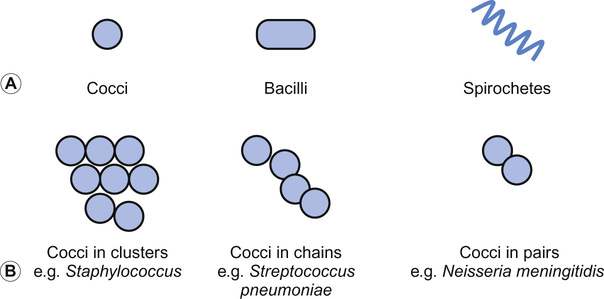
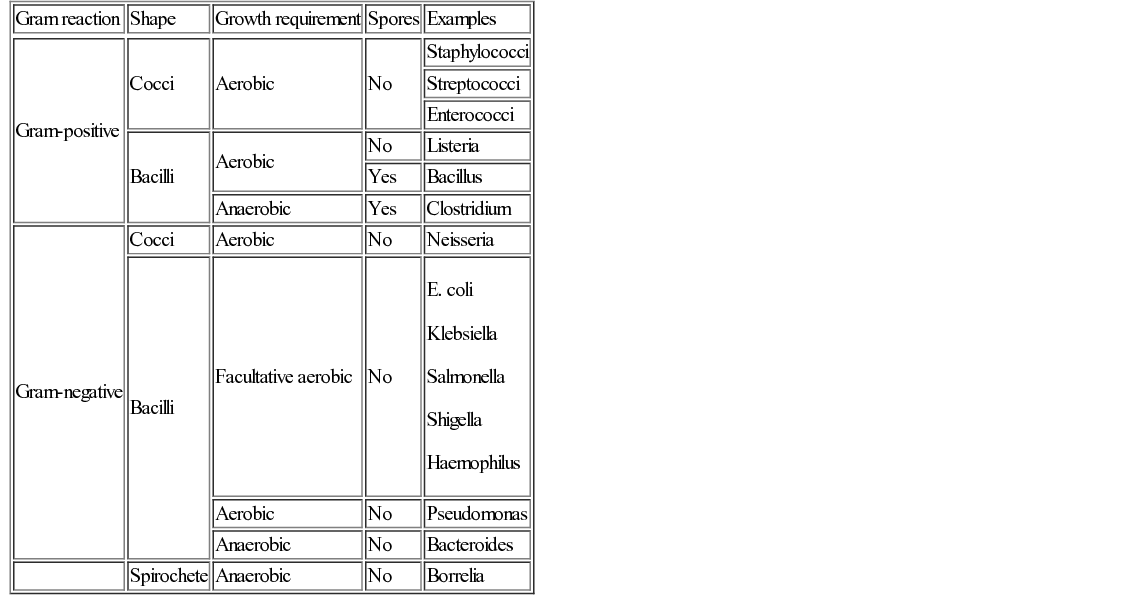
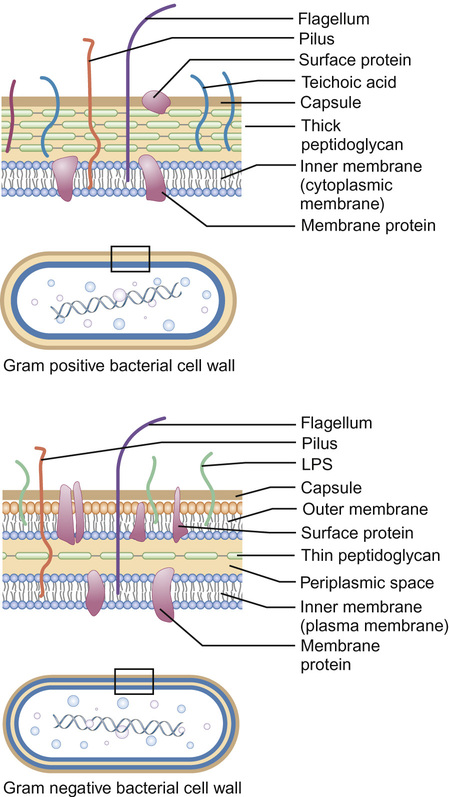
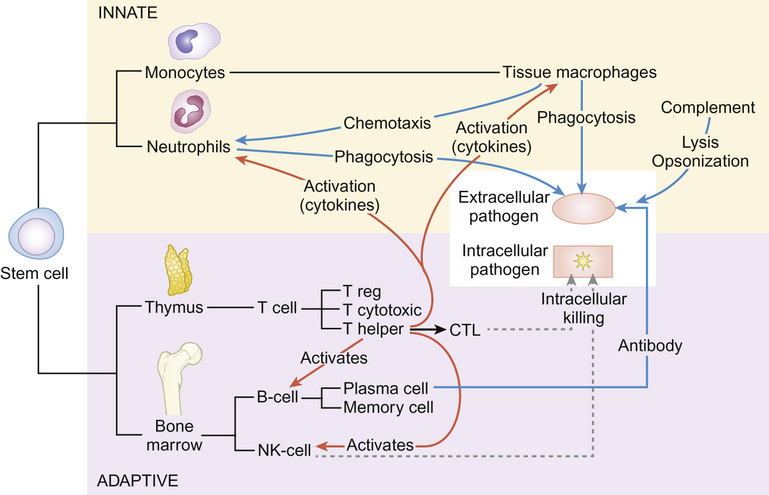
Christine E Jones, Manish Sadarangani, Graham Davies With contributions by, Mike Sharland, Omendra Narayan, Daniel Langer, Christian Harkensee, Chris Barton, Kirsty Le Doare, Aubrey Cunnington Viruses are the commonest cause of human infection. They are small (20–300 nm) and cannot be visualized with a light microscope. They are unable to synthesize their own energy or proteins and so are dependent on the host cell to replicate. However, once a virus particle (virion) infects a cell, it can replicate within hours to produce hundreds of virions, allowing the virus to rapidly spread from cell to cell. Viruses consist of a core of nucleic acid surrounded by a protective protein coating known as the capsid. The capsid mediates the attachment of the virus to specific host cell receptors and defines the species and organ specificity of the virus. The capsid may induce host immune responses. In some viruses, the capsid is covered by a lipoprotein envelope, which confers instability to the virus. Enveloped viruses, such as RSV, dry out rapidly in the environment and are easily inactivated by detergent and alcohol. Viruses without an envelope, such as rotavirus or Norwalk virus, are less easily eradicated in the environment. The classification of viruses is based on the viral nucleic acid (DNA or RNA), capsid (size, symmetry) and presence of envelope. Viral infection of a host cell may result in a number of consequences: Bacteria vary in size; the smallest bacteria are similar in size to the largest viruses and the largest bacteria are the size of red blood cells. They are round (cocci), rod-like (bacilli) or spiral (spirochetes) in shape and tend to take up specific arrangements (Fig. 15.2). Most bacteria are capable of independent metabolic existence and growth, with the exception of obligate intracellular pathogens such as Chlamydia and Rickettsia. They multiply by binary fission, each cell dividing into two daughter cells, and this allows exponential growth of bacterial colonies from a single bacterium to one million organisms within hours. Bacterial classification is based on four features: Gram reaction, bacterial shape (see Fig. 15.2), growth requirement and the presence of spores (Table 15.1). Most bacteria grow in the presence of oxygen (aerobes), some require it (obligate aerobes) whilst others can still generate energy in the absence of sufficient oxygen (facultative aerobes). A number of bacteria will only grow in an atmosphere containing less than 20% oxygen (anaerobic). Some bacteria produce spores in adverse conditions, allowing the organism to survive when exposed to chemicals and heat (i.e. Clostridium species). A number of important pathogenic bacteria do not fit neatly into this classification as they do not take up the Gram stain: Mycoplasma (no cell wall), Chlamydia and Rickettsia (intracellular bacteria) and Mycobacteria (acid fast staining only). Bacterial cells consist of cytoplasm surrounded by a cell wall. The DNA is free within the cytoplasm as a single chromosome of circular DNA and within plasmids, along with ribosomes and all elements required for growth and pathogenesis. The cell wall is essential for survival and is a key target for antibiotics; differences in the components of the cell wall between Gram-negative and Gram-positive bacteria (Fig. 15.3) are therefore clinically important (see antimicrobial section). Gram-positive bacteria have a thick peptidoglycan layer with no outer membrane, whereas Gram-negative bacteria have a thin peptidoglycan layer surrounded by an outer lipid membrane. The periplasmic space of Gram-negative bacteria may contain β-lactamase, which degrades antibiotics such as penicillin. The cell surface may contain different components. Gram-negative bacteria have