Embryo at day 18 showing the cardiogenic field and the formation of the pericardial cavity
The Heart Tube
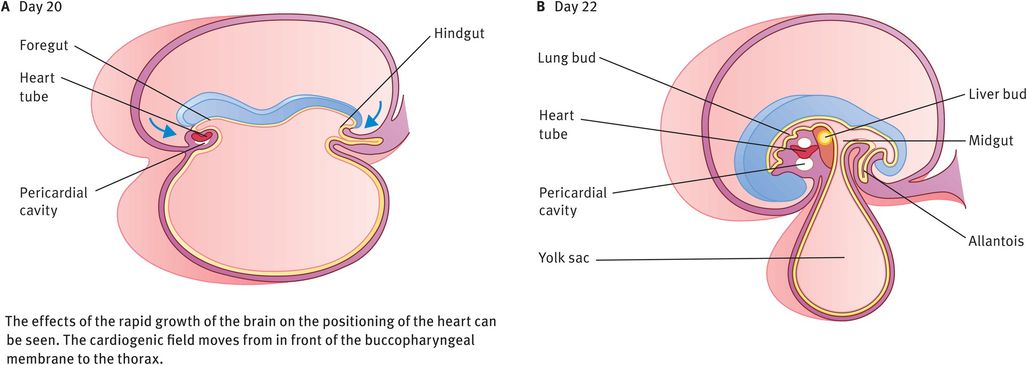
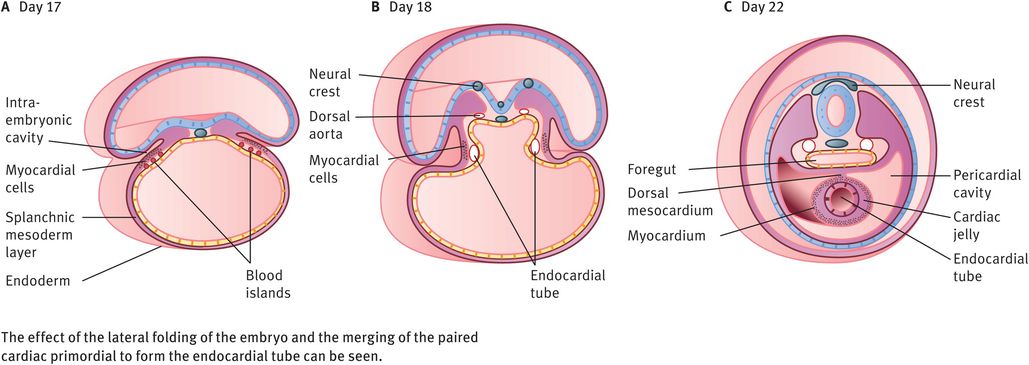
The embryo folds both cephalocaudally and laterally (Figures 14.2 and 14.3). As a result of cephalocaudal folding, as well as the rapid growth of the brain, the cardiogenic area moves caudally to the thorax. As a result of the lateral folding of the embryo, the caudal regions of the paired cardiac primordia merge, except at their caudal-most ends. The heart now becomes a continuous tube consisting of an inner endothelial lining (the future endocardium), which is separated from an outer muscular layer (the future myocardium) by a gelatinous connective tissue called cardiac jelly (Figures 14.3 and 14.4A). The pericardium is formed from mesothelial cells that arise from the external surface of the sinus venosus. This outer layer is responsible for formation of the coronary arteries, including their endothelial lining and smooth muscle.
