Background
In patients with preterm premature rupture of membranes, intrauterine inflammation and/or infection is frequently present, can lead to fetal inflammatory response syndrome, and is associated with adverse neonatal outcome. Clinical decision making requires balancing the potential benefits of pregnancy prolongation against the risk of intrauterine infection. Diagnostic tests in maternal serum are of moderate prediction value and amniocentesis is an invasive procedure. Therefore, markers obtained noninvasively would be helpful in patients with expectant management.
Objectives
To determine the predictive values of amniotic fluid interleukin-6 and tumor necrosis factor-α in vaginal secretions for fetal inflammatory response syndrome and/or histologic funisitis and for adverse neonatal outcome in patients with preterm premature rupture of membranes.
Study Design
In this prospective multicenter case-control study, vaginal secretions were sampled daily with a noninvasive method from 99 women with preterm premature rupture of membranes and expectant management. Amniotic fluid interleukin-6 and tumor necrosis factor-α were measured by 2 different immunoassays (an automated chemiluminescent enzyme immunoassay and a lateral flow immunoassay). After delivery, patients were divided into a control or a fetal inflammatory response syndrome group according to neonatal interleukin-6 in cord plasma and/or the presence of funisitis. Univariate and multivariate regression analyses were performed and prediction models were developed by calculating receiver operating characteristic curves.
Results
Gestational age at delivery was lower and latency period was longer in the fetal inflammatory response syndrome group compared to the control group. The strongest risk factor for composite adverse neonatal outcome was fetal inflammatory response syndrome (odds ratio, 2.48; confidence interval, 1.40–4.38). The median concentrations of amniotic fluid interleukin-6 and tumor necrosis factor-α in vaginal secretions were significantly higher in the fetal inflammatory response group compared to the control group in both immunoassays ( P < .001). The area under the curve of the clinical reference model (including common clinical parameters) was 0.66. Adding interleukin-6 and tumor necrosis factor-α into the model improved the area under the curve to 0.92 (in both assays, interleukin-6 IMMULITE and QuickLine); 0.87 (tumor necrosis factor-α IMMULITE) and 0.94 (tumor necrosis factor-α QuickLine), respectively.
Conclusion
The strongest risk factor for worse neonatal outcome (composite neonatal outcome) was fetal inflammatory response syndrome. Amniotic fluid interleukin-6 and tumor necrosis factor-α seem to be good predictors for fetal inflammatory response syndrome and for histologic funisitis and may improve the clinical management of patients with preterm premature rupture of membranes. The noninvasive technique of sampling amniotic fluid from vaginal secretions facilitates daily measurements and bedside assessment of cytokines and is in this respect preferable to invasive amniocentesis.
Intrauterine inflammation and/or infection is frequently present in patients with preterm premature rupture of membranes (PPROM) and can lead to fetal inflammatory response syndrome (FIRS) and histologic chorioamnionitis (HCA). Several studies have shown that elevated cytokine levels in fetal plasma or amniotic fluid (AF) are associated with preterm delivery and adverse neonatal outcome. The determination of the optimal time of delivery in PPROM patients with expectant management is a difficult obstetrical decision, as it requires balancing the potential benefits of pregnancy prolongation against the risk of intrauterine infection. Amniocentesis is the frequently used method to diagnose intrauterine infections. However, amniocentesis is an invasive procedure with potential complications; it can be technically challenging in the setting of oligohydramnios and should not be repeated on a daily basis. Diagnostic tests in the maternal serum, such as C-reactive protein (CRP) and white blood cell count (WBC), are routinely used despite their (at best) moderate prediction value for intrauterine inflammation/subclinical infection or HCA. Reliable and clinically useful markers for intrauterine infection are not available to date, resulting in the need for early detection of FIRS and for markers that predict adverse outcome. Mercer et al stated that “the availability of markers that predict adverse neonatal outcomes would be helpful in guiding management when PPROM occurs remote from term” and “optimally, such markers would be obtained noninvasively.” The objective of the study was to evaluate AF interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in vaginal secretions, obtained in a noninvasive sampling method and measured by 2 different immunoassays as predictors for fetal inflammatory response syndrome and for adverse neonatal outcome in patients with PPROM and expectant management.
