Diabetes Mellitus and Pregnancy
E. Albert Reece
Carol J. Homko
The discovery of insulin in 1921 brought about a significant improvement in the overall outlook for women with diabetes and their reproductive potential. The incidence of both maternal and perinatal mortality has markedly decreased over the past 80-plus years as a result of this discovery as well as a multitude of other scientific advances, including fetal heart rate and blood glucose monitoring and neonatal intensive care. However, despite these advances, women with diabetes as well as their offspring remain at increased risk for a number of complications. The increased morbidity is directly related to the severity of maternal hyperglycemia. Therefore, the management goal of these pregnancies is strict glycemic control prior to conception and throughout gestation. This is best accomplished through the provision of multidisciplinary team care and targeted self-management education.
Fuel Metabolism
Pregnancy is recognized as having a profound effect on maternal fuel metabolism. These pregnancy-related alterations in maternal metabolism are necessary to meet the demands of the developing fetus. Studies of lean, healthy pregnant women have demonstrated a greater than normal sensitivity to the blood glucose–lowering effect of exogenously administered insulin during early pregnancy as compared with late gestation. In addition, insulin responses to an oral glucose load have been demonstrated to be increased in early pregnancy as compared with the nonpregnant state in the same glucose-tolerant women.
These increases in serum insulin levels and insulin sensitivity produce a milieu during early gestation that favors maternal fat accumulation in preparation for the rise in energy requirements associated with the rapid growth of the fetoplacental unit during late pregnancy (Fig. 15.1). Moreover, the increases in plasma concentrations of estrogen, progestins, and cortisol observed during early pregnancy also are likely to stimulate fat accumulation.
Late gestation is characterized by accelerated growth of the fetoplacental unit, rising plasma concentrations of several diabetogenic hormones including human placental lactogen and estrogens, and increasing insulin resistance. Several investigators have demonstrated increased first- and second-phase insulin release during late gestation as well as increased plasma insulin and glucose ratios. Studies using the euglycemic-hyperinsulinemic clamp and minimal model techniques have reported that peripheral insulin sensitivity is reduced by 33% to 50% during late gestation. During the third trimester of pregnancy, insulin-stimulated carbohydrate oxidation is reduced out of proportion to the decrease observed in insulin-stimulated glucose uptake. Endogenous glucose production also is significantly less inhibited during the third trimester when compared with either the second trimester or the nonpregnant state. Thus, there appears to be general agreement that the second half of pregnancy is associated with increasing insulin resistance both in the periphery (muscle) and at the hepatic level.
The cause or causes of the increased insulin resistance during late pregnancy are not entirely clear (Fig. 15.1). The parallel development of insulin resistance and increases in blood levels of human placental lactogen, a hormone with strong lipolytic and anti-insulin action, suggests that human placental lactogen and perhaps other diabetogenic hormones, including cortisol, progesterone, and estrogens, may be responsible for much of the observed insulin resistance. In addition, there also is evidence to support a role
for plasma free fatty acids and inflammation in the development of insulin resistance during late pregnancy.
for plasma free fatty acids and inflammation in the development of insulin resistance during late pregnancy.
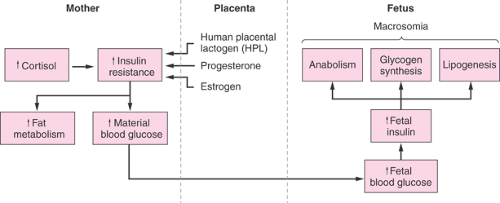 Figure 15.1 Schematic diagram of metabolic alterations in diabetes mellitus in late pregnancy (in absence of appropriate therapy). |
The development of peripheral and hepatic insulin resistance after midpregnancy can be seen as an effort of the mother to adapt to the fuel needs of the rapidly growing fetus. During the third trimester of pregnancy, glucose uptake by the fetus has been estimated to be ∼33 μmol/kg per minute. To satisfy this additional need, peripheral insulin resistance increases in pregnant women, thus reducing maternal glucose utilization. In addition, hepatic insulin resistance increases, which increases hepatic glucose production. Moreover, by decreasing carbohydrate oxidation, much of the glucose entering the muscle is shunted into alanine or lactate, which can be recycled into glucose.