Cephalocaudal sections through an embryo
Transverse sections through an embryo
Ventral views of the developing heart
The Cardiac Loop
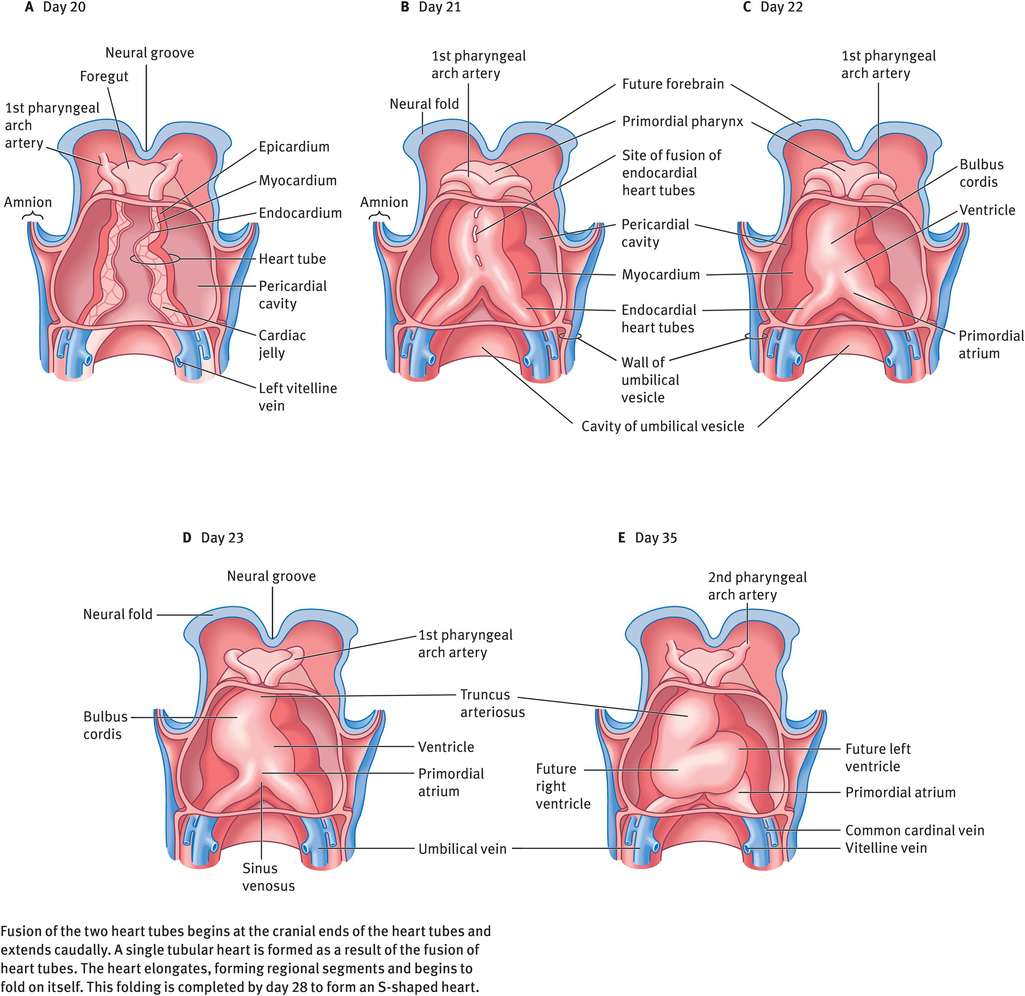
The tubular heart elongates and develops alternate dilatations and constrictions (Figure 14.4). The primordial heart is composed of four chambers – the bulbus cordis (conotruncus), ventricle, atrium and sinus venosus. The cranial part of the bulbus cordis is the truncus arteriosus and is continuous cranially with the aortic sac. The sinus venosus receives the umbilical, vitelline and common cardinal veins from the chorion, umbilical vesicle and embryo, respectively. Because the bulbus cordis and ventricle grow faster than other regions, the heart bends upon itself, forming a S-shaped loop: the cardiac loop or the bulboventricular loop. This is completed by day 28. The developing heart bulges more and more into the pericardial cavity. Eventually, it is suspended in the cavity, remaining attached only at the cranial and caudal ends by blood vessels.
Partitioning of the Primordial Heart
The partitioning of the various chambers of the primordial heart begins simultaneously around the middle of week 4 and is largely completed by the end of week 8.
