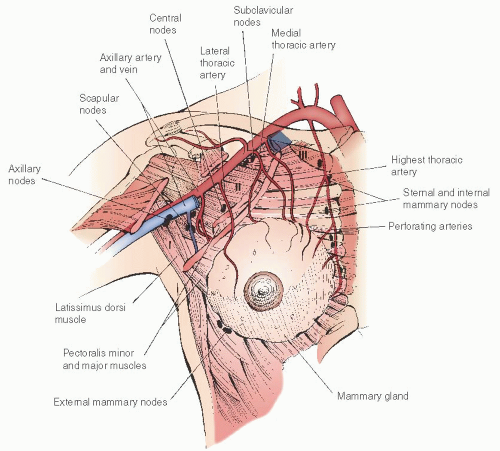
The blood supply to the mammary gland is primarily derived from the internal mammary artery and the lateral thoracic artery. The anterior perforating branches of the internal mammary artery supply the medial and central breast, whereas the lateral thoracic artery supplies the upper outer portion of the gland. Additional vascular supply is derived from branches of the thoracoacromial, intercostal, subscapular, and thoracodorsal arteries
(Fig. 45.4).Venous drainage follows the course of the superficial and deep arterial supply. The medial quadrants primarily drain to the internal thoracic vein, whereas the lateral quadrants drain via branches of the axillary vein and posterior branches of the intercostal vein.
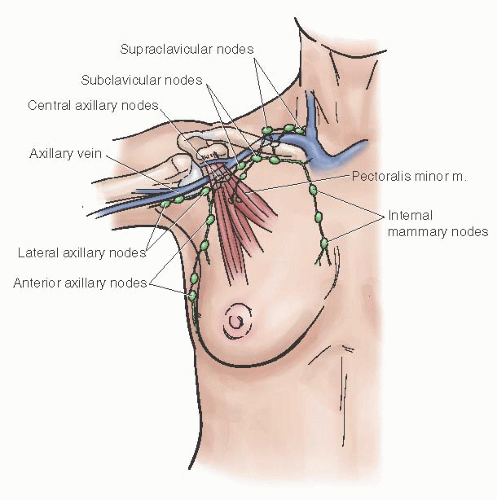
Over the past decade, the routine use of sentinel lymphatic mapping in the treatment of breast cancer has significantly enhanced the understanding of lymphatic drainage of the mammary gland. The subepithelial lymphatic plexus is confluent with the subepithelial plexus over the surface of the body. These vessels merge with the subdermal lymphatic vessels and drain to Sappey plexus beneath the nipple-areolar complex. Lymph then flows through these valveless ducts in only one direction into lymphatics along the lactiferous ducts to the perilobular and deep subcutaneous plexus. Lymph flow then moves in a centrifugal manner toward the axillary and internal mammary nodes. Approximately 97% of the drainage is to the ipsilateral axilla with the remaining 3% of the effluent to the internal mammary nodes.
Approximately 20 to 30 lymph nodes are located within the axillary basin. They consist of five subgroups denoted by their relationship to the axillary structures. The subgroups include the
apical nodes located medial to the pectoralis minor muscle, the
axillary vein group located along the axillary vein extending from the pectoralis minor muscle to the lateral extent of the vein,
interpectoral (Rotter) nodes located between the pectoralis major and minor muscles, the
scapular group located along the subscapular vessels, and the
central nodes located beneath the lateral border of the pectoralis major muscle. An alternative approach to the nodal basin nomenclature is to divide the nodal groups into three levels in relation to the pectoralis minor muscle. Level I nodes are lateral to the lateral border of the pectoralis minor muscle, level II nodes are located beneath this muscle, and level III nodes are located medial to the medial border of the pectoralis minor muscle
(Fig. 45.5).
BREAST HEALTH ASSESSMENT
An intake history should be obtained in which the patient describes her concerns. Care should be taken to elicit information that can help identify a woman at risk for breast cancer.
Breast cancer cannot be excluded by any single fact within the patient’s history; rather, the history can help focus attention on risk factors for the development of breast cancer. The practitioner should obtain detailed information about the patient’s symptoms and other pertinent related positive and negative signs or concerns. Age, menstrual status, use of oral contraceptives, and postmenopausal hormone replacement therapy (HRT) (including the regimen and duration) are important parts of risk assessment. A detailed family history spanning three generations on both the maternal side and paternal side may identify an individual with a possible genetic predisposition. It is important to elicit and document the timing and specific nature of the concerns, whether any cyclic changes have occurred, change in site, and the presence of pain.
All previous breast diagnostic and surgical procedures should be documented. Past medical and surgical history, current medications, and social history (including tobacco use, alcohol intake, and educational level) should also be reviewed.
In the past, the traditional approach for early detection of breast cancer used a combination of breast self-examination (BSE), clinical breast examination (CBE), and mammography. For women with average risk of breast cancer, current guidelines from the American Cancer Society (ACS), the American Congress of Obstetricians and Gynecologists (ACOG), and the U.S. Preventive Services Task Force (USPSTF) are shown in
Table 45.3. Both ACS and ACOG advise annual mammography beginning at the age of 40, CBE beginning in the 20s, and BSE beginning at age 20. Both groups encourage women to know what their breasts look and feel like and promptly report any changes to their health care providers.
BREAST EXAMINATION
Traditionally, BSE and CBE have been considered important facets of a breast screening program. Controversy surrounds the use of both of these physical examinations.
Breast Self-Examination
Recently, the role of BSE has been called into question, as it has been associated in two major studies with increased rates of radiographic imaging and invasive procedures. Controversy exists regarding the utility of routine BSE for increasing the rate of breast cancer detection. A study from Shanghai involving more than 250,000 women, who were randomly assigned to no instruction or intensive BSE instruction, failed to demonstrate any difference in the number of cancers detected or in the stage or size at which they were found (RR 0.97, 95% CI: 0.88 to 1.06). No reduction in mortality was seen, but more than twice as many benign lesions were found in the BSE group. In breast disease, wherein early detection is so clearly related to improved survival, the value of this relatively simple, economical, and minimally invasive technique cannot be overemphasized. ACOG and the ACS support BSE performed monthly beginning at age 20 as optional approach for early detection.
If BSE is advised, a discussion of the benefits, risks, and limitations for the individual patient should take place. Effective instruction of patients in the technique of BSE incorporates description of the procedure while the patient views the health care provider’s performance of the examination. The patient should be encouraged to reiterate her understanding of what has been taught and then demonstrate her mastery of the technique using manufactured breast models to further solidify compliance. The patient should be informed of the significance of breast inspection in various positions and the utility of breast palpation in the standing and supine positions. The circular
method of breast palpation using the pads of her fingers is the easiest to master, although for patients with pendulous breasts, positional changes to ensure positioning of the breast tissue on the chest wall must be emphasized. The best time to perform the examination is usually after the week after the end of the menstrual cycle, although menopausal women should pick a convenient time of the month, such as their birth date or the first day of each month. After hysterectomy, patients with continued estrogenic support of the ovaries should observe for breast fullness or tenderness. Breast examination should then be performed 7 to 10 days after cessation of menstrual breast symptoms.
Clinical Breast Examination
The CBE is an important part of early detection of breast cancer. The ACS, ACOG, and others recommend CBE beginning in the mid-20s for women at average risk for breast cancer. The USPSTF states that insufficient evidence exists concerning the risk or benefit of this practice. CBE has a sensitivity of 57% to 70% in detecting breast cancer.
Strategies to improve a practitioner’s ability to detect a mass include developing a systematic, consistent search mode, increasing the time devoted to the examination, and using a technique with variable degrees of pressure. The more facile and practiced the examiner, the greater the rates of detection of breast lesions.
CBE should be a routine part of the examination of gynecologic and obstetrical patients. Obstetrician-gynecologists should not abdicate their responsibilities by relying on previous examinations performed by other specialists. The practitioner must be mindful of the legal ramifications of errors of omission.
CBE is ideally performed 1 week after the end of the menstrual cycle to diminish the impact of luteal phase breast congestion, which can obscure mass detection. Each portion of the breast examination should be performed with the patient in both the sitting and supine positions because positional changes often expose a lesion that can be masked by the patient’s normal anatomy.
The initial part of the examination should focus on breast inspection, viewing for symmetry, skin retraction, rashes, and nipple retraction or deviation (
Fig. 45.6). Inspection should continue with the patient’s arms raised overhead. This allows visualization of the inferior and lateral aspects of the breast. Inspection with the patient placing her hands on her hips while contracting the pectoralis muscles will highlight any changes in the upper quadrants of the breast. In patients with rheumatoid arthritis or other conditions preventing pressure on the hip joint, other more comfortable options may be used. One may have the patient either grasp her fingertips at a level near the waistline while pulling laterally or press her palms together while extended above the head. Because the breast lies on the pectoralis muscle and Cooper ligaments are attached to the muscle and the skin, tension on the muscle accentuates carcinoma invading these structures. An inspection of the breast for nipple retraction or inversion, which can result from either stromal contraction or direct attachment to the tissues beneath
the nipple, should be performed (
Fig. 45.7). Nipple inversion can be normal, and if with gentle manipulation eversion occurs, it is considered a normal variant. New onset of nipple inversion is concerning and warrants additional evaluation.