Materials and Methods
Study population
The study was conducted in the Department of Obstetrics and Gynecology, Medical Center – University of Freiburg, Germany and in the Department of Obstetrics and Gynecology, Diakoniekrankenhaus Henriettenstiftung, Perinatalzentrum Hannover, Germany. Patients with PPROM were identified at these 2 tertiary referral care centers from January 2010 to February 2014. Inclusion criteria were: PPROM from 23-0/7 to 33-1/7 weeks of gestational age, and a viable singleton or twin pregnancy without congenital anomalies and with planned expectant management. In twin gestations, we included only the fetus diagnosed with ruptured membranes in the analysis; in all study cases PPROM occurred in the presenting fetus. Exclusion criteria were: nonreassuring fetal status, congenital fetal malformations, active labor with cervical dilatation ≥3 cm, clinical chorioamnionitis or other evident infection on admission. We also excluded outborn neonates owing to missing samples and neonatal data. The study was approved by the Institutional Review Board Committee of the University Medical Center Freiburg (no. 312/2001) and of the University of Hannover (no. 003/2010), respectively. Written informed consent was obtained from all women before enrollment. All maternal and neonatal medical records were reviewed and demographic and outcome data were extracted.
Gestational age was determined based on the date of last menstrual period, when reliable, and/or prenatal ultrasound examination in the first 14 weeks of pregnancy. PPROM was diagnosed by the history of amniotic fluid leakage combined with positive vaginal pooling of amniotic fluid on sterile speculum examination, pH testing, and a decreased amniotic fluid index (AFI) on obstetric sonogram. The diagnosis of clinical chorioamnionitis was made by the attending physician and was based on the following criteria: maternal temperature >38.0°C, uterine tenderness, maternal leukocytosis, maternal and/or fetal tachycardia, and malodorous vaginal discharge. Postpartum placentae were examined by a pathologist and HCA was defined as the presence of neutrophil infiltration in the chorion or amnion (maternal inflammatory response) according to the criteria of Redline. Funisitis was defined as the presence of neutrophil infiltration into the wall of the umbilical vein and/or arteries and/ or into Wharton’s jelly (fetal inflammatory response).
Definition of FIRS
Gomez et al defined FIRS by the elevation of IL-6 in fetal plasma. Later the definition got expanded to include funisitis, the histologic counterpart of FIRS.
We defined FIRS as IL-6 in cord plasma at the time of birth >100 pg/mL and/or funisitis present in histopathology based on studies conducted at the Medical Center – University of Freiburg and the literature review.
Classification of groups
The patients were classified into 2 groups according to the neonatal outcome:
- (1)
Control group: IL-6 in fetal cord plasma ≤100 pg/mL, neonate without signs of infection, placental histopathology without signs of funisitis.
- (2)
FIRS group: IL-6 in fetal plasma >100 pg/mL and/or placental histopathology with funisitis.
Neonatal morbidity
Significant neonatal morbidity was defined as the presence of at least 1 of the following diagnoses according to the criteria published previously: Early-onset sepsis was defined as positive blood or cerebrospinal fluid culture in the first 72 hours of life and suspected clinical sepsis was diagnosed by the neonatology attending based on clinical symptoms. Respiratory distress syndrome was defined as a symptomatic infant with typical chest radiograph findings and treatment with surfactant and the need for oxygen or other ventilator support >48 h. Diagnosis of severe necrotizing enterocolitis was based on Bell’s criteria and defined as stage II–III. Cranial ultrasound was performed routinely and intraventricular hemorrhage was graded I–IV based on the Papile classification. The individual neonatal outcome parameters were of low prevalence. Therefore, we used a surrogate composite outcome of mortality and severe morbidity as performed in other neonatal studies. Composite severe neonatal morbidity and mortality was defined as neonatal death, intraventricular hemorrhage grade III or IV, proven or clinical sepsis, severe respiratory distress syndrome (RDS grade III–IV), and severe necrotizing enterocolitis (stage II–III).
Management of patients
We managed all patients with PPROM in a standardized manner. For patients <34-0/7 weeks without signs of clinical chorioamnionitis, placental abruption, or nonreassuring status we generally followed expectant management. All patients received antenatal glucocorticosteroids for fetal lung maturity enhancement and a 7-day course of antibiotics to prolong latency. All neonates were assessed and managed by a certified neonatologist and admitted to the neonatal intensive care unit. Researchers measuring the samples were blinded to the already obtained cytokine results and the clinical course of the patient.
Sample collection and cytokine measurement
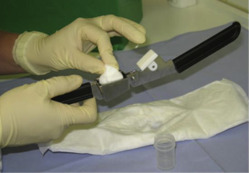
We prospectively sampled AF daily in a noninvasive procedure by squeezing the vaginal secretions out of sanitary napkins/pads (Flockenwindel, Strampelpeter; Paul Hartmann AG, Heidenheim, Germany) with a commercially available garlic press ( Figure 1 ). The samples were stored at −20°C and were measured at least in duplicate after delivery.