Partitioning of the atrioventricular canal
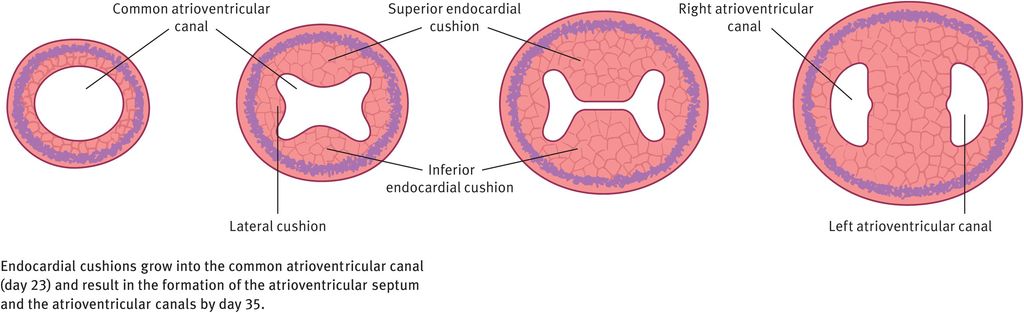
In week 4 of development, endocardial cushions form on the dorsal and ventral walls of the atrioventricular canal. They approach each other and fuse, dividing the common atrioventricular canal in to right and left atrioventricular canals (Figure 14.5). These canals partially separate the primordial atrium from the primordial ventricle.
Partitioning of the atrioventricular canal
Partitioning of the atrium
The partitioning of the atrium occurs between day 27 and day 37 (week 4 to week 6) (Figure 14.6). A thin crescent-shaped membrane, the septum primum, grows from the roof of the primordial atrium towards the endocardial cushions, partially dividing the primordial atrium into right and left halves. This septum primum bears a large opening called the foramen primum (ostium primum), which serves as a shunt, enabling oxygenated blood to pass from the right to left atrium. As the septum primum fuses with the endocardial cushions, the foramen primum becomes progressively smaller and then disappears. Before this can happen, however, perforations appear in the central part of the septum primum, which subsequently coalesce to form a new opening, the foramen secundum (ostium secundum). Thus, although the foramen primum closes, shunting of blood from the right to the left atrium continues via the foramen secundum. Now, a thick crescentic muscular fold grows from the ventrocranial wall of the right atrium, just adjacent to the septum primum. As this grows downwards, the upper part of the septum primum disappears, thus forming a flap-like valve between the two, called the foramen ovale (oval foramen). Owing to the flap-like mechanism, the foramen ovale allows only unidirectional flow of blood from the right atrium to the left during fetal life.