Inspection of the breast is followed by palpation. The patient should be properly gowned, with only the area being examined exposed. The patient must be positioned (by tilting her hips and torso) to allow the portion of the breast tissue being examined to lie directly upon the chest wall. For patients with pendulous breasts, this may require positional changes for each breast. The pads of the fingers and not the fingertips are the most sensitive and should be used in examination. Three circular motions are made with three levels of pressure (superficial, medium, and firm) on each 1-cm square area of the breast. Not only does this improve mass detection but it also prevents masking a mass through excessive pressure.
The circular pattern is the most frequently used; however, the “vertical-strip” and radial or “wheel and spoke” patterns are acceptable techniques. With the circular method of palpation, the examination proceeds clockwise around the full circumference of the breast at its perimeter and gradually moves inward toward the nipple.
During the vertical-strip search pattern, the provider will begin overlapping circular motions in the axilla extending inferiorly (1 cm at a time) to one to two ribs below the breast tissue. The fingers are then moved inward 1 cm, and the pattern of search is extended superiorly, in a straight line, to the clavicle. This pattern is continued until the sternum is reached. The patient’s position may have to be changed several times during this portion of the exam to assure the tissue being examined lies directly upon the chest wall.
The wheel and spoke method requires radially palpating to the clavicle, sternum, and other bony margins and palpating from the nipple or vice versa. Each method should be mastered, as they may be helpful in certain clinical and diagnostic situations, such as patients with large breasts. Regardless of the pattern of breast examination used, the importance of using a consistent and methodical pattern of evaluation and allowing sufficient time for a thorough examination cannot be overemphasized.
Concurrently, the axilla is evaluated for masses. To allow proper assessment of the axilla in the sitting position, the arm should be extended at a 90-degree angle from the chest wall. This maneuver relaxes the area to be examined for a more thorough evaluation. Next, the patient should be placed in a comfortable supine position with her arm—on the same side as the breast being examined—raised above her head to evenly distribute the breast over the chest wall, thereby making its deeper regions of the axillary basin more accessible.
Palpation should extend beyond the actual breast tissue to encompass the supraclavicular and infraclavicular lymph nodes, the area adjacent to the sternum, approximately 1 to 2 cm below the inframammary ridge, and the axillary tail of Spence. In this manner, all the borders of the breast and associated nodal basins are examined. Gentle pressure on the nipple and areolar area can be used to elicit discharge. Excessive breast manipulation can lead to a nipple discharge. Using a milking technique improves the examiner’s ability to elicit a nipple discharge. Each quadrant of the breast is “milked” by sliding the fingers from the outer quadrant in a clockwise fashion toward the nipple and documenting the location of any fluid accumulation.
Careful and comprehensive documentation of the history, examination, and disposition of the case should be filed in the patient’s record. A clear and legible note should record all findings. The use of a diagram is also helpful (
Fig. 45.8). Although positive or abnormal findings are important, it also is valuable to list negative findings in the medical record.
Radiologic Breast Assessment
Imaging tests of the breast can be considered screening or diagnostic in nature. Screening tests are done in asymptomatic patients, while diagnostic tests are performed in patients with clinical findings such as breast masses, pain, or nipple discharge. Screening tests include mammograms, breast MRI, and tomosynthesis. Screening with breast ultrasounds is not commonly performed and is rarely indicated; however, increasing information suggests benefit from the use of ultrasound when mammography demonstrates dense breast tissue. Diagnostic tests include mammogram, breast ultrasound, breast MRI, tomosynthesis, and breast-specific gamma imaging.
Mammography
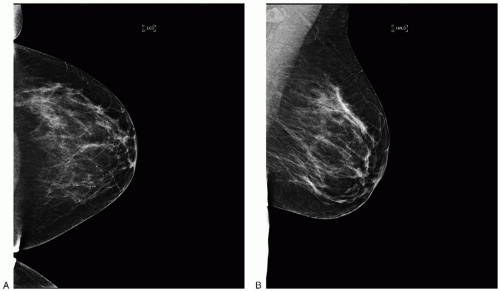
The most common imaging test used for screening is mammography (
Fig. 45.9A, B). Albert Salomon published the first study documenting that x-rays can be used to image breast cancer in 1913; however, many decades passed before mammograms were accepted as standard means of imaging the breast. The first randomized controlled trial of breast cancer screening with mammography, the health insurance plan (HIP) study was initiated in the 1960s. The first data were published in 1971; since then, a total of nine major randomized control trials have been completed and reported. A summary is presented in
Table 45.4.
It is important to note that these trials have been performed in many countries with varying schedules of screening, study population age, and mammographic technique. The HIP study was one of the few studies in which the control group had no screening mammograms. This study enrolled 62,000 patients aged 40 to 64 years and found that 30% fewer deaths in the study group compared to the control group. A major issue with this study is that the prestudy breast cancer prevalence was unclear in the control group. The screening group underwent examinations at study entry and excluded patients with breast cancer, while the control group did not have equivalent assessment. Whether or not the patient had breast cancer prior to entry into the study was determined retrospectively in the control group; thus, it is possible that the control group had more undiagnosed breast cancer at entry. This bias may favor the screening group with regard to breast cancer mortality rate. Another issue is that the trial used outdated mammographic equipment compared to today’s equipment. In Malmo, Sweden, another large randomized controlled trial was initiated in 1976. This trial had two cohorts that were randomized by birth year. This trial enrolled approximately 42,000 patients aged 45 to
69 years who subsequently underwent two-view mammograms every 18 to 24 months for a total of five screening examinations. This study did not find any reduction in deaths between the study and control groups, although a nonsignificant 20% lower breast cancer death rate occurred in the study group for women greater than age 55 years. Lack of a positive finding may have been due to the high number of women in the control group undergoing at least one mammogram (24% for the entire cohort, 35% in women aged 45 to 49 years). Only 70% to 74% of the patients in the study group underwent screening examinations. The Swedish two-county study was a large trial that enrolled patients in two counties in Sweden, Östergötland and Kopparberg. More than 133,000 women between ages 40 and 74 years were enrolled in this study. This trial was different from previous trials in that the four screening intervals were varied by age, every 24 months for women aged 40 to 49 and every 33 months for ages 50 years and older. Data reported after 8, 11, 14, and 20 years of follow-up revealed 30% to 32% fewer breast cancer deaths in the study group compared to the controls. Unlike the HIP and Malmo trials, the Swedish two-county study used a single-view mammogram—a screening test that is not comparable to the current standards of screening mammography in the United States. Another issue with this study is approximately 13% of the control group had mammograms as part of routine care. In Stockholm, Sweden, in 1981, a large trial randomly assigned approximately 40,000 patients aged 40 to 64 years to two rounds of screening every 28 months with a single-view mammogram. The screening group was found to have 29% to 26% fewer breast cancer deaths compared to the controls at 7.4 and 11.4 years of follow-up, respectively. However, these differences were not statistically significant. There were concerns about the randomization methods in this study, and the number of patients in each arm varied significantly. In 1979, a randomized controlled trial was initiated in Edinburgh, United Kingdom, and enrolled approximately 25,000 patients aged 45 to 64 years. The screening strategy consisted
of a two-view mammogram and clinical breast exam and then annual CBE followed by a single-view mammogram in years 3, 5, and 7. Socioeconomic differences found between the general medical practices were not recognized until the study ended; adjustments were then made for these differences. A nonsignificant 21% reduction in deaths from breast cancer was found between the study and control group. A significant 29% reduction in deaths from breast cancer was noted when breast cancer deaths that occurred 3 years after the end of the study were censored. Only 61% of the screening group underwent screening. A randomized controlled trial performed in Gothenburg, Sweden, in over 51,000 women aged 39 to 59 years randomly assigned participants to five rounds of screening. The screening group underwent a two-view mammogram every 18 months unless a prior screening test indicated that a single-view mammogram was sufficient (this accounted for 30% of the women screened). At 11 years of follow-up, the study group had a 44% statistically significant reduction in breast cancer mortality among women aged 39 to 49 years. Issues with this study included a delay of 3 to 8 months in the end-of-study mammogram in the control group compared to the study group and a complex randomization scheme that resulted in unequal numbers of patients in the study and control groups. Two large Canadian randomized controlled trials, National Breast Screening Study (NBSS) I and II trials, were initiated in 1980. These trials were designed to evaluate the role of screening mammograms in women aged 40 to 49 years and 50 to 59 years, respectively. The NBSS I trial is unique in that it was the first trial designed specifically to test the efficacy of mammographic screening in women younger than 50 years. In this trial, approximately 50,000 patients were randomly assigned to an annual two-view mammogram or CBE for 4 to 5 years. Compliance in the study group was good, with 85.5% of patients undergoing screening mammograms by year 5. In the control group, noncompliance was high: Only 26.4% of patients had at least one mammogram. Surprisingly, this trial revealed more breast cancer-related deaths in the screening group at 7 and 10.5 years of follow-up, 36% breast cancer-related deaths in the study group compared to 14% in the control group. At 11 to 16 years of follow-up, the increase in breast cancer-related mortality persisted, with 7% more breast cancer-related deaths in the study group. The NBSS II study had similar results in the 50-to 59-year-old cohort, with 3% and 2% more breast cancer-related deaths in the study group at 7 and 13 years of follow-up, respectively. There has been much speculation as to why these results have differed from all the other randomized controlled trials. One possible explanation was the statistically significant higher number of advanced cancers in the study group. While this may account for the high death rates, it is not clear why this occurred. Concerns have been expressed with regard to the equipment, technical adequacy of the mammograms, and training of the radiologists. An external independent review found some inadequacy with regard to the mammographic views from 1980 to 1985. The last randomized controlled trial published to date is the UK Age Trial designed to evaluate the role of mammographic screening starting at the age of 40 years. This trial was unique in that it avoided contamination that can occur by screening episodes after the age of 50 years by starting annual screening at the age of 39 to 41 years and comparing this group to women who were screened beginning at age 50 years. At 10.7 years of follow-up, a statistically nonsignificant reduction occurred in breast cancer mortality in both relative and absolute terms, with a relative risk of 0.83 (95% CI: 0.66 to 1.04) and absolute risk reduction of 0.40 per 1,000 women invited for screening (95% CI: 0.07 to 0.87). The major issue with this trial was that patients underwent a two-view mammogram at entry; subsequent screenings utilized single-view mammograms. The study was stopped early due to funding issues with lower post hoc power analysis than expected (72% vs. 80%).