endotoxin (lipopolysaccharide, LPS) in their outer membrane, which can induce septic shock. Teichoic acid is only found in Gram-positive bacteria and this can also induce septic shock. Numerous pili, hair-like structures, facilitate adhesion and acquisition of external DNA. Flagella are important in locomotion and may help with bacterial identification. Other proteins act as sensors, receptors and adhesins. Bacteria such as Streptococcus pneumoniae, Neisseria meningitidis, Klebsiella pneumoniae and Escherichia coli are surrounded by a polysaccharide capsule, which enables them to evade phagocytosis. The spleen forms an important role in clearing these bacteria, therefore individuals who are hyposplenic, such as children with sickle cell, are more susceptible to these organisms. Some bacteria produce slime in addition to the capsule and this helps with the formation of biofilms. This tough protective matrix is very difficult for antibiotics to penetrate and may form on foreign material. Bacteria may be transmitted via the respiratory, gastrointestinal, urogenital or cutaneous route. Once transmitted, bacteria adhere to mucosal sites, facilitated by pili and surface proteins. Once a stable population of bacteria has been established, the host is colonized. In some instances, invasion occurs and the bacteria penetrate host cells and tissues. Not all strains of bacteria are equally pathogenic, for example, there are six serotypes of Haemophilus influenzae, but type b (Hib) causes the most serious disease. Moreover, different strains with differing virulence determinants cause distinct patterns of infection; for example, E. coli may cause disease in the gastrointestinal tract, meningitis, sepsis or a UTI. Protozoa, fungi and helminths are eukaryotic organisms, in contrast to viruses and bacteria. The DNA of eukaryotes is contained within a nucleus. Protozoa are unicellular organisms that can exist in a vast range of environments. The cytoplasm is surrounded by a plasma membrane, which may have external structures such as a cell wall to enable the organism to survive outside the host (Giardia intestinalis) or flagella (Leishmania) to propel the protozoa. They can be divided into three main groups that cause disease in humans: 1. Spore-forming (sporozoa): Plasmodium, Toxoplasma gondii 2. Flagellates: Giardia intestinalis and Trichomonas 3. Amoeboid: Entamoeba histolytica (causes amoebic dysentery or liver abscess) They reproduce sexually and/or by binary fission. Their life cycle may involve vectors; for example, Plasmodium parasites are transmitted by mosquitoes (vector) and cause malaria in humans. Other protozoa are waterborne and only involve humans (Entamoeba histolytica). Fungi are widespread in the environment and can survive in hostile environments. They are saprophytes, living off dead matter in soil and water. Their cell wall contains chitin polysaccharide and not peptidoglycan. As a result, fungi are not sensitive to antibiotics that inhibit peptidoglycan synthesis. The cell wall also contains the polysaccharide β-glucan and ergosterol, which are targeted by various antifungal drugs (see antimicrobial section). Fungi can be classified into yeasts, moulds and dimorphic fungi. Yeasts are simple unicellular organisms that reproduce by asexual budding, Candida albicans is responsible for most disease caused by yeasts in humans. Moulds grow as long filaments. Their branching filamentous hyphae assist with reproduction and acquisition of nutrients. They produce germinative spores, which enable them to colonize new environments. Airborne spores of Aspergillus fumigatus may be inhaled and cause infection in immunocompromised hosts. Dimorphic fungi take the form of moulds at room temperature, but transform into yeasts at body temperature. Histoplasma capsulatum is a dimorphic fungus that may cause disease in individuals with HIV infection. Fungal infections (mycoses) mainly