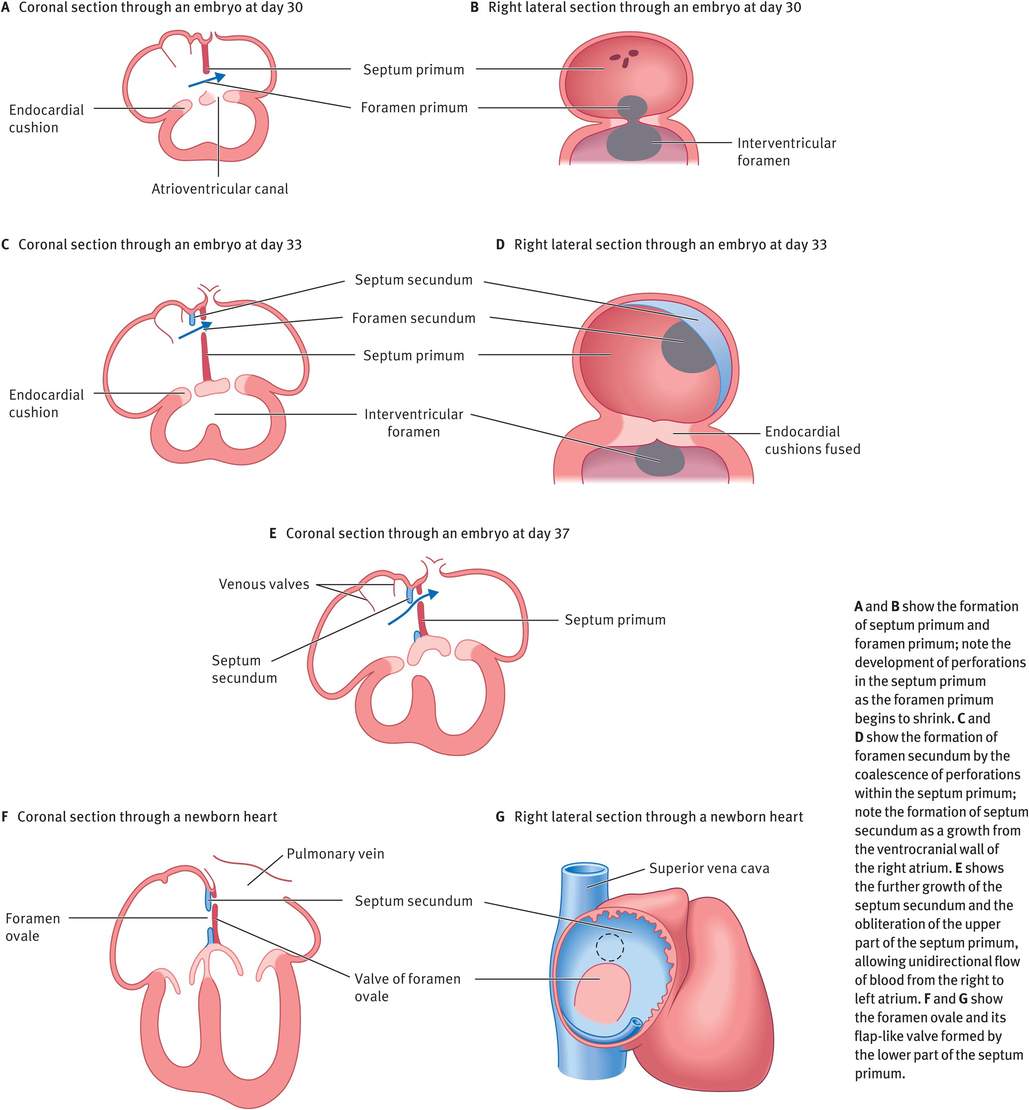
Partitioning of the atrium
Partitioning of the sinus venosus
Partitioning of the sinus venosus occurs between week 4 and week 10 (Figure 14.7). Initially, the sinus venosus opens into the centre of the dorsal wall of the primordial atrium and its right and left horns are approximately of the same size. During weeks 4–5, left-to-right shunts in the venous system cause the right sinus horn to enlarge progressively. The left sinus horn rapidly loses its importance as the right umbilical vein and the left vitelline vein obliterates during week 5. At week 10, the left common cardinal vein also obliterates and now all that remains of the left sinus horn is the oblique vein of the left atrium and the coronary sinus. The enlarged right horn begins to receive all blood from the cranial and caudal regions of the body via the superior and inferior vena cava and gets incorporated into the wall of the right atrium forming the smooth-walled part of the right atrium, the sinus venarum. The remainder of the right atrial wall has a rough trabeculated appearance. The smooth and rough parts are demarcated internally by a vertical ridge, the crista terminalis, and externally by a shallow groove, the sulcus terminalis.
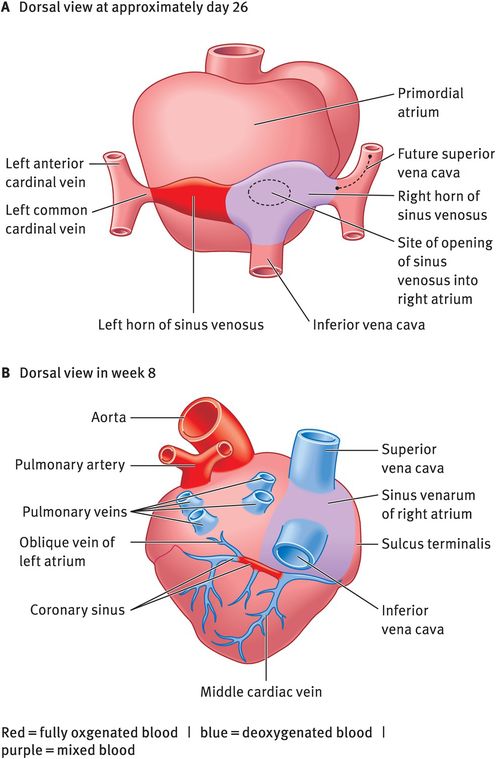
Partitioning of the sinus venosus
Most of the wall of the left atrium is formed by incorporation of the primordial pulmonary vein and is therefore smooth (Figure 14.8).