Controversies with Regard to Mammographic Screening
There has been much debate over when mammographic screening should be initiated. The main issues with all randomized trials have been the heterogeneity of the type of screening (single-vs. two-view mammograms), length of time between screening exams (range of 12 to 28 months), and variation in the age of the study population. Due to these issues, there have been multiple meta-analyses of the randomized controlled trial data to date, all confirmed that mammographic screening decreased breast cancer mortality rate in patients who were invited for screening. The analysis by Wald and colleagues found a 26% reduction in breast cancer mortality in patients older than 50 years. Hendrick and colleagues found an 18% reduction in mortality rate in patients aged 40 to 49 years. The same authors found a 24% reduction in mortality rate in patients aged 50 to 74 years. The most recent update of the meta-analysis of the Swedish trials found a 21% reduction in breast cancer mortality between the study and control group; however, when various age groups were taken into account, the reduction in breast cancer mortality was not significant in patients younger than 50 years. In a 2004 meta-analysis of all the randomized controlled trials, Smith and colleagues found a 20% reduction in breast cancer mortality in women aged 40 to 74 years, a 15% reduction (P < 0.05) in women aged 40 to 49 years, and a 22% reduction (P < 0.05) in women aged 50 to 74 years. Given that many women who were invited to have mammographic screening did not have any screening done, the authors went on to analyze the actual effect of having a mammographic screen. When the results were analyzed only for women undergoing screening, the overall effect of mammographic screening was even higher, with a 40% reduction in mortality. Recently, the National Cancer Institute applied mathematical modeling with regard to outcomes of mammographic screening using six models with varying assumptions. All the models found that screening starting at the age of 40 years will lead to reductions in breast cancer mortality from 2% to 10% (median 3%) with annual mammographic screening. This would result in one breast cancer-related death averted per 1,000 women screened and 33 life years gained per 1,000 women. The life years gained per 1,000 women was lower at 25 years if biennial screening was done, but there was a reduction of approximately 50% in false-positive rates and unnecessary biopsies.
Overall, these data suggest that screening starting at the age of 40 years leads to reductions in breast cancer mortality rates but at increased cost. The National Cancer Institute (NCI) mathematical models suggest there would be approximately 1,250 false-positive results per 1,000 women screened. This has the potential to lead to approximately 88 unnecessary biopsies per 1,000 women. There is also a risk of radiation-induced mortality. It is estimated that that risk of radiation-induced breast cancer death is about 0.22 to 0.50 per 1,000 women screened. With regard to absolute benet-to-risk ratio, screening starting at age 40 years would still be expected to be of benefit; 1,894 women would need to be screened to prevent one breast cancer-related death, and one radiation-induced breast cancer-related death would likely occur per 6,456 women screened. The recent recommendation from the USPSTF states that screening should start at age 50 years and be conducted biennially. Although starting breast cancer screening at age 40 may result in increase in life years gained, the most efficient means of reducing the breast cancer mortality rate on the analysis of the ratio of benefits to screening exams was to start to screening at
age 50 years; hence, this was the basis for the recommendation. Additionally, biennial screening examinations maintained 81% of the benefit compared to annual exams while reducing the number of false-positive exams by nearly 50%. These recommendations are controversial as breast cancers in women younger than age 50 years are thought to be more aggressive and faster growing; thus, a biennial screening schedule may be too long. A recent analysis of the Surveillance, Epidemiology and End Results (SEER) database found that the biennial screening interval was associated with an increased risk of late stage disease (odds ratio 1.35, 95% CI: 1.01 to 1.81). Another study attempted to use the SEER database to evaluate the effect of screening mammogram on breast cancer incidence between 1976 and 2008. The authors found a significant increase in the number of early-stage breast cancer cases (112 to 234 cases per 100,000 women, i.e., absolute increase of 122 cases), while there has been a decrease of 8% in the number of late-stage disease (decrease of 102 to 94 cases per 100,000 women, i.e., absolute decrease of 8 cases). The authors concluded that only 8 of the 122 additional cases were expected to progress to advanced cancer; therefore, screening mammograms may be leading to an overdiagnosis of breast cancer. The main issue with this paper is that the SEER database does not record how the cancers were diagnosed or who within this dataset had screening mammograms. In a different analysis, the authors looked at the utility of mammographic screening in absolute numbers (
Fig. 45.10). In this analysis, one life may be extended per 1,700 to 5,000 women screened and followed for 15 years for women aged 50 to 70 years. The same analysis would predict that one or two lives may be extended per 5,000 to 10,000 mammograms in women aged 40 to 49 years. Recommendations from various national organizations are highlighted in
Table 45.5. All of these various screening recommendations are reasonable with varying risks and benefits.
Breast Magnetic Resonance Imaging for Screening
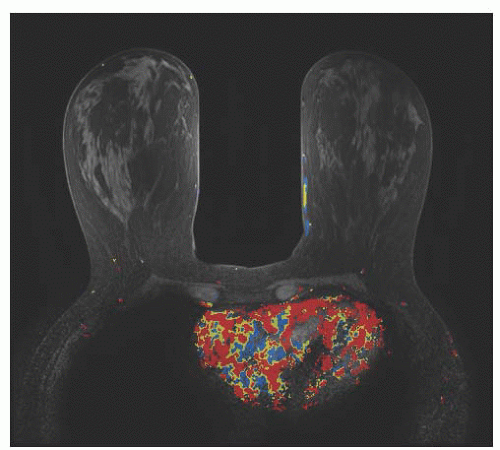
Breast magnetic resonance imaging (MRI) is typically used for diagnostic evaluation, that is, workup of breast masses or physical findings
(Fig. 45.11). The best data for using breast MRI as a screening test come from studies in high-risk populations. In 2004, Kriege and colleagues reported on 1,909 women at high risk for breast cancer (358 of whom were
carriers of germline mutations) who were screened with both breast MRI and mammograms. The authors found that the sensitivities of mammography and MRI in detecting breast tumors were 33.3% and 79.5%, respectively. It is important to note that while breast MRI sensitivity is higher than that of mammography, the tests were considered complementary as some breast cancers that were missed by breast MRI were detected by mammography. The overall sensitivity of testing was higher when the results of both tests were used, resulting in a combined sensitivity of 88%. This study demonstrated breast MRI had lower sensitivity for picking up DCIS compared to mammography (17% vs. 83%, respectively). A large study in Canada found that breast MRI was more sensitive than was mammography in the high-risk population. In this study, 236 women underwent screening with breast ultrasound, mammography, and MRI all on the same day. The authors found that breast MRI was the most sensitive test at 77% followed by mammography at 36% and ultrasound at 33%. Again, these tests appeared to be complementary as the sensitivity was improved to 95% when results from all three modalities were combined. Other studies have confirmed these results, and at present, the addition of breast MRI as part of a screening regimen is recommended for patients at high risk for developing breast cancer. The American Cancer Society’s most recent guidelines state that women should undergo screening with breast MRI if they have a BRCA1 or BRCA2 mutation or have a first-degree relative (parent, sibling, child) with a BRCA1 or BRCA2 mutation (even if they have yet to be tested themselves), if their lifetime risk of breast cancer has been scored at 20% to 25% or greater based on one of several accepted risk assessment tools for evaluating family history and other factors, if they have received radiation to the chest between the ages of 10 and 30, if they have a male relative with history of breast cancer at any age, or if they have Li-Fraumeni syndrome, Cowden syndrome, or Bannayan-Riley-Ruvalcaba syndrome or may have one of these syndromes based on history in a first-degree relative. The presence of dense breast tissue is not a reason for using breast MRI for screening despite the lower sensitivity of mammography in these patients. It may be reasonable to use breast MRI as a screening test for women with a personal history of lobular carcinoma of the breast as these cancers are difficult to detect via mammography. Another scenario in which screening with breast MRI may be appropriate is in women whose original cancer was not visible on mammography.