cause superficial infections that are localized to epidermis (tinea corporis if it affects the body, tinea pedis if it affects the feet), hair (tinea capitis) and nails (tinea unguium). All forms of tinea that affect the skin may be referred to as ringworm; these are typically caused by dermatophytes. Dermatophytes belong to one of three fungal groups: Trichophyton, Microsporum and Epidermophyton. Systemic infections are most commonly due to opportunistic fungi in immunocompromised hosts. Helminths are complex multicellular parasitic worms that range in size from microscopic filarial parasites to tapeworms several metres in length. They reproduce sexually and have complex lifecycles. Helminths typically cause chronic rather than acute diseases. They can be classified into nematodes (round worms) and platyhelminths (flatworms), which include cestodes (tapeworms) and trematodes (flukes). Nematodes appear worm-like and cause infection in the intestine (Enterobius vermicularis, causing pruritus ani in children), blood (Filaria, such as Wuchereria bancrofti causing lymphatic filariasis) and tissues (Onchocerca volvulus, causing river blindness). Cestodes (e.g. Taenia solium, T. saginata) are ribbon-like worms and can grow up to 10 m in length. The excreted eggs are ingested by an intermediate host, such as a cow or pig; humans become infected by eating meat from this animal. Trematodes are flat, leaf-like organisms. Humans are the definitive host and freshwater snails are the intermediate host. Schistosoma species are a medically important example of trematodes. An understanding of the pharmacology of antimicrobial agents is important to ensure adequate concentrations at the site of infection and therefore efficacy of the drug. An understanding of pharmacokinetic–pharmacodynamic profiles guides dosage and dose frequency (Box 15.1). For example, antibiotics with a short half-life, such as penicillins, need to be given more frequently. Azithromycin has a very long half-life and is administered once daily. Another factor that guides decisions related to dose frequency is the mechanism of action of an antibiotic. The activity may be related to the time that the concentration exceeds the minimum inhibitory concentration (MIC), e.g. penicillin. The activity of other antibiotics, such as gentamicin, is related to the peak antibiotic concentration reached. The therapeutic index describes how likely the drug is to cause toxicity to the host. It is the maximal tolerated dose that can be tolerated by the patient divided by the minimum effective dose (i.e. the lowest dose that will give the required MIC at the site of infection). The higher the therapeutic index, the less likely the drug is to cause toxicity, e.g. β-lactam agents have a high therapeutic index. Where the therapeutic index is low, serum level monitoring and dose adjustment is required (e.g. aminoglycosides). Therapeutic index can be seen as the balance between safety and efficacy. Antibiotics exert their antimicrobial effect in four major ways: β-Lactam and glycopeptide agents prevent cross-linkage of peptidoglycan, a key component of the bacterial cell wall. The bacterium is then killed by osmotic lysis. β-Lactams such as penicillin are predominantly used to treat Gram-positive infections caused by Streptococci. Third-generation cephalosporins such as ceftriaxone are active against a much broader spectrum of bacteria, including Gram-positive and Gram-negative organisms; however, ceftriaxone has poor action against Pseudomonas and Enterococci. Glycopeptide activity is limited to Gram-positive bacteria, as the large molecules are not able to penetrate the outer membrane of Gram-negative bacteria. Protein synthesis is inhibited by macrolides, tetracylines, aminoglycosides and clindamycin at the level of the ribosome. As a group, these antibiotics are active against a wide range of bacteria. Macrolides have a similar spectrum