Simon Bomken, Josef Vormoor • Understand the different research settings and the phases of clinical trials • Understand how to develop a research question and frame this as a null hypothesis to be tested • Be aware of the regulatory bodies and processes involved in trial design and approval • Understand how to enrol a young person on a clinical trial • Be aware of the monitoring requirements of a clinical trial • Have a basic understanding of the approach to statistical analysis of trial data
Clinical research
There has been a dramatic improvement in child health outcomes throughout the second half of the last century. This has mainly been driven by improvements in nutrition, housing, child safety, immunization and other public health developments. These important approaches are now being complemented by clinical trials, fuelled by the development of new ‘omics’ technologies, which are uncovering the molecular origins of disease faster than we are able to translate the new information into clinical benefit for patients.
Paediatricians are a critical component in ensuring that our ever improving understanding of fundamental molecular biology is translated into improvements in the health of young people. This requires that paediatricians continue to identify, and seek to answer, key questions relating to childhood development and disease. Identifying clinically relevant problems, framing questions and searching for answers from established research is at the heart of evidence-based medicine (see Chapter 39, Evidence-based paediatrics). However, many questions remain unanswered. Designing studies to address these unanswered questions is the essence of clinical research.
This chapter aims to introduce clinical research, providing a broad understanding of what underpins clinical trial conception and design, the process of obtaining trial approval, recruitment, monitoring and finally reporting of trial findings.
The role of research
Research into the causes of childhood illness underpins much of what we see as standard practice today. However, many of our daily clinical decisions are based on little or no evidence. Children are not small adults, but individuals with specific and ever changing developmental and physiological needs. Despite this, paediatricians are often obliged to extrapolate from adult trials and to prescribe drugs for which there is little clinical trial data from children. Challenges facing the development of research for young people are listed in Box 37.1.
How, then, can child-specific clinical research be driven and what does it offer to the health of young people? Paediatricians involved in caring for children with rare, complex or life-threatening disorders are constantly looking to understand that disease in more detail, to identify high-risk patients and to develop innovative management strategies. For example, molecular characterization of malignant diseases has opened the door to the application of targeted therapies in place of non-selective cytotoxic chemotherapies. Cellular and genetic manipulation of donated stem cells will reduce the risk of rejection or graft-versus-host disease in recipients. Delivery of the wild type CFTR gene will reconstitute normal function, preventing the destructive lung disease seen in cystic fibrosis. However, the majority of child health research seeks to provide evidence