Breast Ultrasound
The most common use of breast ultrasound is as an adjunct to mammography. In one analysis of the six studies of screening ultrasounds with a total of 42,838 exams, only 150 (0.35%) additional cancers were only visible via ultrasonography. The largest study to date was performed by the American College of Radiology Imaging Network (ACRIN Protocol 6666). This study enrolled 2,809 women with dense breasts who had elevated risk of breast cancer (i.e., a personal history of breast cancer, lifetime risk >25% by Gail or Claus model, atypical ductal hyperplasia [ADH], atypical lobular hyperplasia [ALH], lobular carcinoma in situ [LCIS], atypical papilloma, presence of BRCA mutation, elevated 5-year Gail model risk, or history of chest radiation). This study found that the addition of screening ultrasound will increase the detection of breast cancer by 4.2 per 1,000 women screened, but will substantially increase the number of false-positive exams. At present, the most common use of breast ultrasound is to further evaluate abnormal findings on mammography and not necessarily as a primary screening tool. It may be of benefit in women with dense breasts; however, the American College of Radiology states that “the addition of ultrasound to screening mammography may be useful for incremental cancer detection.” Due to the increase in false-positive rates and decrease in positive predictive value, it is not considered the standard of care for primary screening in women with dense breasts.
Digital Mammography
Digital mammography is a newer technology in which a digital detector is used in place of standard film. The images are then processed by a computer and displayed on a monitor. This results in images with greater resolution that can be easily magnified on a workstation. The contrast can also be adjusted to greater degree than in standard film. The ease in which the images can be manipulated is thought to lead to potential improvements in sensitivity. One of the largest studies to date is the Digital Mammographic Imaging Screening Trial (DMIST). The study compared film with digital mammography in over 49,000 women. Overall, the sensitivities of digital versus film mammography were similar (70% vs. 66%, respectively). Digital mammography was found to be 15% to 30% more sensitive for the detection of breast cancer in premenopausal women and in women with dense breasts (improvement in sensitivity from 51% to 55% to 70% to 78%, respectively). In another study that randomly assigned over 25,000 women aged 45 to 69 years old to either digital mammography or film mammography, a higher prevalence of breast cancer was found with digital mammography (0.59% vs. 0.38%, respectively). Recall rates were higher with digital mammography in comparison to film mammography (4.2% vs. 2.5%, respectively), but no difference in positive predictive value was observed between the two types of mammography. Unlike the DMIST trial, overall sensitivity was higher in the digital mammography arm at 77.4% versus 61.5% seen in the film mammography group. At the present time, digital mammography appears to be useful for breast cancer screening especially in younger patients and patients with dense breasts.
Breast Tomosynthesis
Breast tomosynthesis is a modification of digital mammography that utilizes a moving x-ray source and digital detector. This allows for three-dimensional images to be generated using
computer algorithms (similar to CT images of the body) that can overcome the limitation of overlapping tissue that can decrease the sensitivity of traditional film mammography. This technique can avoid additional compression views and recall mammograms. A number of studies have compared breast tomosynthesis to routine two-view mammography with the majority of studies consisting of small case series. At the present time, there is no consensus of whether or not the sensitivity of breast tomosynthesis is higher than that of the traditional twoview mammography as some studies have shown decreased sensitivity while others have shown increased sensitivity. Notably, all of these studies demonstrated the specificity of breast tomosynthesis to be higher than that of two-view mammography. In one study, the use of breast tomosynthesis reduced falsepositive recalls by 40%. The best role for breast tomosynthesis may be to use it in conjunction with two-view mammography. When breast tomosynthesis is performed in combination with two-view mammography, superior accuracy can be achieved.
Breast-Specific Gamma Imaging
Breast-specific gamma imaging (BSGI) is a nuclear medicine technique that measures the mitochondrial density of breast tissue through the use of technetium-99m sestamibi as a tracer. It is expected when there is increased mitochondrial density in breast cancer tissue, and by tagging the mitochondria at a cellular level, breast cancers can be detected. This technology is still relatively new in comparison to the other breast imaging techniques, and as such, there are still limited data on its utility. In general, the majority of the studies have found BSGI to have similar sensitivity to that of breast MRI. In one retrospective review, 159 women with a concerning physical exam and/or mammograms underwent additional testing with BSGI to assess for occult disease; BSGI was able to detect additional suspicious lesions in 29% of the women of whom 3% had occult cancer in the contralateral breast. In a study of 146 women at high risk for breast cancer, BSGI was found to have an overall sensitivity of 96.4% and specificity of 59.5%. A more recent study of 149 women also demonstrated a similar sensitivity rate of 98.0%. In one study that directly compared BSGI to breast MRI, the sensitivities were similar (88.8% vs. 92.3%, respectively), but BSGI appears to have improved the specificity to 90% compared to 39.4% in breast MRI. The main advantage of BSGI is that the study does not require the patient to lie in a prone position and it may be easier to perform in a patient with severe claustrophobia. However, the data are limited given the small cohorts in these studies. More studies are needed to determine the true sensitivity and specificity of BSGI to establish its role in breast imaging.
Positron Emission Mammography
Positron emission mammography (PEM) was designed as a way to use typical whole-body PET technology in breast imaging. Whole-body PET scanning is not particularly useful in imaging the breast due to its low resolution. Resolution with PEM is increased through the use of two detectors that are arranged in similar fashion to conventional mammogram. The dye used is the same as that used in PET scans, a positron-emitting isotope of fluorine,18F-2-deoxy-2-fluoro-D-glucose (FDG). Early small studies of PEM demonstrated sensitivities of 80% to 86%. One of the larger studies conducted in 94 women found that PEM had a sensitivity of 91% and specificity of 93% when interpreted with mammographic and clinical findings. However, the role of PEM in breast imaging requires further investigation; a recent study found PEM to be inferior to breast MRI in detecting contralateral breast cancers.
Thermography
This test is designed to measure the skin temperature over the breast, as it is thought that the presence of breast cancer may cause elevated localized skin temperature. No data exist to support the use of breast thermography, and such testing is not supported by guidelines from any major organizations. The U.S. Food and Drug Administration issued a communication in June of 2011 stating that breast thermography is not a replacement for mammography as a means of breast cancer screening and is not considered to be an effective screening tool.
Computer-Aided Detection
Computer-aided detection (CAD) uses software-based artificial intelligence to function as a “second reader” of mammographic films (
Fig. 45.12). Second or double reading of films can improve sensitivity but with some loss of specificity. Improved detection rates upward of 15% have been reported but with an associated increase in false-positive or recall rates up to 45%. It functions as a second reader by identifying areas of concern that the radiologist must then characterize. It is estimated that CAD can correctly identify approximately 80% of cancers. Like double reading, CAD was shown to increase sensitivity but with a decline in specificity.
BENIGN PATHOLOGY
Fibrocystic Change
Fibrocystic change (FCC) of the breast is the most frequently encountered benign breast disorder. It occurs most often in women of reproductive age between 30 and 50 years. FCC does not increase the risk of developing breast cancer, but it can make the physical examination of the patient more difficult.
In 85% to 90% of cases of significant FCC, breast discomfort is the leading symptom. Women often present with a history of bilateral, menstrual-related, painful, tender, and nodular breasts, most often localized to the upper outer quadrants. Typically, the pain is most severe just before menses as a result of the normal physiologic stromal edema and ductal dilation.
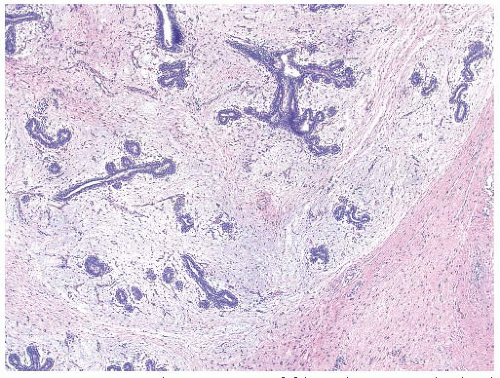
FCC encompasses several histopathologic categories, including microcyst and macrocyst formation, hyperplasia of ductal epithelium, apocrine metaplasia, papillomatosis, duct ectasia, sclerosing adenosis (dense fibrotic tissue surrounds the acini), and stromal fibrosis. In essence, it has no single defining histologic entity (
Fig. 45.13). Providing no atypia is identified, there is no increased risk of developing breast cancer when FCC is present.
Table 45.6 lists benign pathologic findings and subsequent risk of breast cancer development. An explanation of relative risk is provided in
Table 45.7.
Fibroadenoma
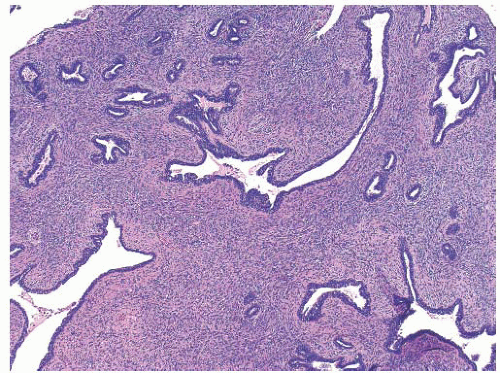
Fibroadenomas are benign tumors that consist of both fibrous and stromal elements. These are common lesions particularly in adolescents but can be seen in woman of all ages particularly prior to menopause. These lesions are thought to represent an exaggerated response to estrogen. Clinically, the patient presents with a palpable mass that is well circumscribed, rubbery in consistency, and often multilobulated. Lesions are generally 2 to 3 cm. They are commonly found in the upper outer quadrant, and in approximately 25% of cases, there are additional lesions that are present in either breast. Imaging modalities can include ultrasound and, if age appropriate, mammography. On sonography, the lesions are avascular and well circumscribed. Mammogram will often detect a solitary nodule with smooth borders with a halo around the mass. In the adolescent population, mammography should be avoided unless the index of suspicion for malignancy exists. Fine needle aspirate or core needle biopsy can confirm the diagnosis. Observation and reassurance are reasonable options for management. Excision should be considered for enlarging masses or discordant imaging or pathology finding or if the patient desires removal. Histologic evaluation demonstrates a well-circumscribed lesion with both epithelial and stromal elements. One or the other element often dominates. The epithelial component has well-defined, glandlike, and ductlike spaces, whereas the stromal component consists of bands of collagen (
Fig. 45.14).
Phyllodes
These fibroepithelial lesions have diverse biologic behavior. Originally, this entity was termed cystosarcoma phyllodes,
but this terminology was deemed confusing as less than 5% of these lesions actually behave in a malignant fashion. Clinically, they are more common in women in their 30s and 40s, but presentation can mimic fibroadenomas with the findings of a solitary, well-circumscribed, mobile, multilobulated mass with a rubbery to firm texture. Phyllodes tumors tend to be larger and exhibit more rapid growth. Radiographic imaging with ultrasound and mammography can be similar to fibroadenomas. Determination of the diagnosis on percutaneous sampling can be difficult given similarity to fibroadenomas. On gross examination, the lesions can be gray or tan and tend to be plump and fleshy in texture, and a branching pattern can be seen (
Fig. 45.15). On histologic inspection of the lesions, they lack a true capsule. A leaflike pattern involving both the epithelial and stromal components is diagnostic (
Fig. 45.16). The stromal component determines the grading of the tumor to include benign, borderline, and malignant entities. Stromal cellularity, atypia, pushing or infiltrative borders, and the presence of stromal overgrowth can be useful in evaluating prognosis and predicting behavior. Surgical excision with a margin of normal breast tissue is recommended. Local recurrence rates of 20% have been reported in instances of inadequate margins with less risk of recurrence for those with benign features.
Adenomas
Adenomas are well-circumscribed benign lesions primarily composed of epithelium with rare stroma. This distinguishes them from the diagnosis of fibroadenoma. Radiographic imaging findings with ultrasound and mammography can be similar to fibroadenomas. There are two basic types: tubular adenomas and lactating adenomas. Generally seen in young women, tubular adenomas tend to ovoid with a pseudocapsule and minimal stroma. An inner epithelial layer and an outer myoepithelial layer line the tubules. Clinical presentation is similar to fibroadenomas with a localized well-defined lesion. Lactating adenomas are seen in pregnancy or postpartum state. In these circumstances, there may be more than one lesion. On gross examination, they are tan in color and softer in texture than a tubular adenoma. Microscopically, cuboidal cells with secretory activity line the tubules. Diagnosis of these entities can be made on percutaneous biopsy. Radiographic imaging in a pregnant woman should be restricted to sonography unless a high suspicion of malignancy exists. Additionally, mammographic imaging of the breast of gravid or lactating women will be compromised due to increased density. Percutaneous or excisional biopsy during pregnancy or lactation is difficult due to increased vascularity. The presence of milk enhances risk of infection and milk fistula formation.
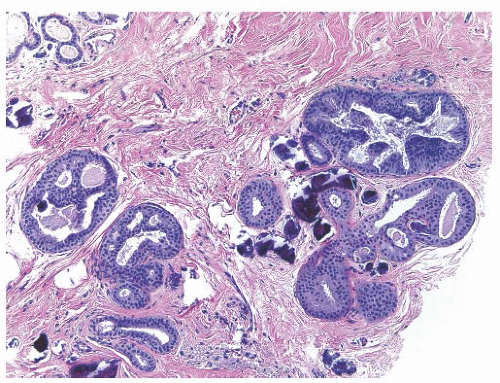
Papilloma
Papillomas are hyperplastic lesions categorized as central (solitary) or peripheral (multiple). Solitary papillomas are found in the major lactiferous ducts and are most often associated with the bloody nipple discharge. These are commonly seen in women between 30 and 50 years of age. They vary in size from a few millimeters to 1 cm, are attached to the duct walls by a thin stalk, and are prone to infarction. Histologically, they are composed of multiple, branching, and interanastomosing papillae arranged around a fibrovascular core. There can be areas of atypia that will classify them as atypical papilloma; on rare occasions, they are associated with DCIS or invasive cancer. From an epidemiologic point of view, solitary papillomas do not appear to markedly elevate the risk of subsequent breast cancer development.
Peripheral intraductal papillomas tend to be multiple and bilateral. The condition affects younger women and is not usually associated with nipple discharge. The condition itself raises the individual’s risk of developing breast cancer, suggesting they are subject to malignant transformation.
Atypical Hyperplasia
Atypical hyperplasia encompasses both ADH and ALH. ADH is often found on percutaneous biopsy where the targeted lesion contains microcalcifications. ALH is often an incidental finding without discrete radiographic changes.
On histologic inspection, ADH has a proliferation of uniform epithelial cells with round nuclei filling part of the duct (
Fig. 45.17). ALH has proliferation of monomorphic, evenly spaced cells filling part but not all of the lobules and sometimes the surrounding ducts. Both ALH and ADH share similar architectural and cytologic features with their in situ counterparts.
When ADH is found on percutaneous core biopsy, the recommendation is for excisional biopsy. Ideally, the site has been previously marked at the time of biopsy with a titanium clip. Utilizing dye, wire, or radioactive seed localization can help the surgeon identify the site of concern. The basis of excisional biopsy is the concern of upgrading to either DCIS or invasive cancer, which occurs in 10% to 20% of cases. Larger core needles and a larger number of cores are associated with a lower incidence of DCIS and invasive cancer at excisional biopsy.
The presence of ADH increases the individual’s personal risk of breast cancer (RR 3.7 to 5.5). This is conferred to either the ipsilateral or contralateral breast. Risk reduction strategies including the use of chemopreventative agents and lifestyle modification can be employed.
For ALH found on percutaneous biopsy, a similar approach to the management for ADH is utilized. Recently, the role of additional excision for areas of ALH has been called into question. For some women with concordant mammogram and pathologic findings, additional excision may be unnecessary.
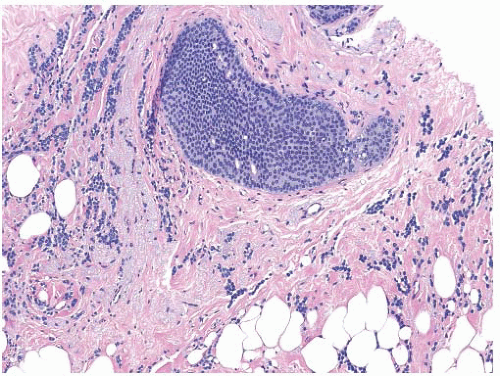
Lobular Carcinoma In Situ
Originally described in 1941 as a rare form of mammary cancer, LCIS is now considered a benign condition. It is histologically characterized by the involvement of terminal duct lobular unit with small cells with round nuclei that distorts these spaces (
Fig. 45.18). The condition tends to be multifocal in the affected breast and often an incidental finding. At the time of this initial report, recommendation for this condition was at least a total mastectomy. Contralateral breast involvement has been reported to be approximately 30%. The condition is often an incidental finding on percutaneous core needle biopsy and has no mammographic or clinical correlate. At times, mammographic microcalcifications can be an indicator of its presence. Average age of diagnosis is between 40 and 50 years. The lesion is associated with increasing an individual’s breast cancer risk estimated to be 1% to 2% per year with a lifetime risk of 30% to 40%. The cancer may be ductal or lobular in nature and involve either breast. The term LCIS is often associated with the diagnosis of carcinoma; hence, there have been efforts to group both ALH and LCIS into the term lobular intraepithelial neoplasia (LIN), which accounts for a spectrum of conditions and is graded LIN1, LIN2, and LIN3 depending on morphology and clinical outcome. This classification system has not been universally accepted. Current clinical management of LCIS is individualized and includes the use of observation, chemopreventative agents, and surgical management of bilateral total mastectomy.
COMMON CLINICAL SCENARIOS
Several clinical scenarios occur in the evaluation of a patient for breast-related issues. Patients may present with complaints of a mass or thickening, abnormal mammogram, nipple discharge, or breast pain. Management and workup of each of these clinical scenarios is described below.
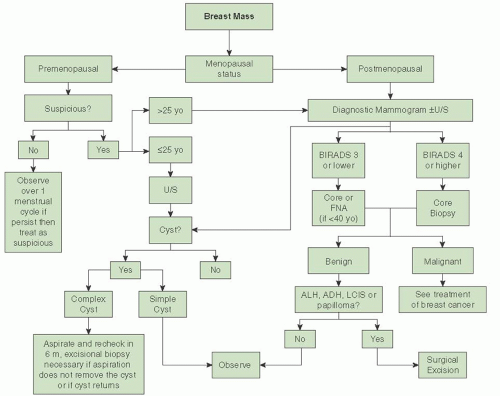
Evaluation of a Breast Mass
Many potential causes of breast mass exist, most of which are not related to a breast malignancy. One should always consider the patient’s menopausal status during the evaluation of a breast mass. The likelihood that a mass is malignant is based on the patient’s history. A thorough history and physical examination should be performed as part of the initial workup of a breast mass. The main point in the history-taking process is to get a sense of how long the mass has been present and whether it fluctuates with her menstrual cycle (if she is premenopausal) or mass has increased in size or whether any trauma to her breast has occurred. Masses that fluctuate with the patient’s menstrual cycle are more likely to be benign. Breast trauma leading to fat necrosis can also lead to a breast mass; a commonly overlooked cause of breast trauma is a shoulder strap/seatbelt injury. Surgeries to the breast can cause scarring and fat necrosis that mimics a carcinoma. Patients with a personal history of breast cancer are at risk for recurrence of breast cancer. It is estimated that the risk of recurrence is approximately 0.7% per year and this risk is considered to be cumulative. Careful attention should be paid to the patient’s family history and ethnicity. Ashkenazi Jewish patients have higher risks for harboring a BRCA mutation as it is estimated that the baseline risk in this population can be as high as 1 in 40. This risk can increase markedly depending on what age breast cancer is diagnosed. Strong family history with multiple members with breast and/or ovarian cancer also increases the patient’s
risk for harboring BRCA mutations.
Table 45.8 is a summary of the salient points to consider in the history taking process. Enlarging masses are concerning as are masses in patients with strong family histories of breast cancer.
A thorough physical examination should be conducted, identifying the location of the area of concern and documenting the size of the mass, the presence of any skin retraction/changes, nipple discharge, presence or absence of axillary and/or supraclavicular adenopathy, whether the lesion is mobile, whether the mass is firm or rubbery, and whether there is breast symmetry. Physical findings concerning for malignancy include a hard, irregularly shaped mass, presence of axillary and/or supraclavicular adenopathy, retraction or redness of the overlying skin, or an associated bloody nipple discharge. While breast cancer can present as a fixed mass, this is an uncommon finding as most breast tumors, unless very posterior, are not fixed to the pectoralis major until late in their course. At times, especially in a patient with very firm breasts, the tumor can be deceptively difficult to palpate and the only obvious finding is breast asymmetry with the involved breast being either larger or smaller (if there is retraction of the surrounding tissue). See
Table 45.9 for the summary of the salient parts of the physical exam. Typically, masses that are concerning for malignancy are firm masses with associated skin retraction, masses in axilla, or masses causing breast asymmetry.
Once the history and physical examination is complete, the next part of the evaluation is to determine the role of imaging tests. This assessment is based on the patient’s menopausal status. Imaging tests are appropriate in patients who are postmenopausal even if the mass does not seem to be suspicious. Mammographic evaluation is the initial step, even if the patient has had a mammogram within the past year. Additional testing with breast ultrasound may be helpful especially in patients with dense breasts or if breast cysts are suspected. In a premenopausal patient, a nonsuspicious mass (smooth, mobile, ballotable) can be observed over one menstrual cycle. If the mass persists, imaging tests are recommended. In young patients (<25 years), mammograms are of little use due to the high breast density. In these patients, ultrasound should be the initial test. Regardless of menopausal status, if the mass on ultrasound is a cyst, a fine needle aspiration (FNA) of the cyst is indicated only if it is complex in nature or if the patient is symptomatic. Repeat imaging with ultrasound should be performed in 6 months to assess for cyst recurrence. If aspiration does not resolve the cyst completely, if it returns in 6 months, or if the aspirated fluid is bloody in nature, then an excisional biopsy should be performed. Simple cysts are not associated with malignancy, and no further workup is required. If the mass in not found to be a cyst, then either a percutaneous core or excisional biopsy (when a core is not possible) of the mass should be performed. In patients younger than 40 years, FNA can be utilized if examination and imaging test indicate a benign process. If the FNA is negative, the risk of the patient for having a breast malignancy is extremely low as the physical examination and imaging tests are concordant with the biopsy results. If the patient is 40 years old or older, then an image-guided core biopsy with either mammographic guidance (stereotactic core biopsy) or ultrasound guidance is recommended. Subsequent treatment is dependent on the results of the core biopsy. In general, most benign lesions do not require additional treatment unless atypical or papillary cells are found. These lesions are hyperplastic and can be associated with underlying malignancy; therefore, an excisional biopsy is required. The other “benign” lesion that requires an excisional biopsy is LCIS. While these lesions are not malignant, concurrent cancer may be found at the time of the excisional biopsy. Additionally, excisional biopsy should be performed if the results of the physical exam, pathology, and imaging results are not concordant; this is called the “triple test.” The radiologist should review all imaging tests after the pathology report is issued to assess for concordance. If the radiologist does not feel that the biopsy explains the radiographic findings, an excisional biopsy is mandated. See
Figure 45.19 for algorithm of workup of a breast mass.
Abnormal Screening Evaluation
A common clinical scenario is a patient presenting with an abnormal screening mammogram. Further evaluation and treatment of such abnormal mammographic findings should be based on Breast Imaging Reporting and Data System (BI-RADS) score. This American College of Radiology classification lexicon should be included in all mammogram reports.
Table 45.10 details the BI-RADS classification system. Further workup is dependent on the BI-RADS category associated with the mammogram. BI-RADS 0 signifies that further work with spot compression views, breast ultrasound, or other imaging modalities are required to complete the evaluation. BI-RADS 1 indicates that the mammogram looks completely normal without any mammographic findings. BI-RADS 2 indicates a mammographic finding associated with benign disease (e.g., fibroadenoma, simple cyst). An inherent false-negative rate exists, and a negative mammogram (i.e., BI-RADS 1 or BI-RADS 2) does not rule out malignancy if a palpable mass
is present. (Refer to
Fig. 45.19 for evaluation of a palpable mass.) A mammogram interpreted as having findings that do not appear suspicious is classified as BI-RADS 3; by definition, these findings should have a 2% or less risk of malignancy; a repeat mammogram in 6 months is reasonable given the very low risk of malignancy. A BI-RADS 4 report indicates findings that are suspicious for malignancy. This category is further divided into 4A, 4B, and 4C. The risk of malignancy can be as high as 95% based on the subcategory. In these cases, a core biopsy should be considered in order to rule out an underlying malignancy. The same workup should be performed for BI-RADS 5, which indicates the findings are highly suspicious for malignancy (
Fig. 45.20).
In general, when a biopsy is warranted as part of the additional workup, a percutaneous core biopsy should be performed as it provides histopathology. Ideally, the biopsy should be done with the imaging modality in which the mass was discovered. Studies have shown a concordance rate close to 100% between an open excisional biopsy and a core biopsy. Core biopsies have the advantage of being less invasive and resulting in a smaller scar. Even in cases of BI-RAD 5 in which underlying cancer is almost a certainty, a core biopsy allows the surgeon to strategize the operative approach; a diagnosis of DCIS in a patient undergoing breast-conserving surgery does not require sentinel mapping where an invasive cancer would require both partial mastectomy and a sentinel lymph node biopsy (SLNB).
Stereotactic core biopsies may not be possible in some cases (e.g., the breast size is small and compresses to less than 17 mm or if the lesion is superficial or close to the chest wall). Given that the core needle mechanism extends at least 1.5 cm beyond the needle tip, the issue of a through and through injury exists in this circumstance. In these cases, if the lesion is visible on ultrasound, an assessment of feasibility of core biopsy without compression is an option versus a localization and excisional biopsy procedure.
Further treatment depends on the histologic diagnosis. High-risk proliferative lesions such as atypical lobular/ductal hyperplasia, papillary lesions, and LCIS should be treated with an excisional biopsy. Treatment of breast cancer is discussed elsewhere in this chapter.
Evaluation of Nipple Discharge
One of the most common breast complaints is nipple discharge with up to 80% of women reporting this symptom. Of note, manipulation of the breast and nipple can yield a small amount of clear discharge in most premenopausal women. This section will discuss the management of galactorrhea and pathologic nipple discharge.