of activity as penicillin but Mycoplasma, Mycobacteria and Chlamydia are also sensitive to macrolides. Aminoglycosides have excellent Gram-negative activity. Fluroquinolones inhibit enzymes involved in the coiling and uncoiling of DNA, thereby inhibiting DNA replication. Fluroquinolones have good activity against Gram-negative organisms, but poor activity against Gram-positive organisms, such as Streptococci and Staphylococci. Bacteria produce folate for the synthesis of DNA as they cannot absorb folate from the host. Trimethoprim inhibits the conversion of dihydrofolate to tetrahydrofolate, thereby preventing purine and pyrimidine metabolism and DNA formation. It is active against Gram-negative and Gram-positive organsims and is most commonly used to treat urinary infections due to its excretion and high concentrations in the urine compared to blood. Antibiotics may be considered as narrow or broad spectrum according to the range of bacteria they are active against. Broad-spectrum antibiotics should be reserved for when a wide range of bacteria could be responsible for an infection or when polymicrobial infection may be present. Antibiotics such as β-lactams and aminoglycosides are bactericidal and kill the bacteria they are effective against; these should be selected for serious infections or immunosuppressed patients. Bacteriostatic antibiotics, such as tetracyclines or trimethoprim, inhibit bacterial growth but do not kill them, and therefore rely on the immune system to erradicate the organism. A bacterium is considered resistant when its growth cannot be inhibited by a concentration of drug that is achievable in the blood. Resistance may be innate; for example, Pseudomonas is innately resistant to penicillin. Alternatively, resistance may be acquired as a result of genetic change. If genetic change results in a survival advantage, then the population of resistant bacteria may outgrow the sensitive population. Antibiotics exert a considerable selection pressure on bacterial populations, favouring populations that are able to withstand them. Inappropriate and overuse of antibiotics is the main driver for the emergence of resistant bacteria and given the shortage of new antibiotics, it is essential to maintain the efficacy of current drugs. The principles of selection of antibiotics (see Key points) and of antimicrobial stewardship (Box 15.2) should be followed to ensure reduction in the inappropriate use of antibiotics. Viral replication depends on host cellular processes, therefore drug antiviral selectivity is harder to achieve than with antibacterial drugs and fewer antiviral agents are available. Another limitation of antiviral therapy is that viruses replicate rapidly and infection is often widespread before clinical symptoms are apparent and treatment is initiated. Antiviral drugs target different stages in virus-specific replication, from entry into the cell, transcription, nucleic acid and protein synthesis to the final stages of package and release of virions. Agents are considered here according to the virus they are most commonly used to treat. Most antivirals in clinical use are nucleoside analogues, i.e. they look like the basic nucleosides (e.g. guanosine or thymidine) but have been chemically altered to stop viral replication. Aciclovir is able to induce selective toxicity against viruses because it is a pro-drug which requires a viral enzyme to help convert it to the active form by phosphorylation. In clinical practice, it is used for herpes simplex virus (HSV) and VZV. Valaciclovir is absorbed orally and becomes hydrolysed to aciclovir in the blood. Ganciclovir is active against cytomegalovirus (CMV) and also requires phosphorylation using an enzyme present in CMV. It is more toxic than aciclovir, as host cells can also add the phosphate group. Valganciclovir is better absorbed orally than ganciclovir. Oseltamivir inhibits newly formed influenza virions