relevant to the clinical management of children with more common diseases, forming the evidence base from which we develop our practice, and is the domain of all paediatricians (Box 37.2). Whether as chief investigator, recruiting patients or as someone committed to improving local practice, understanding the principles and practice of clinical research is a key component of paediatrics. All paediatricians have a responsibility to improve children’s healthcare and research is an essential part of this process.
Research methods
Research setting
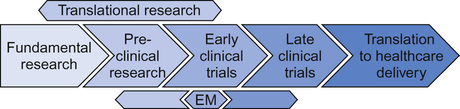
Medical research is conducted across a number of different settings. Different components flow, in two directions, with clinical problems and observations being investigated at a fundamental level and improved fundamental knowledge being applied to the patient setting. The resultant interplay of ideas, questions, solutions and new questions (Fig. 37.1) is what defines the process of medical research. Whilst the boundaries can merge, broadly the style of research being conducted allows these different settings to be considered separately.
Fundamental (or basic) research seeks to develop our understanding and knowledge of the genetic, molecular, environmental or societal basis of disease. Examples include:
Translational research is a two-way process which provides a bridge between fundamental research and applied clinical research and adheres to the philosophy of ‘bench-to-bedside and back again’. In the forward direction, it aims to generate supportive pre-clinical data prior to investigating the clinical application of:
In reverse, it aims to identify:
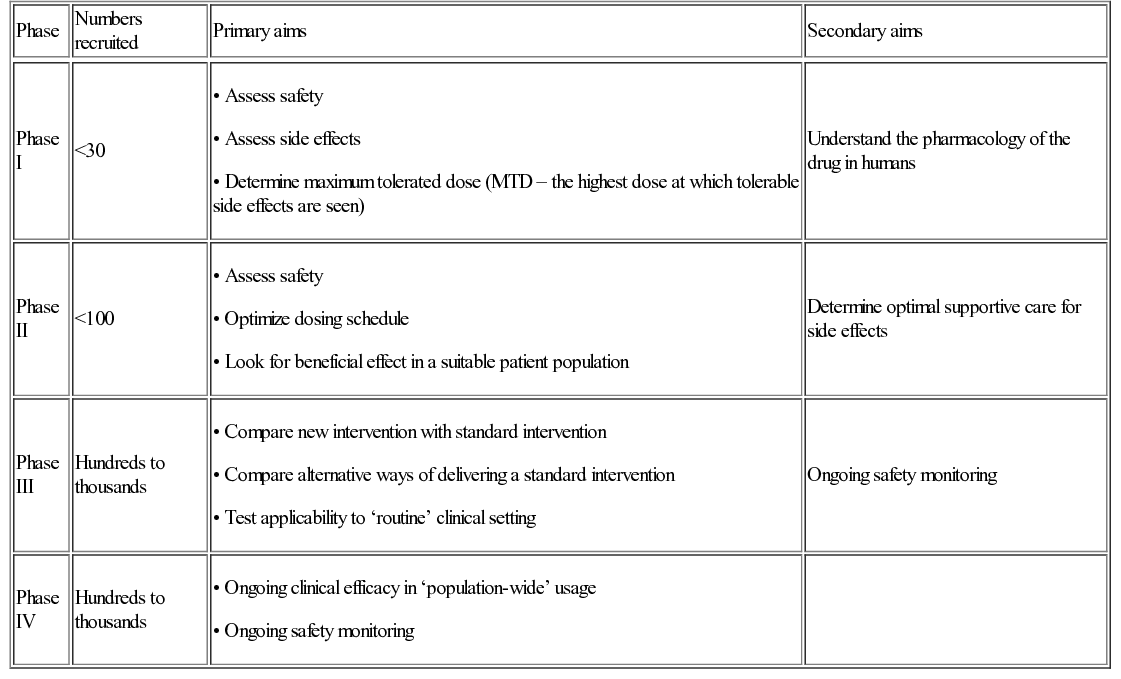
Clinical research seeks to take potential diagnostic, monitoring or therapeutic strategies, identified by basic and translational research, into a representative clinical setting. It asks the question, ‘Does this treatment improve the health and well-being of real patients?’ Addressing this question will normally be performed in steps, or phases, with different aims at each phase (Table 37.1). Phase I and II (early phase) trials recruiting small numbers of patients and focusing on determining the safety and appropriate dosing/schedule of a new intervention, may be combined so that patients are initially recruited to a phase I element followed by progression to a phase II element. This is increasingly common in trials of new therapies designed specifically for rare patient or disease/molecular subgroups.
Increasingly, experimental medicine (see Fig. 37.1, and see Personalized medicine, below) seeks to use human trials to generate pre-clinical data. For example, a clinical trial with a primary aim of investigating the effect of a new cytotoxic anti-cancer drug can also be used to assess the pharmacokinetics/pharmacodynamics of that drug and the effect on similar, potentially cross-reactive pathways in normal tissue. These studies cannot be performed in vitro or in healthy human volunteers due to the potential toxicity.
Common trial designs
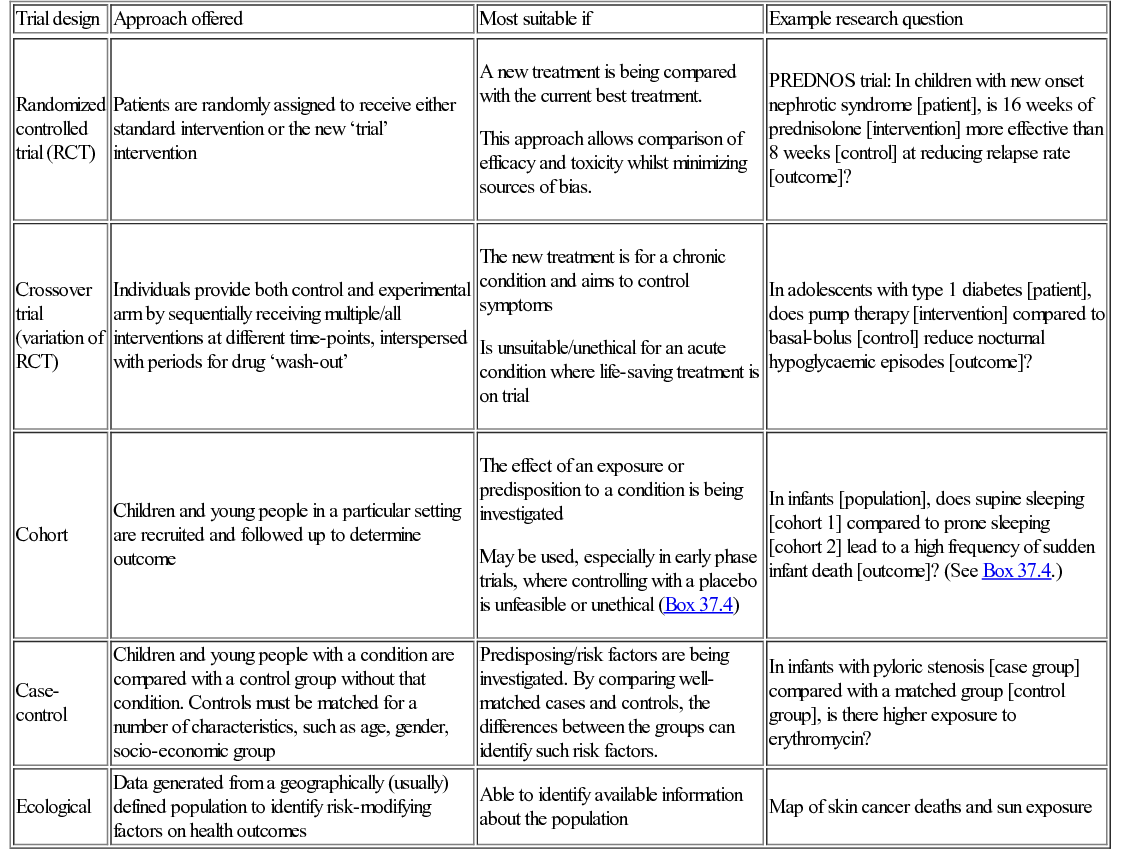
A number of different quantitative trial designs exist. Whilst the randomized controlled trial is frequently seen as the gold standard approach for investigating a new treatment intervention, each of the designs can be the most appropriate choice for a given clinical setting (Table 37.2).