Quantitative cytokine concentrations were measured by an automated chemiluminescent enzyme immunoassay (IMMULITE System; Siemens Healthcare, Erlangen, Germany) and by a lateral flow immunoassay, a point of care (POC) test (QuickLine, Milenia; Biotec GmbH, Gießen, Germany). For analysis by IMMULITE assays, samples were diluted 1:50 (IL-6) and 1:5 (TNF-α), or higher if necessary. Diluents were provided by the manufacturer. For analysis by QuickLine rapid tests (IL-6 and TNF-α) the undiluted samples (100 μL) were applied using Milenia POCScan Reader (Biotec GmbH) after 20 minutes. As described by Kacerovsky et al, the measurement range for IL-6 in the QuickLine was 50–10,000 pg/mL and the intralot and interlot variation was 12.1% and 15.5%, respectively. Values of >10,000 pg/mL were registered as 10,001 pg/mL, <50 as 49, and values <200 pg/mL (in TNF-α QuickLine considered negative) were counted as 199 pg/mL. IL-6 levels of the neonate in cord blood were extracted from the medical files of the neonatal intensive care unit. For statistical analysis, we evaluated the IL-6 and TNF-α concentration from the last measurement before delivery (<24 hours before delivery).
Statistical analyses
The primary outcome was the presence or absence of FIRS and/or histologic funisitis, diagnosed after delivery, and the prediction of FIRS via AF IL-6 and AF TNF-α. The secondary outcome was neonatal morbidity and mortality. Comparison between controls (no FIRS) and cases (FIRS) was conducted descriptively and inferentially by conducting univariate analyses, using the chi-square test, Mann-Whitney test, and Fisher exact test wherever appropriate ( Table 1 ). Potential effect measure modification was addressed by stratification according to gestational age (weeks) at time of delivery.
| Variable | Control group (n = 45) | FIRS group (n = 54) | P value |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age (y) | 31.3 ± 5.7 | 32.4 ± 4.8 | n.s. |
| Gravidity | 2.0 ± 1.1 | 2.8 ± 1.7 | <.05 |
| Parity | 0.71 ± 0.79 | 0.98 ± 1.22 | n.s. |
| History of preterm birth | 5 (11.1%) | 14 (25.9%) | n.s. |
| Ethnicity, n (%) | n.s. | ||
| White | 40 (88.9%) | 48 (88.9%) | |
| African | 3 (6.7%) | 2 (3.7%) | |
| Asian | 1 (2.2%) | 2 (3.7%) | |
| Other or unknown | 1 (2.2%) | 2 (3.7%) | |
| Singleton pregnancy, n (%) | 38 (84%) | 47 (87%) | n.s. |
| PPROM to delivery interval (days) | 9.9 ± 10.4 | 16.3 ± 19.2 | <.05 |
| Gestational age at delivery (weeks) | 30.3 ± 2.2 | 28.2 ± 2.9 | <.001 |
| Neonatal outcome | |||
| Birthweight (g) | 1624 ± 400 | 1262 ± 485 | <.001 |
| Sex (female) | 17 (38%) | 27 (50%) | n.s. |
| 5-minute Apgar score | 8.1 ± 1.3 | 7.3 ± 1.7 | <.05 |
| 10-minute Apgar score | 8.9 ± 1.1 | 8.0 ± 1.6 | .001 |
| Umbilical pH | 7.31 ± 0.09 | 7.36 ± 0.24 | n.s. |
| Neonatal outcome, n (%) | <.001 | ||
| No morbidity | 37 (82.2%) | 17 (31.5%) | |
| Sepsis | 0 | 3 (5.6%) | |
| Clinical sepsis | 0 | 17 (31.5%) | |
| IVH | 0 | 6 (11.1%) | |
| RDS | 8 (17.8%) | 8 (14.8%) | |
| NEC | 0 | 3 (5.6%) | |
| Neonatal mortality | 0 | 4 (7.4%) | |
| Composite neonatal outcome | 8 (17.8%) | 37 (68.5%) | <.001 |
| Placental histology, n (%) | <.001 | ||
| No chorioamnionitis | 33 (73.3%) | 2 (3.7%) | |
| Chorioamnionitis | 12 (26.7%) | 6 (11.1%) | |
| Funisitis and chorioamnionitis | 0 | 46 (85.2%) |
Multiple multivariate analyses were conducted to identify independent risk factors for developing FIRS, including a backward selection model (all variables), a directed acyclic graph (DAG) model, and a clinical reference model (common clinical parameters that are used to predict a starting FIRS in daily clinical practice: fever, maternal CRP, and WBC).