Classifying Diabetes
Diabetes mellitus is generally classified into the following categories: type 1 or insulin-dependent diabetes mellitus, type 2 or noninsulin-dependent diabetes mellitus, and gestational diabetes mellitus (GDM). Approximately 10% of all individuals with diabetes mellitus have type 1 diabetes. Beta cell destruction, with resulting insulin deficiency, is the hallmark of this disorder. Onset is generally before the age of 30 years, and as a result, this type of diabetes is frequently encountered in women of childbearing age. It is estimated that type 1 diabetes complicates approximately 0.2% of all pregnancies in the United States annually.
Type 2 diabetes is the most common form of the disease, affecting nearly 90% of all individuals with diabetes. Type 2 diabetes is characterized by defects in both insulin action and secretion. It typically is seen in individuals over the age of 40 years and therefore in the past was felt to be uncommon in women of childbearing age. However, in recent years, the incidence of type 2 diabetes has been increasing steadily among younger individuals, and data from the National Maternal and Infant Health Survey indicates that type 2 diabetes complicates 0.3% of all pregnancies in the United States. Gestational diabetes mellitus is defined as carbohydrate intolerance of variable severity with onset or first recognition during the index pregnancy.
If the abnormality in glucose tolerance persists after pregnancy, the patient’s diagnosis is revised to type 1, type 2, or impaired glucose tolerance (IGT).
Gestational Diabetes Mellitus
GDM is a common problem that complicates approximately 5% of all pregnancies in the United States. The likelihood of developing GDM is significantly increased among certain subgroups, including individuals with a positive family history of type 2 diabetes, advancing maternal age, obesity, and. nonwhite ethnicity. Excess risks for both GDM and IGT have been demonstrated in black, Hispanic, and Native American women as well as in women from the Indian subcontinent and the Middle East.
Screening and Diagnosis for Gestational Diabetes Mellitus
Screening of pregnant women for GDM remains a topic of great debate, both in this country and throughout the world. In 1998, the Fourth International Workshop-Conference on GDM acknowledged that there were certain populations of low-risk women in whom it may not be cost-effective to routinely screen for GDM. This low-risk group includes women who were not members of ethnic minorities, were less than 25 years of age, had no first-degree relatives with diabetes, and are of normal body weight. Although selective screening may be appropriate in certain populations with a low prevalence of type 2 diabetes, universal screening is advocated in most centers. The American College of Obstetricians and Gynecologists (ACOG) recommends that all pregnant patients be screened for GDM whether by patient’s history, clinical risk factors, or a laboratory screening test. However, the American, Canadian,
and British Diabetes Associations recommend biochemical screening.
and British Diabetes Associations recommend biochemical screening.
Screening should be performed between 24 and 28 weeks gestation, although women with significant risk factors may benefit from being screened earlier in pregnancy. A 50-g glucose challenge test is performed without regard to the time of day or interval since the last meal. A venous plasma glucose is measured 1 hour later, with a value of ≥140 mg/dL indicating the need for a 3-hour 100-g oral glucose tolerance test (OGTT). However, lowering the cutoff value to 130 to 135 mg/dL increases the yield of cases of GDM by 10%. Thus, by lowering the cutoff, the sensitivity is increased yet the number of false-positive screening tests increases as well. The use of either threshold is acceptable.
The OGTT is performed after an overnight fast and 3 days of an unrestricted carbohydrate diet. A fasting blood glucose level is drawn, and a 100-g glucose load is then administered. Plasma glucose levels are drawn 1, 2, and 3 hours following ingestion of the glucose solution. Diagnosis requires that at least two of the four glucose levels of the OGTT meet or exceed the upper limits of normal. Currently, there are two sets of diagnostic criteria in use in the United States—those of the National Diabetes Data Group and those of Carpenter and Coustan. The Fourth International Workshop Conference believed that there was sufficient evidence to suggest that the Carpenter-Coustan criteria, with its lower cutoff values, more accurately predict neonatal risks and has recommended the use of these criteria. ACOG guidelines support the use of either set of criteria.