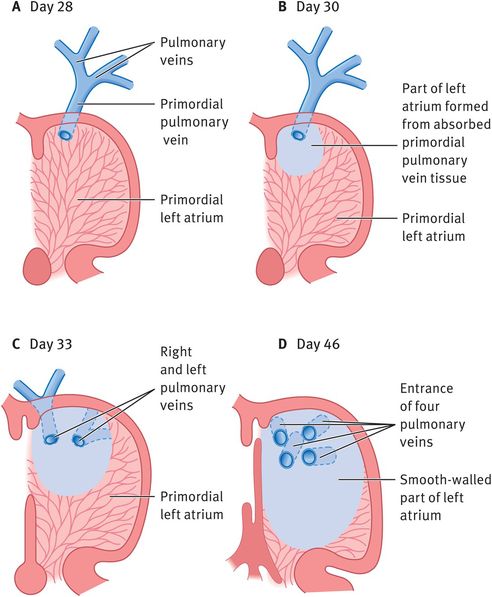
Development of the left atrium
Partitioning of the ventricles
A muscular septum arises in the floor of the primordial ventricle, near its apex. This interventricular septum contains a crescent-shaped interventricular foramen, which permits communication between the two ventricles, until week 7. After the closure of this foramen, the pulmonary trunk communicates with the right ventricle and the aorta with the left ventricle (Figure 14.9).
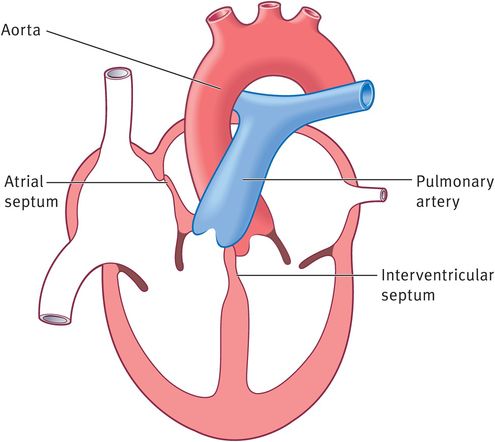
Closure of the interventricular foramen
Partitioning of the bulbus cordis and truncus arteriosus
During week 5, mesenchymal cells in the walls of the bulbus cordis proliferate actively and form bulbar ridges. Similar ridges that are continuous with the bulbar ridges form in the truncus arteriosus. The bulbar and truncal ridges (conotruncal ridges) then undergo 180-degrees spiralling, resulting in the formation of a spiral aorticopulmonary septum when the ridges fuse. This septum divides the bulbus cordis and the truncus arteriosus into two distinct channels, the ascending aorta and pulmonary trunk. Because of the spiralling of the aorticopulmonary septum, the pulmonary trunk twists around the ascending aorta (Figure 14.10).
