Clinical Research and Evidence-based Pediatric Surgery
R. Lawrence Moss
Shawn J. Rangel
Section of Pediatric Surgery, Yale University School of Medicine, New Haven, Connecticut 06520-8062.
Section of Pediatric Surgery, Yale University School of Medicine, New Haven, Connecticut 06520-8062.
Since the advent of the concept that surgical care of children was fundamentally different than that of adults, the discipline of pediatric surgery has placed the needs of sick children above all other considerations. Beginning with the pioneering efforts of Drs. Ladd and Gross in the United States and others abroad, pediatric surgical research was focused on defining the field (1,2). The standard operations for childhood surgical conditions had to be developed. The basic principles of neonatal and pediatric physiology as applied to postoperative care had to be established. This groundbreaking work was based primarily on careful clinical observation.
On the shoulders of the remarkable achievements since the mid-1950s, the field of pediatric surgery is now presented with the opportunity to define the next stage in its development through sound clinical research efforts. We are now presented with the challenge of shifting our investigative efforts from careful clinical observation to rigorous scientific inquiry. As our specialty matures, it is no longer optimal or even acceptable to choose therapies because, “that’s how I was trained,” or because, “it has always worked well for our group,” or because “a recent published series from a major center suggested success.” If we are to truly continue to place the needs of sick children above all other considerations, we must demand the best available evidence when making important treatment decisions.
Evidence-based medicine was defined by Sackett as “the integration of best research evidence with clinical expertise and patient values” (3). This chapter is devoted to reviewing the principles of sound clinical research and addressing the role of evidence-based practice in the field of pediatric surgery. Our objectives are to review current research paradigms for answering surgical questions, to illustrate the existing status of clinical research in pediatric surgery, to explain the techniques for understanding and evaluating clinical research reports, and to discuss how current initiatives in clinical research will enhance our discipline.
CURRENT PARADIGMS OF CLINICAL RESEARCH IN SURGERY
From the late nineteenth century on, when William Stewart Halsted introduced the concept of surgery as a viable academic discipline, surgical research has been responsible for ongoing advancements in patient care (4,5). Since this time, the cornerstone of clinical research in surgery has been observation only (6,7). Observation begins with careful assessment of the results of one’s own outcomes in his or her patients and extends to the “case-series” study that is currently the backbone of clinical research in surgery (8,9).
Observational studies have provided much valuable information advancing the cause of patient care. However, overreliance on the results of these studies to the exclusion of applying more scientifically rigorous methods has also allowed ineffective and even harmful treatments to persist for many years. Barnes reviewed an extensive series of surgical procedures that were supported by published observational studies, but that were subsequently found to be ineffective and were abandoned (10). A modern example of a procedure widely supported by observational studies until subjected to rigorous scientific inquiry is the extracranial-intracranial bypass operation. This operation was shown in many different observational studies to reduce stroke risk over medical therapy for intracranial carotid disease (11,12). A subsequent randomized controlled trial showed it to be ineffective, and that bias in the observational studies had led to the wrong conclusion and subsequently the wrong therapy for many patients (13). The evolution of
breast cancer therapy in the United States provides an example of how rigorous scientific inquiry can dramatically alter a previously accepted approach to surgical disease (14,15,16). Prospective randomized clinical trials have resulted in significant advances in the surgical management of carotid artery disease, adenocarcinoma of the colon, and many other diseases (17,18,19,20,21). Despite the dramatic impact of these studies on surgical care, the application of rigorous scientific inquiry to clinical questions in surgery remains the exception rather than the rule (22,23,24).
breast cancer therapy in the United States provides an example of how rigorous scientific inquiry can dramatically alter a previously accepted approach to surgical disease (14,15,16). Prospective randomized clinical trials have resulted in significant advances in the surgical management of carotid artery disease, adenocarcinoma of the colon, and many other diseases (17,18,19,20,21). Despite the dramatic impact of these studies on surgical care, the application of rigorous scientific inquiry to clinical questions in surgery remains the exception rather than the rule (22,23,24).
CLINICAL RESEARCH IN PEDIATRIC SURGERY
To date, clinical research in pediatric surgery has been comprised predominantly of retrospective nonrandomized observational studies. The most comprehensive review of the subject was undertaken by Hardin et. al. in 1999 (25). These investigators reviewed and categorized more than 9,000 manuscripts in the major pediatric surgical journals during a 22-year period (Fig. 5-1). When basic laboratory studies (11.8% of the total) were excluded, 97.5% of all studies were either case reports or single institution case series studies. Prospective studies were infrequently performed and randomized controlled trials (RCTs) were rare. Moss et. al. focused on the use of RCTs in pediatric surgery and found that only 0.17% of more than 80,000 studies in the field were clinical trials (26). Furthermore, the majority of these RCTs suffered from major design and execution flaws, and most did not directly study surgical therapy (Table 5-1). Thakur and colleagues confirmed that most pediatric surgical studies are observational, and reported finding serious methodological defects in both randomized and nonrandomized studies (27).