In addition, the Fourth International Workshop-Conference acknowledged an alterative to the two-step screening/diagnostic procedure described previously. The evaluation of glucose intolerance during pregnancy may be made by using a one-step approach or 2-hour 75-g OGTT. This approach is considered most applicable in high-risk populations. Currently, studies are under way to establish the relationship between the various diagnostic criteria and perinatal outcomes in these pregnancies.
Women with GDM should be evaluated at the first postpartum visit by a 2-hour OGTT using a 75-g load. Greater than 90% of women will convert to normal glucose tolerance following delivery. However, studies indicate that the risk for overt diabetes may be as high as 20% to 50% in this population. Long-term annual follow-up is therefore indicated. GDM is influenced greatly by body weight, with the highest rate occurring in obese patients. Women with a history of GDM should be counseled regarding the use of lifestyle changes such as weight loss and regular exercise to reduce this risk.
Maternal Complications
Despite improvements in pregnancy outcomes, women with both GDM and pre-GDM are at greater risk for a number of pregnancy-related complications (Table 15.1). These include preterm labor, infectious morbidities, hydramnios, and hypertensive disorders. In addition, multiple investigators have reported a significant association between poor glycemic control and hypertensive disorders, preterm labor, and infections. Furthermore women with pre-GDM also are at risk for the acute complications of diabetes because of the metabolic alterations associated with pregnancy as well as the effects of strict glycemic control. The vascular alterations associated with long-term diabetes also contribute to the higher morbidity rates observed in women with diabetes. Both diabetic nephropathy and retinopathy may progress during pregnancy and should be monitored closely.
TABLE 15.1 Pregnancy Complications in Diabetes | ||
|---|---|---|
|
Current data would seem to indicate that pregnancy is an independent risk factor for diabetic retinopathy. Hypertension, poor control early in pregnancy, and rapid normalization all appear to be associated with the potential to accelerate retinal deterioration. Furthermore, women with more advanced forms of retinopathy and a longer duration of diabetes are at highest risk for progression. All women with type 1 diabetes for 5 years or more or type 2 diabetes at diagnosis require a thorough dilated ophthalmologic evaluation. This may necessitate preconception fluorescein angiography, as dye studies generally are contraindicated during pregnancy. Ideally, these evaluations should be completed prior to attempting conception. Laser therapy, if indicated, also needs to be completed prior to conception.
In contrast, most studies that have observed the effects of pregnancy on diabetic nephropathy have suggested that pregnancy is not associated with either the development
or progression of preexisting nephropathy in women with mild to moderate disease. However, diabetic nephropathy is the complication of diabetes that is most likely to affect pregnancy outcomes. There are increased risks for pregnancy-induced hypertension and/or a progression of already existing hypertension, intrauterine growth retardation resulting in small-for-gestational-age infants, preterm deliveries secondary to fetal distress, and a 10-fold increase in the incidence of stillbirth over women with diabetes but without nephropathy. Preeclampsia is the most frequent, serious complication of maternal nephropathy, with implications for both mother and fetus. Close monitoring of blood pressure, with addition or adjustment of antihypertensive agents as needed, is recommended. The drugs of choice are methyldopa, calcium channel blockers, and β-blockers. Select calcium channel inhibitors, such as diltiazem, induce mild reductions in blood pressure but have a potent effect on decreasing excess protein excretion.
or progression of preexisting nephropathy in women with mild to moderate disease. However, diabetic nephropathy is the complication of diabetes that is most likely to affect pregnancy outcomes. There are increased risks for pregnancy-induced hypertension and/or a progression of already existing hypertension, intrauterine growth retardation resulting in small-for-gestational-age infants, preterm deliveries secondary to fetal distress, and a 10-fold increase in the incidence of stillbirth over women with diabetes but without nephropathy. Preeclampsia is the most frequent, serious complication of maternal nephropathy, with implications for both mother and fetus. Close monitoring of blood pressure, with addition or adjustment of antihypertensive agents as needed, is recommended. The drugs of choice are methyldopa, calcium channel blockers, and β-blockers. Select calcium channel inhibitors, such as diltiazem, induce mild reductions in blood pressure but have a potent effect on decreasing excess protein excretion.