from being released from host cells by inhibiting viral neuraminidase (surface proteins). There are five main classes of antiretroviral drugs: nucleoside analogue reverse transcriptase inhibitors (NRTIs, e.g. AZT) and non-nucleoside analogue reverse transcriptase inhibitors (NNRTI, e.g. nevirapine), protease inhibitors (PI, e.g. ritonavir), fusion inhibitors (FI, e.g. maraviroc) and integrase inhibitors (e.g. raltegravir). Side effects can limit their tolerability, e.g. on glucose and lipid metabolism, as seen with the protease inhibitors. Mutations in the virus can lead to drug resistance, thus the need for combination treatment and good adherence. A growing number of antifungal agents exist, however some are limited to topical application due to their toxicity. Polyenes bind to ergosterol in the fungal cell wall, causing lysis of the cell membrane and cell death. Some polyenes are toxic only to fungi, whilst others also cause toxicity in the host. For example, amphotericin also binds to cholesterol in the kidneys causing nephrotoxicity, however this effect is reduced in the lipid preparations. They have a broad spectrum of antifungal activity. Azoles block the synthesis of ergosterol, leading to fungal cell wall dysfunction. Fluconazole is often used to treat Candida infections, but where activity against Aspergillus is required, voriconazole is the treatment of choice. Echinocandins inhibit an enzyme responsible for β-glucan synthesis and prevent fungal cell wall synthesis leading to cell death. They are active against Candida and Aspergillus. Antifungal agents may exert a fungostatic or fungicidal effect on fungi and this effect varies between different fungi; for example, voriconazole is fungicidal against Aspergillus sp., but fungostatic against Candida sp. Effective agents with antihelminth activity are limited. Intestinal nematodes are treated with mebendazole, a poorly absorbed agent that is active locally, causing parasite immobilization and death. Albenazole is better absorbed and therefore is active against blood and tissue nematodes. The treatment of cestodes and trematodes are limited to praziquantel. A number of antimalarial agents are available, however geographic resistance limits their use. Knowledge of malaria species and travel destination are essential for appropriate prescribing. Options for intestinal protozoa are more limited. Robust mechanical, biochemical and microbiological barriers (such as skin, bile acid and host flora) fend off many microorganisms before they come in contact with the immune system. The lymphoreticular system represents the main anatomical structure of the immune system. This includes the bone marrow from which all cells of the immune system are produced, the thymus (where T cells mature), lymph nodes, tonsils, Peyer’s patches, appendix and spleen. The immune system can be considered as the innate and adaptive immune system (Fig. 15.4).
Infection and immunity
The classification and essential features of infectious agents
Viruses
Structure (Fig. 15.1)
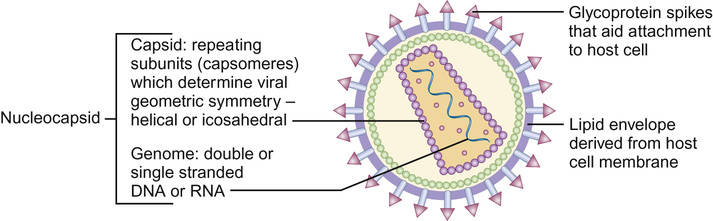
Pathogenesis
Bacteria
Classification
Structure
Pathogenesis
Eukaryotes
Protozoa
Fungi
Helminths
Pharmacology and the rational use of antibiotics
Key concepts in antimicrobial pharmacology
i) Disruption of bacterial cell wall
ii) Inhibition of protein synthesis
iii) Inhibition of DNA replication
iv) Interruption of microbial chemical pathways
Resistance
Antiviral therapy
Herpesviruses
Influenza
HIV-1
Antifungal therapy
Polyenes (e.g. nystatin, amphotericin B)
Azoles (e.g. fluconazole, itraconazole, voriconazole)
Echinocandins (e.g. caspofungin, micafungin)
Antihelminth agents
Anti-protozoal agents
Host defence mechanisms and their pattern of development
The structure of the immune system
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Infection and immunity
Chapter 15
Learning objectives
By the end of this chapter the reader should: