Clinical Laboratory Procedures
Meta Carroll
James F. Wiley II
Introduction
This chapter deals with laboratory tests that may be commonly performed by a physician or other health care provider during the emergent care of a child. The focus is on the scientific background of each test, the equipment required, the procedure, and the interpretation of results. Since January 1995, with a recent update in 2003, significant federal oversight has governed the performance of all clinical laboratory tests. This chapter specifically deals with physician-performed microscopy that is not waived under federal regulation.
Regulations under the Clinical Laboratory Improvement Act (CLIA) of 1988 require that all sites of laboratory testing register with the Health Care Financing Administration (HCFA) and obtain a provider number. According to CLIA, tests are classified as waived, physician-performed microscopy, moderate complexity, and high complexity. Waived tests require certification only, without any further requirement except to agree to random inspection intended to ensure that only waived tests are being performed. Urine beta human choriogonadotropin, rapid urine dipstick, fecal occult blood, spun hematocrit, and HemoCue tests are some of the more common waived tests utilized in the emergency department (ED) (1). Laboratories performing physician-performed microscopy are subject to quality control and assurance regulations but are exempt from routine laboratory inspections. All other tests are classified as moderate complexity or high complexity and require external proficiency testing, establishment of written quality control and quality assurance procedures, special personnel requirements, and laboratory inspections every 2 years (2,3). In some instances, the hospital clinical laboratory can administer the satellite laboratory activities in the ED. This arrangement allows for resources to comply with CLIA regulations.
Infectious Disease
Gram Stain
The Gram stain, named for Hans Christian Gram, differentiates two large groups of bacterial species: Gram-positive and Gram-negative organisms. The procedure requires applying crystal violet, a dye taken up by the bacterial cell wall, then Gram’s iodine, which aids in bonding dye to the cell wall. Next, decolorization with an acetone-alcohol agent and counterstaining with safranin solution are performed. The Gram-positive organisms retain the crystal violet dye and resist decolorization, thus appearing purple in color. Gram-negative organisms take up the dye but are then susceptible to decolorization and on microscopic examination appear pink. The ability to resist decolorization is due to the cell wall composition of each organism. The Gram-positive cell wall contains a thick peptidoglycan layer with numerous teichoic acid cross-links. These cross-links contribute to cell wall resistance to alcohol decolorization. In contrast, the cell wall of the Gram-negative organism has a thin layer of peptidoglycan and a thick external coat of lipopolysaccharides and protein islands (4,5).
Other cells in a Gram-stained specimen include erythrocytes and leukocytes, which initially stain with the application of crystal violet; the stain then washes out with an application of decolorizer. Subsequent safranin counterstain results in a pink or red cell appearance. Yeast cells, resisting decolorization and staining purple, are Gram-positive in their Gram reaction, but fungal mycelia are Gram variable (4). This procedure is designated as moderately complex by the U.S. Food and Drug Administration (FDA) and thus requires proficiency testing, designated personnel, written quality control and assurance measures, and laboratory inspection every 2 years.
Equipment
Glass slide and coverslip
Crystal violet solution
Ethyl alcohol 95% (optional: acetone-ethyl alcohol mixture)
Gram’s iodine
Safranin solution
Heat source (optional: 95% methanol)
Procedure
Gram staining requires specimen collection, preparation of the smear, staining, decolorization, counterstain application, drying, and finally microscopic examination. The primary goal of specimen collection is to obtain the body fluid or exudate material with minimal contamination. For example, careful cleaning of the surrounding skin before obtaining a wound exudate specimen helps to eliminate skin bacterial contaminants. Material collected in a syringe or plastic container is preferable to using cotton swabs, which can absorb components of the specimen. The specimen is thinly applied on a clean glass slide and air dried. Most specimens will adhere to the slide and do not require heat fixing. To heat-fix, the slide is passed over a gentle flame several times. The slide should feel warm to the touch and should not be overheated to avoid damaging the specimen. Alternatively, fixation can be performed with 95% methanol. The methanol is applied to the slide, allowed to run off for 1 minute, then air dried before staining. The following steps are then performed (5,6).
The slide is flooded with crystal violet solution for 30 to 60 seconds and rinsed with tap water. Then the slide is flooded with Gram’s iodine solution for 30 to 60 seconds and rinsed with tap water. Next, the decolorizer agent (95% ethyl alcohol or acetone-ethyl alcohol mixture) is used to rinse the slide for 5 to 30 seconds, until the drops running off the slide are no longer blue. For decolorizing agents that have higher acetone content, the decolorization is much more rapid (5 to 10 seconds). The agent used will dictate the rinsing time required for adequate decolorization. Thicker smears also may require more time for decolorization. Finally, the slide is flooded with safranin solution for 30 to 60 seconds, rinsed with tap water and air dried. If a paper towel or filter paper is used to facilitate drying the slide, it is important to ensure that the slide is blotted, as wiping may damage the specimen. The specimen is examined under first a low-power and then a high-power objective. Using an oil immersion lens allows better visualization of leukocytes, erythrocytes, and bacteria.
TABLE 123.1 Gram Stain Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
A more rapid method of Gram staining can be used. First, crystal violet is applied for a few seconds, followed by Gram’s iodine solution for a few seconds. The slide is rinsed with decolorizing agent, and safranin solution (counterstain) is applied for a few seconds. The slide is then rinsed with tap water and dried.
Interpretation
Using the Gram stain technique serves two primary purposes: (a) classification of bacteria on the basis of the Gram reaction (i.e., Gram-positive vs. Gram-negative) and (b) identification of numbers and morphology of bacteria (e.g., cocci, bacilli, etc.). These two factors are valuable clues to the cause of disease and may help guide early treatment. Of note, appropriate antibiotic therapy should not be dictated by the Gram stain alone but should be chosen based on all available clinical information. Gram staining, as it provides an opportunity for direct examination of the specimen, also determines the adequacy of the specimen for culture. For example, a sputum sample with more than 10 epithelial cells per low-power field indicates contamination of the sample with oropharyngeal flora and is thus inadequate for culture.
The Gram stain is useful in many clinical settings, including infections of the lower respiratory tract, genitourinary tract, skin and soft tissues, and joint spaces. Bacterial morphology and Gram reaction may help guide therapy in cases of septic arthritis, cystitis, and skin infections. Gram stain findings for respiratory and gynecological pathogens are summarized in Table 123.1 (7,8). Gram stains of cerebrospinal fluid and the buffy coat of peripheral blood (in the setting of meningitis and septicemia, respectively) are generally performed in the hospital laboratory rather than the ED.
The decolorizing step, as previously described, distinguishes Gram-positive from Gram-negative organisms. Failure to perform this step correctly yields erroneous results. Underdecolorization occurs when rinsing with the decolorizing agent is not performed an adequate length of time. Thus cellular elements retain the crystal violet dye even in the absence of the thick peptidoglycan layer of Gram-positive organisms. Overdecolorization also may occur. The specimen that is decolorized for too long a period (especially when using the rapid-acting, high–acetone content decolorizer) may remove crystal violet dye and lead to the erroneous interpretation of Gram-negative staining. Of note, any loss in cell wall integrity of Gram-positive organisms may allow the crystal violet to rinse away with the decolorizing step. The teichoic acid cross-links may be affected by such factors as antibiotic treatment, the action of autolytic enzymes, or the age of the organism itself (4,6). In light of the potential for misinterpretation and the frequent difficulty in obtaining an adequate, contaminant-free specimen, the Gram stain is always recommended as a diagnostic adjunct, not the sole determinant, in guiding appropriate presumptive antibiotic therapy.
Potassium Hydroxide Preparation
The potassium hydroxide (KOH) preparation provides direct microscopic examination of clinical specimens in suspected fungal infections. Fungi have a polysaccharide-containing cell wall. The KOH solution is an alkali that digests proteinaceous material, such as host cellular material, while leaving the fungal cell wall intact (4). Thus using the KOH preparation can help confirm the diagnosis in clinically suspected fungal infections. The KOH preparation is designated as a physician-performed microscopy procedure and requires proficiency testing, test management, and quality control and assurance policies.
Equipment
Glass slide and coverslip
Scalpel, No. 15 blade
KOH solution (10%)
Heat source (optional)
TABLE 123.2 Microscopic Examination of Fungi | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Procedure
The specimen is obtained and placed on a glass slide. For cutaneous infections, the site is cleaned with an alcohol swab and the active edge of the lesion is scraped with the edge of a microscope slide or scalpel blade (see also Chapter 122). If the infection involves scalp and hair, one may select a short broken hair and remove it using a hemostat or gently scrape the scalp using the slide or a scalpel. For vaginal infection, the specimen is collected using a cotton swab. Care must be used in viewing such specimens, as the cotton strands may resemble fungal hyphae on microscopic examination (9).
The specimen is covered with one to two drops of KOH solution and topped with the coverslip. Alternatively, the coverslip may be placed on the specimen and one drop of KOH solution placed at the edge of the preparation, allowing the solution to flow under the coverslip (9). One should wait at least 5 minutes before proceeding with the microscopic examination. If proteinaceous debris remains, potentially obscuring fungal elements, 5 to 10 minutes longer is allowed before re-examining the specimen microscopically. Alternatively, the clinician can pass the specimen quickly over a flame, which may speed the alkali digestion. It is important not to overheat or boil the material, as this will damage the specimen. The specimen is examined using a low-power objective (10× magnification), then high-power objective to identify fungal elements.
Interpretation
Fungi are divided into yeasts and molds, which differ in the macroscopic appearance of colonies formed on culture media, microscopic morphologic features, the mode of sporulation, and the characteristics of the spores produced. Most of the clinically significant fungi reproduce by asexual sporulation. Three types of spores—arthroconidia, chlamydospore, and blastoconidia—may be identified by microscopic examination (Table 123.2 and Fig. 123.1) (4,9).
As previously mentioned, care must be taken in preparing the specimen and interpreting microscopic findings. Overheating may damage the specimen and obliterate fungal elements. Foreign material such as the cotton fibers from a swab
may masquerade as hyphae. Inadequate time allowed for the KOH preparation may impair fungi visualization, as cellular debris obscures fungal elements. Such debris also may resemble fungal elements, further impeding accurate identification.
may masquerade as hyphae. Inadequate time allowed for the KOH preparation may impair fungi visualization, as cellular debris obscures fungal elements. Such debris also may resemble fungal elements, further impeding accurate identification.
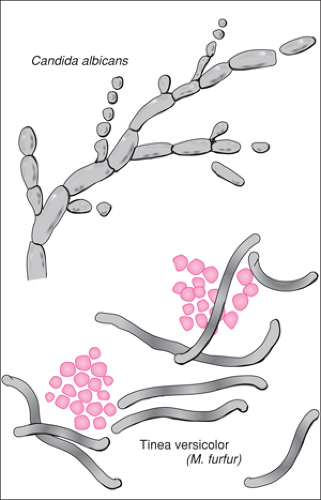 Figure 123.1 Identification of fungal elements (40× magnification).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|