An estimated 10% to 15% of patients who present with nipple discharge have an underlying malignancy. Discharge can be classified as related to lactation, physiologic or pathologic (
Table 45.11). Nipple discharge that is concerning can present as unilateral, bloody, and/or spontaneous discharge arising from a single duct. Discharges that come from multiple ducts or those that are bilateral tend to be less often associated with malignancy. Discharge from breast can be milky, clear, green, yellow, brown, or bloody. Intraductal papillomas are the most common cause of nipple discharge followed by ductal ectasia and cancer. These anatomic lesions present with unilateral nipple discharge, while physiologic causes such as galactorrhea will present with bilateral nipple discharge. Physiologic causes of galactorrhea include prolactinomas, pharmacologic agents, and any condition that can lead to increased prolactin production. Breast infections can also lead to nipple discharge. The initial workup of nipple discharge entails a thorough history and physical examination. One should get a sense of how long the discharge has been present and whether it was spontaneous, bloody, or unilateral. One also should ask if the discharge was seen coming from one or more ducts. Questions concerning headaches and vision changes should be considered as a
means to check for the presence of a symptomatic prolactinoma. A thorough evaluation of the patient’s medications is important as common medications such as cimetidine, metoclopramide, and some antipsychotics can also lead to galactorrhea
(Table 45.12). In examining the breast, one should pay attention to how many ducts the discharge is coming from, whether or not the discharge is reproducible, and if it is unilateral or bloody. An evaluation should also be made to see if a mass is present and whether or not there are skins changes consistent with Paget disease by the nipple (i.e., scaly rash originating from the center of the nipple spreading outward) or if any signs of infection are present. Guaiac assessment for occult blood (Hemoccult test) may be helpful in the evaluation, but the absence of does not preclude an underlying malignancy. A mammogram and ultrasound should be ordered as part of the workup. A ductogram may help localize the lesion more accurately. Breast MRI may be helpful, but normal imaging cannot rule out an underlying malignancy. If any lesion is found on these imaging tests, an image-guided biopsy should be performed. If no lesions are found, duct excision is still considered standard treatment. If the duct with the discharge can easily be identified, a limited resection of the duct can be done by placing a lacrimal duct probe or fine suture in the affected duct and performing a directed excision. Approximately 2 to 5 cm of tissue distal from the nipple should be removed at the time of surgery.
Figure 45.21 demonstrates an algorithm for evaluation of nipple discharge.
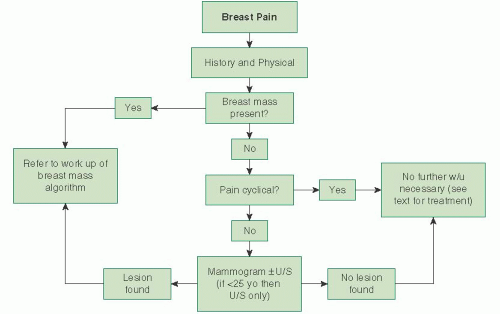
Evaluation of Mastalgia
Breast pain or mastalgia is one of the most common breast complaints. In one survey of premenopausal women, 75% of respondents stated that they had this symptom; 30% stated it was moderate to severe in nature. Typically, mastalgia is not considered to be a sign of breast cancer as the frequency of underlying breast cancer in women with breast pain is between 1.2% and 6.7%. Of note, the literature indicates that breast pain is a presenting symptom in 5% to 17% of patients with breast cancer. In one series, mastalgia was the only symptom in 7% of patients. The evaluation of the patient with breast pain should begin with the determination of whether or not the pain is cyclical in nature. Cyclical mastalgia is thought to be due to hormonal stimulation of the breasts. It is not uncommon to have some minor breast discomfort toward the latter half of the menstrual cycle, and this presents as diffuse bilateral breast pain. Cyclic mastalgia is not associated with cancer, and unless there are physical findings of concern, further workup is not necessary. By definition, noncyclical breast pain is not associated with the menstrual cycle. Despite a thorough workup, etiology of the pain often is not found. Noncyclical breast pain may be due to the stretching of the Cooper ligament, mastitis,
ductal ectasia, hidradenitis suppurativa, medications (e.g., hormone replacement), increased intake of caffeine, chest musculoskeletal issues, previous surgery to the breast, or in rare circumstances inflammatory breast cancer (IBC).
The workup for breast pain begins with a thorough history and physical examination. Assessment should include the location, severity, and duration of the pain, whether it is bilateral, associated skin changes, previous breast surgery and/or other trauma, and if the pain limits her ability to perform daily activities. The patient’s medications should be reviewed with particular attention given to oral contraceptive pills or hormone replacement medications, which can cause breast pain. Physical examination should assess the patient for any signs of trauma, skin changes, masses, or nipple discharge. The axillary and supraclavicular area should be examined for signs of adenopathy.
Figure 45.22 depicts an algorithm for evaluation of mastalgia.
Patients with IBC can present with a firm, tender, and erythematous breast. Often, the onset is rapid, and the skin may have the typical peau d’orange appearance (dimpling and thickening of the skin) with or without an underlying mass. Mastitis or breast abscess can be associated with mastalgia. This often occurs in lactating women although subareolar abscesses also can occur in nonlactating women. Typically, the abscess will present as a painful mass close to the nipple or with skin changes over the nipple consistent with cellulitis. On occasion, painful masses can be due to hidradenitis suppurativa. This is thought to be due to occlusion of the terminal parts of the follicular acroinfundibulum and can present either on the breast or in the axilla. The lesions can present as solitary painful nodules without an area of central necrosis and can have secondary cellulitis.
Attention to the chest wall during physical examination is essential, as costochondritis can mimic breast pain. Costochondritis pain is parasternal and reproduced by pressing on the costochondral junctions. Most often, the physical exam will be normal with no masses or skin changes found to suggest breast cancer, cellulitis, breast abscess, or hidradenitis suppurativa. It is reasonable to order a breast mammogram and a targeted ultrasound of the tender area, though the yield of such testing is low; one study in 987 women found only four cancers (a prevalence of 0.4%). Breast MRI is not indicated, as no studies suggest any clinical utility at this time.
Treatment of mastalgia will be dependent on the underlying cause. Antibiotics treatment for a breast abscess and cellulitis is generally successful, but refractory cases may require incision and drainage. If possible, a culture of the infected area can be taken and antibiotics regimens adjusted accordingly. In cases in which no underlying cause is found, treatment should be aimed at improving support of the breast through the use of better fitting bras. In patients with large pendulous breasts, a bra with steel underwire may provide relief. Reducing caffeine intake may benefit some patients; however a large case-control study did not find an association between caffeine and breast pain. Several medications have been prescribed for mastalgia. Medical management with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen should be the first line of treatment. In cases in which medications have not been effective, a topical NSAID such as Aspercreme (Sanofi, Chattanooga, TN) or the Flector Patch (diclofenac epolamine topical patch (13%) Pfizer, Mission, KS) can be tried. Evening primrose oil at 3,000 mg/day or vitamin E at 1,200 IU/day also can be prescribed; however, efficacy may be due to a placebo effect as multiple randomized trials have not proven them to be effective treatment. Randomized trials have demonstrated that tamoxifen 10 mg/day decreases mastalgia, but this drug can be associated with side effects such as hot flashes, vaginal dryness, leg cramps, increased risk of venous thrombolic events, and development of endometrial cancer. Danazol, a synthetic testosterone (100 to 400 mg/day in two divided doses), also has proved effective for the treatment of mastalgia but can have significant androgenic side effects.
TREATMENT OF BENIGN BREAST DISEASE
Most benign lesions can be treated with observation alone, providing there is concordance between the clinical findings, radiographic imaging, and the cytologic or histologic results. Using these three criteria is referred to as the “triple test.” Cytopathology and histology can be obtained through
percutaneous fine needle aspirate and core biopsy, respectively. If all components of the test are concordant with a benign entity, the chance of breast cancer is low (0.7%). Indeterminate or suspicious findings in any one of the components is an indication for further evaluation until confirmation of either a benign or malignant entity is found. Excisional biopsy without percutaneous sampling is a reasonable alternative when the patient desires removal of a mass with benign features.
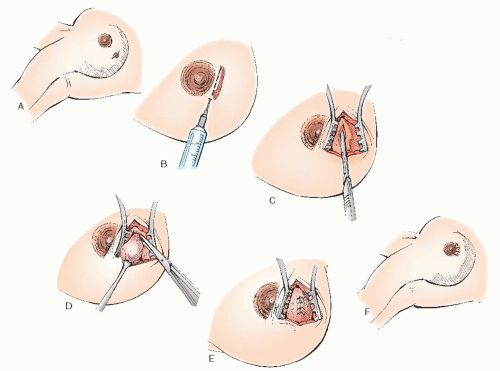
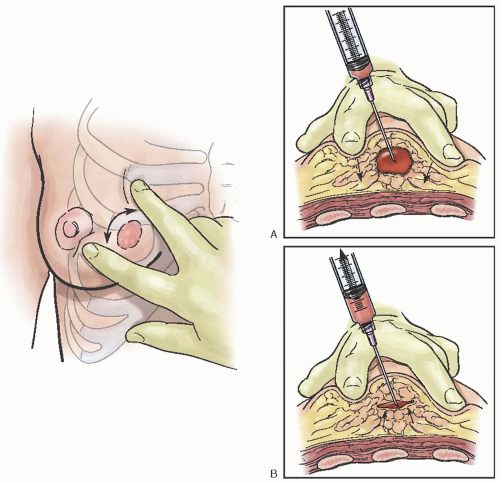
Fine Needle Aspiration
Fine needle aspiration (FNA) is an established diagnostic method in the evaluation of palpable breast lesions. In response to the increasing frequency with which women are consulting their obstetrician-gynecologists about concerns related to their breasts, the American Board of Obstetrics and Gynecology (ABOG) has specific educational requirements for resident training in the various aspects of diagnosing and treating breast disease. Technical proficiency in this procedure is recommended. In most areas where dedicated breast imaging centers exist, it is often the radiologist that performs this procedure.