Correlation analyses between IMMULITE and QuickLine (POC) tests were performed using Spearman and Pearson correlation test statistics.
Prediction models were developed by creating receiver operating characteristic (ROC) curves and according c-statistics for each multivariate model. The area under the curve (AUC) was calculated and compared among the different models. The clinical model was chosen as the model of reference, since this model is used in daily practice. The improvement of the model by the markers was evaluated using the predictor as a continuous variable. The ROC curve was used as a tool for displaying the discriminatory ability of the markers in distinguishing between FIRS and no FIRS. ROC curves were created for multiple (at least 10) cutoff values for all 4 markers and corresponding areas under the ROC curves were calculated and compared. The value with the highest corresponding AUC value was chosen as the final cutoff value. In addition, predictors for the composite outcome “severe neonatal morbidity” (as defined above) were assessed in a multivariate analysis including FIRS as a predictor. Data analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
In total, 110 patients were enrolled in the study. We excluded 11 patients: 3 were excluded secondary to logistic problems (missing samples at <24 hours prior to delivery), 4 because of missing IL-6 in neonatal cord blood and/or placental pathology, 2 owing to transfers to other hospitals before delivery, and 2 owing to inadequate specimens (no AF collection possible <24 hours prior delivery). We analyzed the vaginal secretions of 99 patients (78 at Freiburg and 21 at Hannover). A daily collection of an adequate sample was technically feasible in 96 of 99 cases (97%).
Fifty-four patients were included in the FIRS group and 45 women were included in the control group. Table 1 presents the maternal demographics, clinical characteristics, and neonatal outcomes according to the study groups. The gestational age at delivery was lower and the latency period longer in the FIRS group compared to the control group ( P < .05). Accordingly, the birthweight was lower in the FIRS group. All patients received corticosteroids and antibiotics to prolong latency. Newborns with FIRS had worse outcomes (lower Apgar scores, more major morbidities, and composite outcome) compared to infants in the control group. This was still the case after adjusting for gestational age. The presence of HCA was observed in 52 of 54 (93%) of the FIRS group and in 12 of 45 (27%) of the control group. Funisitis was detected in 46 of 54 (85%) of the FIRS group and in none of the control group.
We analyzed the risk factors for composite neonatal outcome. The strongest associated variables were the presence of FIRS (OR, 2.48; CI, 1.40–4.38), higher gestational age (OR, 0.68; CI, 0.54–0.85), and longer latency period (OR, 1.07; CI, 1.01–1.12). Maternal serum leukocytes (OR, 0.98; CI, 0.85–1.14), maternal serum CRP (OR, 1.01; CI, 0.98–1.04), or fever (OR, 1.20; CI, 0.20–7.30) had no influence on the occurrence of composite neonatal outcome.
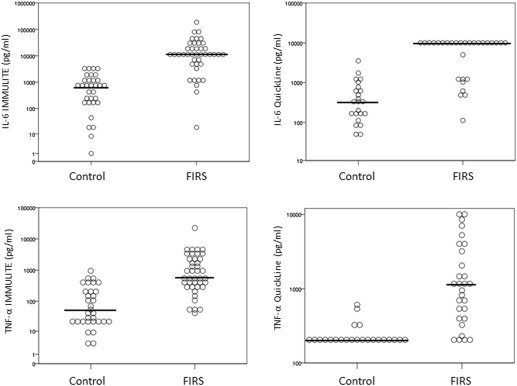
The median concentrations of IL-6 and TNF-α in both immunoassays were significantly higher in the FIRS group compared to the control group ( Figure 2 ). The median IL-6 IMMULITE concentration was 682.5 pg/mL (control) vs 10,001 pg/mL (FIRS group) ( P < .001); IL-6 QuickLine was 329 pg/mL (control) vs 10,001 pg/mL (FIRS group) ( P < .001). For TNF-α tested with IMMULITE the median was 51 pg/mL (control) vs 591 pg/mL (FIRS group) ( P < .001); with TNF α QuickLine, 199 pg/mL (control) vs 1140 pg/mL (FIRS group) ( P < .001). Median of maternal WBC was 12.4 × 10 3 /μL (control) vs 13.8 × 10 3 /μL (FIRS, P = .03) and maternal CRP was 4.0 mg/L (control) vs 8.0 mg/L (FIRS, P = .036).