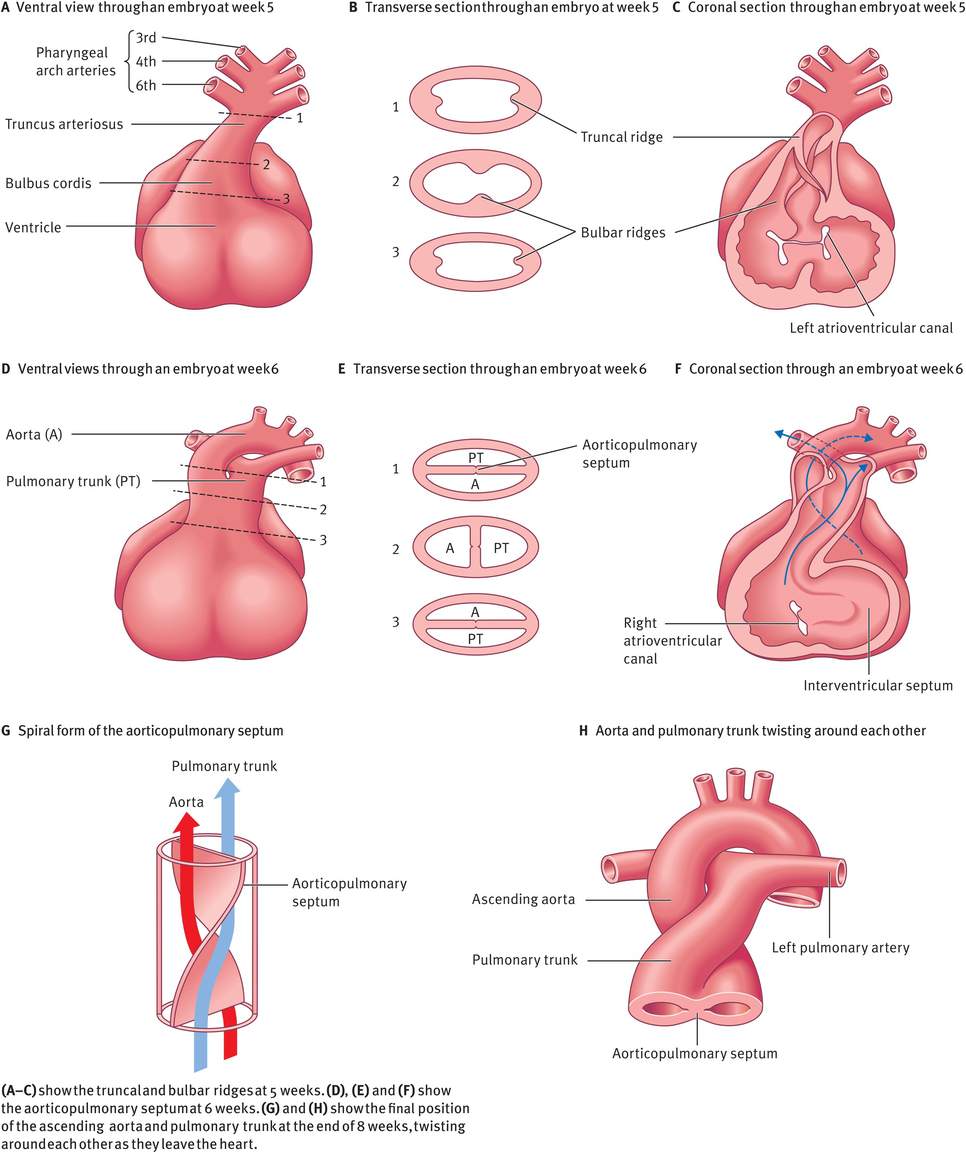
Partitioning of the bulbus cordis and truncus arteriosus
Formation of the cardiac valves
Atrioventricular Valves
After the fusion of the atrioventricular endocardial cushions, each atrioventricular orifice is surrounded by proliferations of mesenchymal tissue. The bloodstream hollows out and thins these tissue proliferations on the ventricular surface, resulting in the formation of valves. These valves remain attached to the ventricular wall by muscular cords. Over time, the muscular tissue in the cords degenerates and is replaced by dense connective tissue. The valves then consist of connective tissue covered by endocardium. They are connected to thick trabeculae in the wall of the ventricle, the papillary muscles, by means of chordae tendineae (Figure 14.11). In this manner, two valve leaflets (mitral valve) form in the left atrioventricular canal, and three valve leaflets (tricuspid valve) form on the right side.