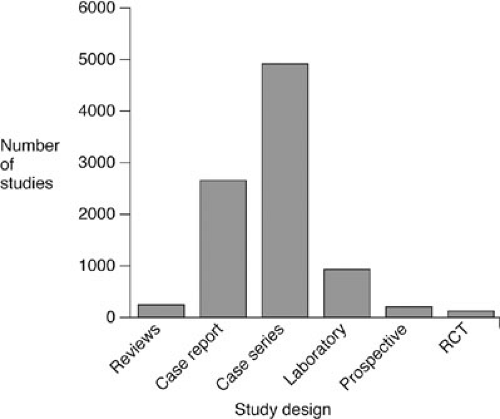 FIGURE 5-1. Types of clinical research published in the pediatric surgical journals. RCT, randomized controlled trial. |
Why is there a paucity of high-quality evidence in pediatric surgery? Rangel et. al. identified several barriers impeding the widespread use of randomized trials and other high-quality prospective studies in pediatric surgery:
Requirement of logistically challenging multicenter designs due to the infrequency of pediatric surgical conditions
TABLE 5-1 Percentage of Trials Judged to be Adequate on Essential Quality Attributes.
Attributes
% (All RCT)
% (Excluding Analgesia)
Data
Therapeutic regimens adequately defined
93
93
Inclusion and exclusion criteria specified
32
51
Reject log tracked eligible points not enrolled
25
42
Data on points withdrawn from trial provided
20
20
Randomization
Patients blinded to treatment
50
23
Evaluating physician blinded to treatment
6
14
Proper randomization technique used
43
41
Statistical analysis
Proper retrospective analysis of data
30
32
Proper analysis of side effect
14
16
Sample size determined prior to trial start
16
17
Test statistic and observed probability reported
12
13
Statistical power reported for negative trial
5
7
From Moss RL, Henry MC, Dimmitt RA, et al. The role of prospective randomized clinical trials in pediatric surgery: state of the art? J Pediatr Surg 2001;36(8):1182–1186, with permission.
Lack of existing infrastructure and resources to perform multicenter studies
Difficulty in obtaining funding for clinical research in surgery
Lack of equipoise in the surgical community regarding standards of care
Challenges in randomization and blinding when specific to surgical procedures
Challenges in standardizing operative technique and perioperative care
Reluctance of parents to randomize children into potentially invasive treatment arms
Lack of willingness on the part of surgeons to randomize their patients
There is no question that conducting the rigorous clinical studies necessary to generate high-quality evidence in pediatric surgery is rarely possible at a single institution. Even the largest children’s hospitals do not have sufficient numbers of patients to study most of the important clinical questions. In other disciplines where the study of infrequent diseases is common, the use of multicenter collaborative study groups have been employed to generate the necessary sample size for meaningful scientific inquiry. The success of the cooperative Children’s Oncology Group is testament to this (28,29). Through large cooperative trials, this group has accomplished a dramatic increase in survival for children with cancer. Perhaps the most notable accomplishment is a 70% decrease in mortality from childhood acute lymphocytic leukemia without the development of a single new chemotherapeutic agent (30). This advance occurred by the careful modification of existing therapeutic regimens based on the results of rigorous multicenter randomized clinical trials. In the United States, 78% of children with cancer are currently enrolled in a clinical trial (31). This is in stark contrast to our own field, where less than 0.1% of children undergoing major surgical procedures are enrolled in a trial (26). The recent success of the Cystic Fibrosis Therapeutics Development Network provides an additional example of a consortium’s effectiveness at accomplishing research advancing care for an uncommon disease (32,33).
The development of large-scale cooperative groups such as those mentioned previously requires significant financial resources. This is a particular challenge in pediatric surgery. Studies have shown that the National Institutes of Health (NIH) is less likely to fund clinical compared with basic research, and surgical compared with nonsurgical research (34,35). The difficulty in obtaining funding for research in uncommon diseases is well documented (36). To obtain funding for infrastructure, cooperative groups typically must demonstrate a track record of success. However, one of the key elements required for success is adequate infrastructure. For small disciplines such as pediatric surgery, these issues can be prohibitive.
Obtaining the state of equipoise in the surgical community necessary to conduct rigorous clinical research is challenging. Equipoise is defined as “a state of mind characterized by legitimate uncertainty or indecision as to choice or course of action because of a balance of potential gains versus losses or of benefits versus risks” (37,38,39). The difficulty in obtaining equipoise in the pediatric surgical community stems from two factors. First, there is a long-standing tradition of basing therapeutic decisions on the results of observational data (40). Second, although indecision is fundamental to evaluating the absence of available evidence for a treatment plan, indecision with respect to management of an individual patient or with respect to an intraoperative decision is counterproductive and decidedly nonsurgical (30). Surgeons committed to evidence-based practice face the challenge of maintaining intellectual equipoise with respect to evaluating therapies, while maintaining the decisiveness necessary to be an effective surgeon (41).