Neonatal Complications
The offspring of women with diabetes remain at increased risk for a number of complications, which includes congenital anomalies, fetal macrosomia, respiratory distress syndrome (RDS), and metabolic abnormalities as well as long-term sequelae (Table 15.1).
Perinatal Mortality
The two major causes of perinatal mortality are unexplained fetal death and congenital malformations. The causes of unexpected death are not well understood. In animal models, sustained hyperglycemia has been associated with increased insulin secretion, elevated fetal oxygen consumption, acidosis, and death. It has been postulated that fetal polycythemia and increased platelet aggregation could explain the increased incidence of intravascular thrombosis in infants of diabetic mothers and that thrombotic episodes could be the underlying cause for late unexplained intrauterine deaths.
Approximately 40% of perinatal deaths that occur among infants of women with diabetes can be attributed to malformations. Diabetes mellitus is one of the most common maternal conditions that results in anomalous offspring. The frequency of major congenital anomalies is increased two- to threefold over the general population. Great diversity is seen in the types of malformations associated with insulin-dependent diabetes mellitus. The most frequent types of malformations involve the central nervous system and cardiovascular, gastrointestinal, genitourinary, and skeletal systems, with cardiac malformations being the most common.
The defects most often associated with diabetes occur during organogenesis before 7 weeks gestation. Clinical series in humans have shown an association between malformations and glucose control early in pregnancy. In addition, other investigators have demonstrated that tight glucose control either prior to conception or very early during pregnancy can effectively reduce the rate of major malformations. As a result of these and other studies, the management goal for diabetic pregnancies has become the establishment and maintenance of near euglycemia beginning with the preconceptual period and continuing throughout gestation.
Altered Fetal Growth
Macrosomia is a classic hallmark of the pregnancy complicated by diabetes and is reported to occur in 20% to 25% of pregnancies complicated by diabetes. Macrosomia is defined as excessive birth weight (>90%) for gestational age or as a birth weight >4000 g. Increased adiposity is the primary cause of the increased birth weight seen in the offspring of diabetic women. Numerous studies have established a relationship between the level of maternal glucose control and macrosomia. Mothers of macrosomic infants usually have significantly elevated plasma glucose levels at term, indicating increased glucose availability to the fetus, with hyperinsulinemia a likely intermediate step, during the third trimester. Other factors associated with an increased risk for fetal macrosomia include increased maternal weight, increased parity, previous delivery of a macrosomic infant, and insulin requirements >80 U per day.
Macrosomic fetuses have higher perinatal and neonatal mortality and morbidity rates. Approximately 10% of infants weighing over 4,500 g at birth will require admission to a neonatal intensive care nursery. In addition, the reported perinatal mortality is two to five times higher in this group of children than in average-sized children. Delivery of a macrosomic infant is dangerous because of the risk for birth trauma to the head and neck. Fetal asphyxia and meconium aspiration may occur as a result of prolonged labor secondary to unrecognized cephalopelvic disproportion and shoulder dystocia.
At the other extreme, women with type 1 diabetes also are at increased risk for delivering a small-for-gestational-age infant. In general, the risk of growth retardation increases with the severity of the mother’s clinical diabetes. Vascular complications, such as retinopathy and nephropathy, are believed to be associated with uteroplacental insufficiency in pregnant women with diabetes. Poor maternal renal function, hypertension, and placental lesions have all been associated with intrauterine growth retardation in the offspring of diabetic mothers. However, more recent evidence suggests that the growth retardation may be related to disturbances in maternal fuels during organogenesis.
Metabolic Abnormalities
Hypoglycemia occurs when plasma glucose levels fall below 35 mg/dL in the term infant and 25 mg/dL in the




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