FNA has a sensitivity of 96% and specificity of 98% (similar to core biopsies) as well as a 99% positive predictive value and 94% negative predictive value. It has a false-negative rate as high as 20% most notably in the detection of lobular cancers and carcinoma in situ. Performance of the procedure, preparation of the material for analysis, and the facility of the pathologist can affect these rates.
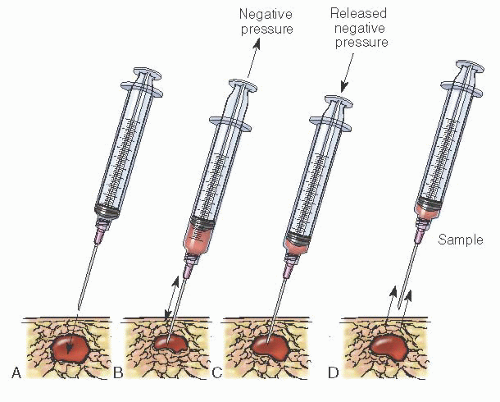
FNA has multiple advantages
(Table 45.13) over excisional biopsy and is helpful in confirming the clinical impression of both benign and malignant breast disease. Nevertheless, a negative cytologic report, as with a negative mammographic report, must not be relied on to rule out malignancy in a clinically suspicious lesion. Any clinically suspicious mass in a patient with negative FNA findings requires additional evaluation, including core or excisional biopsy.
FNA is an easily mastered technique, but the adequacy of specimens is improved with experience and training.
Table 45.14 lists suggested equipment for this approach. Procedure risks include infection, bleeding, and bruising at the site. Because the chest wall is immediately beneath the site, pneumothorax is a potential complication. The small size of the needle and assuring the mass is stabilized over a rib during the procedure significantly decreases this complication.
After the procedure is explained to the patient and consent has been obtained, the skin is cleansed. The lesion is identified and stabilized, preferably over a rib, between the fingers of the nondominant hand. The area can be anesthetized by injecting and raising a wheal of 0.5% to 1.0% lidocaine in the skin over the mass. A 21- to 23-gauge needle with a clear hub attached to a 5- to 20-mL syringe is then inserted into the central portion of the mass; the smaller the syringe, the greater the pressure that can be generated during aspiration. Increasing the needle gauge does not necessarily increase the sample size as it can raise the risk of bleeding and hematoma formation. Two fingers are placed under the piston of the syringe, and the thumb is used against the body of the syringe to pull the piston toward the examiner, applying negative pressure
(Fig. 45.23). A syringe pistol grip holder may be used to facilitate this procedure. With use of the holder, the grips of the holder are pulled together to apply negative pressure.
When a breast cyst is encountered, complete drainage of the mass should be attempted. During the aspiration, the fluid will appear in the hub and then fill the syringe. Most often, the fluid is clear but at times can be cloudy, gray, or green. This fluid can be appropriately discarded. By employing this approach, an immediate diagnosis of cyst can be made and the patient relieved of her concern of over the mass. If the cyst aspirate is bloody, the mass does not disappear, or reoccurs, the patient should be considered a candidate for excisional biopsy. It is important to reinforce to the patient the benefits of following recommended screening guidelines.
After complete sampling along each axis of the mass, suction is released and the needle is removed from the lesion. Releasing the suction before removing the needle is a critical aspect of this procedure. Continued negative pressure upon removing the needle (without release of suction) results in aspiration of the specimen into the body of the syringe that can be difficult to retrieve. Once the needle is removed, pressure is applied to the puncture site. A sterile dressing is then applied to the site.
The material should be expelled onto a glass slide with the needle bevel facing down to prevent scatter of the specimen. To release additional sample, the needle is detached, air is drawn into the syringe, the needle is reattached, and further expulsive efforts are accomplished. The “detach, draw air, and reattach” process is repeated until all the cellular material has been expelled. A second slide is placed on top, and the material is smeared between the two slides. Immediate fixation with 95% ethanol or a spray fixative should occur to prevent drying of the cells. The examiner must provide the cytopathologist with an accurate clinical history.
In the instance of a solid mass, the lesion is sampled by moving the needle up and down within the mass several times. Several passes should be made into every portion of the mass to prevent false-negative samples. Valuable cytologic material is harbored within the needle and hub and is then used for evaluation
(Fig. 45.24).Although FNA is cost-effective, several factors have led to diminished use of this technique, including the inability to diagnosis invasive versus in situ disease, the variability in sampling based on the provider’s abilities, the limited training of pathologists in cytopathology interpretation, and the rising rates of percutaneous core biopsy. Current preference is for histologic assessment, and this can be obtained through large core needle biopsy.
Core Needle Breast Biopsy
It is estimated that approximately one million breast biopsies are performed yearly to diagnose approximately 200,000 breast cancers. Percutaneous core needle biopsy may spare many of these women the need for more deforming, invasive, and expensive surgical procedures. With this procedure, a
histopathologic diagnosis is rendered compared with cytologic evaluation with FNA. Studies indicate a high degree of accuracy, with a sensitivity of 89%, specificity of 100%, positive predictive value of 98%, and an 80% negative predictive value in confirming invasive disease. False-negative results may occur, but false-positive results are rare except with radial scars. Increasing the number and the size of core specimens has increased the accuracy of the procedure.
Core needle biopsy may be performed under tactile, stereotactic, or ultrasound guidance. Less tissue is removed during core needle biopsy compared with excisional biopsy, resulting in no deformity in the breast and minimal to no scarring on subsequent mammograms. Another advantage is the ability to distinguish between invasive and in situ carcinoma. Because special training is not required to interpret the histologic material obtained from core needle biopsy, this obviates the need for skills of a cytopathologist. Given the larger needle size, local anesthesia is required, higher rates of postprocedure bleeding and hematoma formation occur, and there have been instances in which the track of the biopsy has been “seeded” with tumor leading to recurrences at the skin puncture sites. The use of an introducer needle has minimized the latter risk.
At times, core needle biopsy may not be possible due to lesions that are close to the nipple, lesions that are very posterior or superficial, or when the breast compresses to less than 1.7 cm.
This biopsy procedure is often used in the assessment of BI-RADS category 4 and 5 lesions. If core biopsy of a category 4 lesion yields a benign diagnosis concordant with the imaging characteristics, the woman is spared a diagnostic surgical biopsy. Although most often performed for nonpalpable lesions, percutaneous image-guided core biopsy also can be helpful in the evaluation of palpable breast masses, particularly those that are deep, mobile, or vaguely palpable. An advantage of image guidance is the ability to obtain radiographic documentation of the procedure. For select health care providers, this procedure can be performed in their office with or without ultrasound guidance.
Stereotactic core needle biopsy requires dedicated equipment but is helpful in evaluating suspicious mammographic findings that have no palpable or ultrasound correlates. Ultrasoundguided core needle biopsy has several advantages, including real-time visualization of the needle, lack of ionizing radiation, accessibility to all parts of the breast and axilla, multipurpose use of equipment, and lower cost. However, it is limited in detection of lesions less than 1 cm and has decreased sensitivity in detection of microcalcifications. Vacuum-assisted devices (e.g., Mammotome, Cincinnati, OH) may improve tissue sampling by obtaining larger intact samples. The choice of approach depends on several factors, including lesion visibility and accessibility, equipment availability, and preferences of the radiologist.
The area is cleansed and administration of local anesthesia is performed. An introducer needle is placed into the site of interest, and then the coring device is inserted through this conduit
(Fig. 45.25). This can minimize the risk of seeding
the biopsy track with malignant cells and allows for the core needle to be reintroduced multiple times without increasing the risk of further tissue disruption. As the core needle is introduced into the mass, the tissue inside the core of the needle is cut from the surrounding tissue as the outer sheath is advanced. The core needle device is spring loaded allowing for a mechanized capture of the tissue. After removal of the sheath and core needle, a pressure dressing is applied. In some circumstances, the entire lesion can be removed. After the core biopsies are obtained, a titanium clip is deployed into the biopsy site to identify the area for future localization if excisional biopsy is required. A variety of shapes of clips are available, allowing the radiologist to perform multiple biopsies to aid in further identification of these sites. The histopathologic findings then dictate whether observation or further assessment should occur. Disconcordant histology, that is, the imaging is concerning for malignancy but the biopsy is benign, is an indication for excisional biopsy. The radiologist should provide an addendum to the report when the pathology from the biopsy is available stating what the findings are and whether they confirm a benign entity and observation is acceptable or if further assessment is required. In instances where a cancer has been diagnosed, the core specimen can be assessed to determine the hormone and HER2 receptor status.
Excisional Biopsy
This procedure is primarily employed when a benign lesion such as a fibroadenoma or papilloma is present. The technique may be used with or without a localization procedure. The lesion in question is removed in its entirety often without additional breast tissue or margin. The surgeon should choose an incision that offers ready access to the site with minimal cosmetic disturbance (
Fig. 45.26). Curvilinear, circumareolar, and radial incisions are chosen depending on location and the size of the lesion. If the incision is made along Langer lines, an improved cosmetic outcome is achieved (
Fig. 45.27A, B).