Clinical research involving surgical therapy presents unique challenges to the design and conduct of clinical studies. Blinding of patients and surgeons to treatment assignments is often impossible. Furthermore, the well-documented placebo effect of surgical treatment can present challenges in detecting the true benefits of novel interventions (42,43). Nevertheless, effective randomization can still be achieved, and blinding of study personnel during the procurement and analysis of outcomes data is possible even for surgical interventions. Standardization of surgical procedures is essential to the validity of clinical research studies. This challenge may be overcome by establishing standardized training centers, or by disseminating study protocols of operative technique over the Internet (44,45).
The final issue of the willingness of health care providers to randomize their patients is one of individual leadership and intent. In contrast to trials investigating nonoperative therapy, surgical trials are associated with interventions that are irreversible and potentially disfiguring. In this regard, parents may be very reluctant to randomize their children into trials with potentially invasive treatment arms. Nevertheless, with a thoughtful, compassionate, and well-informing presentation of the study to patients by their physicians, enrollment can be maximized (46,47). Nurses have an enormous opportunity for leadership in this regard because they are often the first line of contact in helping patients and their families understand the benefits of clinical trials to an individual patient and to advancement of medical knowledge (48,49).
Interpreting and Understanding Available Evidence
In this section, we review the basic types of study design, the common means of measuring treatment effects, and
some fundamental issues of statistical analysis. A comprehensive discussion of study design, methodology, and statistical analysis is beyond the scope of this chapter, and the reader is directed to many excellent sources for a detailed description (3,50,51).
some fundamental issues of statistical analysis. A comprehensive discussion of study design, methodology, and statistical analysis is beyond the scope of this chapter, and the reader is directed to many excellent sources for a detailed description (3,50,51).
Types of Study Design
Understanding the advantages and limitations of different study designs is the first step to critically evaluating the evidence available regarding a treatment decision. Several classification schemes have been proposed to characterize a hierarchy of scientific rigor for the clinical evidence provided by these designs. The most widely used are those published by the U.S Services Preventative Task Force shown in Table 5-2 (52). The following categories of study designs are presented in descending order based on their relative quality of evidence.
Prospective, Randomized, Controlled Clinical Trial
In this design, patients from a clearly defined study population with a disease of interest are offered enrollment into a study prior to receiving treatment. Following enrollment, treatment is randomly assigned and the patients are otherwise treated identically during the follow-up period. In a large, well-designed clinical study of this type, any observed differences in outcomes can be attributed to the interventions themselves because they are the only difference between the assigned treatment groups. Randomized trials automatically control for other clinical characteristics that might explain an observed difference outside the interventions being compared. The value of the randomized trial lies in the fact that the investigators themselves do not need to identify or control these potentially confounding characteristics.
The properly performed RCT is considered the highest standard of evidence in clinical research. Its advantages in terms of experimental design are obvious. In addition to being the only true experimental study of treatment options, RCTs offer the ability to carefully and fully observe information about the natural history of the disease under both the control and experimental arms. However, the best way to characterize natural history is through a massive cohort study over years and years of open-ended follow-up. Another proven advantage of RCTs is that patients participating in clinical trials have been shown to have improved outcomes compared with patients outside trials, regardless of treatment assignment (53,54). This “inclusion benefit” of clinical trials is due primarily to the fact that patients in trials are treated by means of standardized clinical pathways, which themselves have been developed from best-practice guidelines (30).
TABLE 5-2 Relative Quality of Evidence Based on Type of Study Design. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Randomized clinical trials have several limitations and weaknesses. They are very expensive to perform, and thus can only be applied in a limited number of circumstances. RCTs take considerable time to perform. In a therapeutic area that is rapidly evolving, the therapy under study may be obsolete before the trial is complete. Most important, the results of a clinical trial may not be generalizable to the population at large. If the inclusion criteria for the trial are so strict that the study population does not represent most individuals with the disease, then the results are of limited value to the practicing clinician. Also, the ability of randomization to adequately control for potential biases in the selection of patients is greatly dependent on the sample size. Smaller trials can potentially suffer from the same maldistribution of unknown covariates as lesser epidemiological studies. From a practical standpoint, multicenter design is necessary to adequately power most potential RCTs for pediatric surgical conditions, and this can present major logistical challenges.
As with any study, proper design is the key to success. The elements of a properly done randomized trial have been well documented. The CONSORT group is an international consortium that developed guidelines for the conduct and reporting of clinical trials (55). Their guidelines have been adopted by most leading medical journals in the world, including the Journal of Pediatric Surgery (56,57).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


