Diabetic Ketoacidosis and Continuous Insulin Infusion Management in Pregnancy
Maribeth Inturrisi
Nancy C. Lintner
Kimberlee Sorem
It has been estimated that approximately 2 million women of reproductive age (18 to 44 years) have diabetes; about one-third of these women do not know they have the disease.1 Diabetes is the most common metabolic disorder complicating pregnancy. In the United States, the incidence of diabetes in pregnancy is estimated to be 7 to 14 percent.1 The type of diabetes most often diagnosed during pregnancy is gestational diabetes mellitus (GDM), which accounts for 95 percent of cases. Pre-gestational diabetes, type 1 or type 2, accounts for approximately 5 percent of diabetes cases seen in pregnancy. Both pre-gestational and gestational diabetes increase the risk for adverse maternal, fetal, and newborn outcomes and future metabolic abnormalities.
Identification and careful management of hyperglycemia during pregnancy can reduce adverse maternal and infant outcomes.2,3 The risk for severe hyperglycemia during pregnancy increases:
as gestation advances
in association with rapid weight gain or obesity
when infection is present
during tocolysis with beta-mimetics and/or high-dose steroid administration to advance fetal lung maturation.
These situations increase the risk of diabetic ketoacidosis (DKA), an acute, life-threatening metabolic complication of diabetes associated with increased risk of maternal and fetal morbidity and mortality. Management of DKA or attentuation of its occurrence often requires continuous insulin infusion. Depending on the urgency of the clinical situation, an intravenous insulin infusion (continuous intravenous insulin infusion, or CIII) or a continuous subcutaneous insulin pump infusion (continuous subcutaneous insulin infusion, or CSII) will be utilized.
A comprehensive discussion of diabetes in pregnancy, including management of diabetes in preterm labor or specific infections is beyond the scope of this chapter. This chapter will focus on the pathophysiology of glucose metabolism related to DKA and factors and events that predispose a patient to the development of DKA. Clinical management strategies for patients with DKA in pregnancy are reviewed in detail. In addition, the clinical management of continuous insulin infusions, both intravenous and subcutaneous, will be briefly discussed.
Diabetic Ketoacidosis
The incidence of DKA in pregnancy is approximately 1 to 3 percent.4,5 The fetal mortality rate in cases of maternal DKA is approximately 9 percent.4 The risk of maternal death has been estimated at less than 1 percent.6 Although more prevalent in patients with type 1 diabetes, there are case reports of DKA in patients with GDM and in patients with type 2 diabetes who have a concomitant illness (e.g., infection) or following administration of medications (e.g., corticosteroid or β2 agonist). Between 78 and 90 percent of cases of DKA during pregnancy occur in the second and third trimesters, typically as a result of increased insulin resistance associated with advancing gestation.4,5 DKA with blood glucose (BG) levels of less than 200 mg/dL does occur during pregnancy and necessitates prompt recognition and treatment.7,8
DKA during pregnancy fortunately has become a relatively uncommon event.9 Kilvert and colleagues reported 11 cases of ketoacidosis in 635 insulin-treated pregnancies between 1971 and 1990.4 One fetal loss and one spontaneous miscarriage occurred in this study. Advances in clinical management and BG monitoring
have contributed to the reduction in DKA. However, when DKA does occur, it represents a medical emergency for mother and fetus.
have contributed to the reduction in DKA. However, when DKA does occur, it represents a medical emergency for mother and fetus.
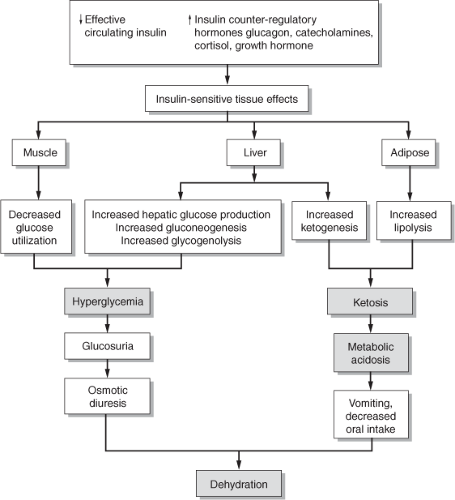 Figure 10-1 Pathophysiology of diabetic ketoacidosis. Adapted from Carroll, M.A., & Yeomans, E. R. (2005). Diabetic ketoacidosis in pregnancy. Critical Care Medicine, 33(Suppl. 10), S347–S353. |
Patients with DKA classically present with a triad of concurrent abnormalities: dehydration, ketosis, and metabolic acidosis.10 It occurs when there is either an absolute or relative deficiency in circulating insulin levels in the context of an excess of insulin counter-regulatory hormones.10 The latter include catecholamines, glucagon, cortisol, and growth hormone, all of which are increased in DKA when compared to baseline.10,11 Additional consequences associated with insulin deficiency include accelerated lipolysis, hepatic glucose and ketone production, hyperglycemia, ketonemia, depletion of water and electrolytes, increased anion gap (greater than 12), and metabolic acidemia (pH less than 7.3; bicarbonate less than 15 mEq/L). Typical symptoms of ketoacidosis include nausea and vomiting (most common during pregnancy), fruity breath, dry mouth, thirst, abdominal pain, polyuria, weakness, alteration in mental status, and labored breathing.10
Pathophysiology of DKA
The underlying pathophysiologic disturbances in DKA need to be assessed carefully throughout the course of treatment. Careful trending of clinical and laboratory data allows for optimal and timely treatment of underlying acidemia and hyperglycemia and prevents potentially lethal complications, such as severe hyponatremia and hypokalemia.
Pathophysiologic changes associated with DKA are depicted in Figure 10-1.10 Overall, a shift from the normal carbohydrate metabolism of the fed state to one of primarily fat metabolism typical of the fasting state initiates the process. When carbohydrates are not available, caused either by a lack of insulin or insulin utilization at the cellular level, the body is unable to transport glucose into cells, a process that is necessary for the maintenance of normal, aerobic metabolism. With an obligatory alternate shift to fat oxidation, free fatty acids (FFAs) are produced in adipocytes and transported to the liver bound to albumin. There they are
broken down into acetate, and then turned into ketoacids (e.g., acetic acid, β-hydroxybutyrate, and acetone).12 In the absence of insulin, the liver maximally initiates this ketogenic pathway such that the supply of these moderately strong acids exceeds peripheral utilization and maternal buffering capacity in the circulation. The presence of excessive ketone bodies, combined with increased lactic acid caused by decreased peripheral uptake of glucose, results in metabolic acidosis.13
broken down into acetate, and then turned into ketoacids (e.g., acetic acid, β-hydroxybutyrate, and acetone).12 In the absence of insulin, the liver maximally initiates this ketogenic pathway such that the supply of these moderately strong acids exceeds peripheral utilization and maternal buffering capacity in the circulation. The presence of excessive ketone bodies, combined with increased lactic acid caused by decreased peripheral uptake of glucose, results in metabolic acidosis.13
As glucagon levels rise, the combination of excess glucagon and insufficient insulin inhibits glycolysis and stimulates gluconeogenesis, which worsens hyperglycemia. As the degree of hyperglycemia and ketonemia increases, there is a rise in serum osmolarity. As a result, hyperglycemia and ketones serve as an osmotic reservoir, leading to diuresis, profound hypovolemia, and hypotension. Hypovolemia then stimulates other counter-regulatory stress hormones (e.g., catecholamines, growth hormone, and cortisol), which further enhance the release of glucagon. This sequence of events contributes to the continued cycle of dehydration, increased serum osmolarity, and increased release of insulin counter-regulatory stress hormones, therefore worsening acidemia that subsequently leads to acidosis.
As a result of osmotic diuresis, the loss of free water can be significant and may approach 100 to 150 mL/kg body weight. For example, a woman who weighs 165 lb (75 kg) may lose over 11 liters of free water from osmotic diuresis. The increased production of urine is accompanied by depletion of electrolytes, especially sodium, potassium, and phosphorus.
Significant dehydration secondary to DKA leads to a significantly depleted potassium level (as much as 5 mEq/kg of body weight). Consequently, low serum potassium levels may be a sign of severe hypokalemia, since potassium is lost not only at the intracellular level but also from within the circulation. Further, sodium levels may be normal, high, or low, and are proportionate to the degree of hydration and hyperglycemia. For example, for each 100 mg/dL of glucose above 100 mg/dL, serum sodium is decreased by approximately 1.6 mEq. Conversely, when glucose falls, serum sodium levels rise by a corresponding amount.
How Pregnancy Affects DKA
Select physiologic changes of pregnancy contribute to an increased predisposition for the development of DKA during pregnancy. An increase in pulmonary minute ventilation is a result of an increased tidal volume. This relative hyperventilation causes a state of respiratory alkalemia which results in a compensatory renal excretion of bicarbonate. The net effect is decreased total buffering capacity, which makes the pregnant diabetic more susceptible to development of metabolic acidemia and subsequently DKA.13 In addition, emesis, commonly present during the first trimester, may result in dehydration significant enough to predispose to the development of DKA. Predisposing factors and events associated with the development of DKA are listed in Table 10-1.13
Table 10.1 Predisposing Factors and Precipitating Events for DKA in Pregnancy | ||||||
|---|---|---|---|---|---|---|
| ||||||
Norbert Freinkel described pregnancy as a state of “accelerated starvation.” He noted that, when pregnant women skipped breakfast and had a prolonged period of fasting, lipolysis was enhanced. Measured plasma glucose concentrations in pregnant women after fasting longer than 14 hours demonstrated that final FFA and β-hydroxybutyrate levels were strongly correlated and inversely related to final plasma glucose levels. These results show why the common practice of meal skipping by personal preference or for medical testing should be avoided in pregnant women.14
Insulin sensitivity has been shown to decrease as gestation advances, particularly in the third trimester with decreased hepatic sensitivity to insulin, postprandial hyperglycemia, and an increased risk for ketoacidosis.9 Increased insulin antagonists from the placenta such as human placental lactogen (HPL), prolactin, cortisol, and tumor necrosis factor (TNF) alpha act on insulin-sensitive tissues to produce alternate substrate for energy use during DKA. A concomitant rise in levels
of stress hormones (e.g., glucagon, corticosteroids, catecholamines and growth hormone) further enhances hepatic gluconeogenesis, glycogenolysis, and lipolysis and contributes to the diabetogenic state.13
of stress hormones (e.g., glucagon, corticosteroids, catecholamines and growth hormone) further enhances hepatic gluconeogenesis, glycogenolysis, and lipolysis and contributes to the diabetogenic state.13
In addition to predisposing factors to DKA, there are also recognized precipitating events to DKA in pregnancy (see Table 10-1) including poor compliance with insulin regimens or meal plans, viral or bacterial infection, obstetrical use of steroids and/or administration of beta-mimetics, insulin administration failures, poor management, diabetic gastroparesis, and newly diagnosed diabetes.13
It is important to emphasize that pregnant women with diabetes are susceptible to the development of DKA at lower glycemic levels than non-pregnant individuals with diabetes. A study reported by Cullen showed that in pregnancies complicated by diabetes, 2 percent of patients demonstrated classic symptoms of DKA during the 10-year study period.4 Ten of 11 patients presenting with DKA (90 percent) demonstrated nausea, vomiting, and decreased caloric intake. Plasma glucose levels of less than 200 mg/dL were present in 4 of the 11 patients (36 percent). Despite contemporary methods of diabetes care, near-normal plasma glucose levels do not preclude DKA. Nausea, vomiting, and decreased caloric intake in an otherwise normal pregnant woman with diabetes requires evaluation to exclude ketosis.4 This has been demonstrated in women with type 1 diabetes who have had insulin withheld due to seemingly normal BG values. Therefore, insulin should not be withheld for more than a few hours in a patient with type 1 diabetes, even with a normal BG value.7
How DKA Affects Pregnancy
DKA in pregnancy occurs more often in women with type 1 diabetes rather than type 2 diabetes, and it is rare in GDM. Some ethnic groups have ketosis-prone type 2 diabetes, such as African Americans and Hispanics, and development is usually associated with the cessation of medication, infection, pancreatitis, or obesity.15 Overall, the prevalence of DKA during pregnancy has declined from 16.7 percent before 1960, to 7.8 percent from 1965 to 1985, and to 1.2 to 4.1 percent from the 1990s to 2003.16
DKA profoundly affects both the mother and the fetus. Literature from the last decade supports a fetal loss rate of approximately 9 percent in pregnancies in which DKA occurred.4 Maternal volume depletion and acidosis leading to decreased uterine blood flow may cause a relative fetal hypoxemia.17 Glucose and ketones readily cross the placenta, at maternal levels.13




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