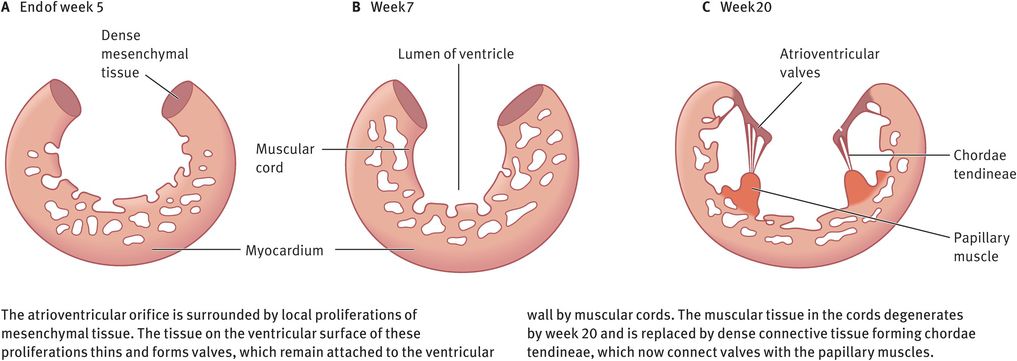
Schematic coronal section through a ventricle, showing the formation of atrioventricular valves
Semilunar Valves
At the time of partitioning of the truncus arteriosus, three swellings of subendocardial tissue develop around the orifices of the aorta and pulmonary trunk. These swellings are hollowed out and reshaped to form three thin-walled cusps, the future semilunar valves (Figure 14.12).
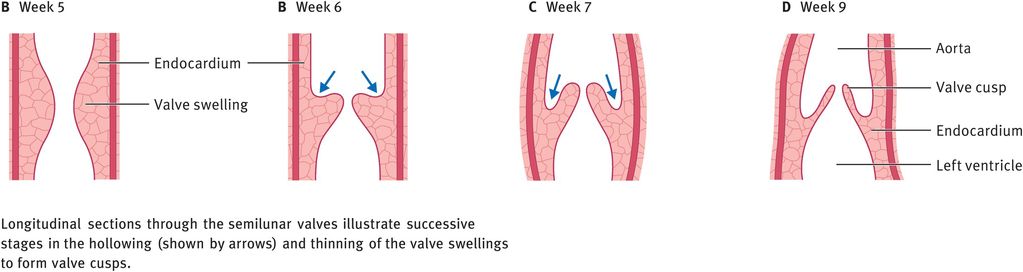
Formation of the semilunar valves
The conducting system of the heart
In the early stages of development, the function of pacemaker is assumed by the atrium and later by the sinus venosus. As the sinus venosus is incorporated into the right atrium, pacemaker tissue comes to lie near the opening of the superior vena cava, resulting in the formation of the sinoatrial node by week 5. The atrioventricular node and bundle (bundle of His) are derived from cells in the left wall of the sinus venosus and the atrioventricular canal. Fibres from the atrioventricular bundle pass from the atrium into the ventricle, split into right and left bundle branches and are distributed throughout the ventricular myocardium (Figure 14.13). Innervation of the sinoatrial node, atrioventricular node and atrioventricular bundle occurs later.
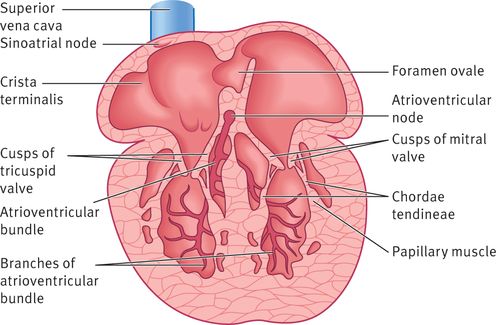
The conducting system of the heart at week 20
Congenital heart defects
Abnormalities of the cardiovascular system account for 1% of malformations among liveborn infants and almost 10% among stillborn babies, making up the largest category of human birth defects. Some defects cause very little disability and their impact may only become apparent at birth. Some others are incompatible with extrauterine life.
Factors Linked with Congenital Heart Defects
The following factors are linked with congenital heart defects.
Genetic and chromosomal factors
It is estimated that 8% of cardiac malformations are due to genetic factors. Congenital heart defects are associated with a number of genetic syndromes such as DiGeorge, Goldenhar and Down syndromes. Of those children with chromosomal abnormalities, 33% have a congenital heart defect, with an incidence of nearly 100% in children with trisomy 18. Of newborns with congenital heart defect, 6–10% have an unbalanced chromosomal abnormality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