Table 37.2
Common trials designs
| Trial design | Approach offered | Most suitable if | Example research question |
| Randomized controlled trial (RCT) | Patients are randomly assigned to receive either standard intervention or the new ‘trial’ intervention | A new treatment is being compared with the current best treatment. This approach allows comparison of efficacy and toxicity whilst minimizing sources of bias. | PREDNOS trial: In children with new onset nephrotic syndrome [patient], is 16 weeks of prednisolone [intervention] more effective than 8 weeks [control] at reducing relapse rate [outcome]? |
| Crossover trial (variation of RCT) | Individuals provide both control and experimental arm by sequentially receiving multiple/all interventions at different time-points, interspersed with periods for drug ‘wash-out’ | The new treatment is for a chronic condition and aims to control symptoms Is unsuitable/unethical for an acute condition where life-saving treatment is on trial | In adolescents with type 1 diabetes [patient], does pump therapy [intervention] compared to basal-bolus [control] reduce nocturnal hypoglycaemic episodes [outcome]? |
| Cohort | Children and young people in a particular setting are recruited and followed up to determine outcome | The effect of an exposure or predisposition to a condition is being investigated May be used, especially in early phase trials, where controlling with a placebo is unfeasible or unethical (Box 37.4) | In infants [population], does supine sleeping [cohort 1] compared to prone sleeping [cohort 2] lead to a high frequency of sudden infant death [outcome]? (See Box 37.4.) |
| Case-control | Children and young people with a condition are compared with a control group without that condition. Controls must be matched for a number of characteristics, such as age, gender, socio-economic group | Predisposing/risk factors are being investigated. By comparing well-matched cases and controls, the differences between the groups can identify such risk factors. | In infants with pyloric stenosis [case group] compared with a matched group [control group], is there higher exposure to erythromycin? |
| Ecological | Data generated from a geographically (usually) defined population to identify risk-modifying factors on health outcomes | Able to identify available information about the population | Map of skin cancer deaths and sun exposure |
Qualitative research
Whilst most clinical research revolves around the quantitative analysis of clinical criteria, the interrogation of qualitative information can also play an important role, both in informing quantitative clinical trial design and in influencing clinical service design and provision.
Qualitative research (Box 37.5) aims to develop understanding of a defined area by collecting information, analysing it and using the output to generate new ideas or hypotheses which may or may not then be suitable for quantitative analysis. Information collecting may involve:
• Review of documented evidence
• Observational approaches – recording uninfluenced behaviours
• Interviews – usually open-ended and defined by topics rather than specific questions
• Group discussion – specifically focusing on the interactions of the group setting
Whilst outcome measures are not restricted in qualitative studies, the study aim, methodology and analysis are no less rigorously defined and validated than in quantitative approaches. Commonly a number of validated methods will be used independently by more than one researcher and the results triangulated to identify areas of agreement, providing a high degree of validity.
Designing a clinical trial
Introduction – identifying a clinical need
The origins of all clinical trials should be an identifiable clinical need. This is one of the key contributions to be made by translational researchers. The process of identifying a need, investigating potential solutions and then validating them is key to framing the clinical research question (Fig. 37.2). Whilst basic scientists may follow lines of investigation which appear interesting in the hope of further defining fundamental elements of biology, a greater expectation must be placed on the question underpinning a clinical trial. Asking a child and their family to consent to novel intervention or additional investigations can only be ethically justified if the benefit outweighs the risk. There must therefore be a reasonable expectation of patient benefit, identified as a clinical need. It may not, for example, be justified to randomize children between two established therapies with an equivalent, well-documented success rate, simply to demonstrate equivalence in a formal setting. If, however, there was a rationale for one treatment being more effective or less toxic than the other, then this would need to be investigated in a randomized clinical trial. The research question becomes:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree