INTRODUCTION
Pelvic masses are common and may involve reproductive organs or nongynecologic structures. Affected women can be symptom-free or may complain of pain, pressure, dysmenorrhea, infertility, or uterine bleeding. Treatment varies with patient age and therapeutic goals. Medical management is possible for many with pelvic masses, but for others, procedural interventions offer highest success rates.
DEMOGRAPHIC FACTORS
Of associated factors, pelvic mass rates and underlying pathology change with age. In prepubertal girls, most gynecologic pelvic masses involve the ovary. Even before puberty, ovaries are active, and masses are often functional, rather than neoplastic, cysts (de Silva, 2004). Of neoplastic lesions, most are benign germ cell tumors, especially mature cystic teratomas (dermoid cysts) (Brown, 1993). Malignant ovarian tumors in children and adolescents are rare, and this age group accounts for only 1.2 percent of all ovarian cancers (National Cancer Institute, 2014). Most cancers are germ cell tumors, and among children and adolescents, rates increase with age (American Cancer Society, 2014).
In adolescents, the incidence and type of ovarian pathology in general mirrors that of prepubertal girls. However, with the onset of reproductive function, pelvic masses in adolescence may also include endometriomas and the sequelae of pelvic inflammatory disease (PID) and pregnancy.
In adult women, the differential diagnosis for a pelvic mass expands. Uterine enlargement due to pregnancy, functional ovarian cysts, and leiomyoma are among the most common. Endometrioma, mature cystic teratoma, acute or chronic tuboovarian abscess (TOA), and ectopic pregnancies are other frequent causes. Most pelvic masses in this age group are benign, but malignancy rates increase with age.
In postmenopausal women, with cessation of reproductive function, the causes of pelvic mass also change. Simple ovarian cysts and leiomyomas are still frequent. Menopause typically results in leiomyoma atrophy, but some uterine bulk may still persist. Importantly, malignancy is a more frequent cause in this demographic group. Ovarian cancer accounts for nearly 3 percent of new cancers among all women (American Cancer Society, 2014). Uterine tumors, including adenocarcinoma and sarcoma, can enlarge the uterus.
LEIOMYOMAS
Uterine enlargement most frequently reflects pregnancy or leiomyomas. Less often, enlargement is from adenomyosis, hematometra, an adhered adnexal mass, or malignancy. Of these, leiomyomas are benign smooth muscle neoplasms that typically originate from the myometrium. They are often referred to as uterine myomas, and they are colloquially called fibroids. Their incidence among women is generally cited as 20 to 25 percent, but is as high as 70 to 80 percent in studies using histologic or sonographic examination (Baird, 2003; Cramer, 1990). The health care consequences of these tumors are substantial. From 1998 to 2005, 27 percent of inpatient gynecologic admissions were for uterine leiomyoma care (Whiteman, 2010).
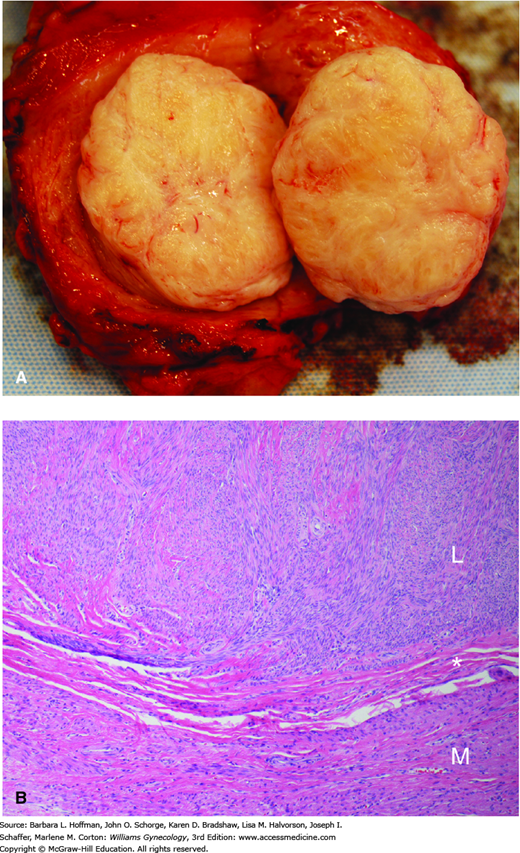
Grossly, leiomyomas are round, rubbery tumors that when bisected display a whorled pattern. They possess a distinct autonomy from their surrounding myometrium because of a thin, outer connective tissue layer (Fig. 9-1). This clinically important cleavage plane allows leiomyomas to be easily “shelled” from the uterus during surgery. Histologically, leiomyomas contain elongated smooth-muscle cells aggregated in dense bundles. Mitotic activity, however, is rare and is a key point in differentiation from malignant leiomyosarcoma.
FIGURE 9-1
The appearance of leiomyomas will vary depending on the degree and type of degeneration present. A. In this bisected uterine fundus, a typical off-white, whorled leiomyoma lies distinct from the surrounding myometrium. B. Microscopically, leiomyomas (L) are composed of bland, spindled smooth-muscle cells characterized by elongate, blunt-ended nuclei and tapered eosinophilic cytoplasm. The cells are arranged in interlacing fascicles that intersect at right angles. Leiomyomas are usually well-circumscribed, and the interface (asterisk) between the myoma and adjacent myometrium can be seen grossly and microscopically. These tumors are usually more cellular than the surrounding myometrium (M). (Used with permission from Dr. Kelley Carrick.)
The typical appearance of leiomyomas may change if smooth muscle is replaced with various degenerative substances following necrosis. This process is collectively termed degeneration, and the replacement substances dictate the naming of these degenerative types. Forms include hyaline, calcific, cystic, myxoid, red, and fatty, and these gross changes should be recognized as normal variants. Necrosis and degeneration develop frequently in leiomyomas because of the tenuous blood supply within these tumors. Leiomyomas have a lower arterial density compared with the surrounding normal myometrium. Moreover, their lack of vascular organization leaves some tumors vulnerable to hypoperfusion and ischemia (Forssman, 1976). As discussed later, acute pain may accompany degeneration.
Each leiomyoma is derived from a single progenitor myocyte. Thus, multiple tumors within the same uterus each show independent cytogenetic origins (Townsend, 1970). Several unique defects involving chromosomes 6, 7, 12, and 14 and others correlate with rates and direction of tumor growth (Brosens, 1998). Of specific gene mutations, those involving MED12 and HMGA2 genes, and less commonly COL4A5-A6 or FH genes, account for most leiomyomas (Mehine, 2014). Of these, fumarate hydratase (FH) gene mutations are rare but lead to the hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. This is characterized by cutaneous and uterine leiomyomas and renal cell cancer (Mann, 2015). Future determination of each mutation’s role in leiomyoma formation is anticipated to aid treatment development.
Following their genesis, uterine leiomyomas are estrogen and progesterone sensitive tumors. Consequently, they develop during the reproductive years. After menopause, leiomyomas generally shrink, and new tumor development is infrequent. These sex steroid hormones likely mediate their effect by stimulating or inhibiting transcription or cellular growth-factor production.
Leiomyomas themselves create a hyperestrogenic environment, which appears requisite for their growth and maintenance. First, compared with normal myometrium, leiomyoma cells contain a greater density of estrogen receptors, which results in greater estradiol binding. Secondly, these tumors convert less estradiol to the weaker estrone (Englund, 1998; Otubu, 1982; Yamamoto, 1993). A third mechanism involves higher levels of cytochrome P450 aromatase in leiomyomas compared with normal myocytes (Bulun, 1994). This specific enzyme catalyzes the conversion of androgens to estrogen (Chap. 15).
Some conditions also provide sustained estrogen exposure that encourages leiomyoma formation. For example, the increased years of persistent estrogen production found with early menarche and with an increased body mass index (BMI) are each linked with a greater leiomyoma risk (Velez Edwards, 2013; Wise, 2005). Obese women produce more estrogens from increased conversion of androgens to estrogen in adipose tissue by aromatase. They also display decreased hepatic production of sex hormone binding globulin (Glass, 1989). Women with polycystic ovarian syndrome (PCOS) have a higher risk of myoma formation, which may stem from the sustained estrogen exposure that accompanies chronic anovulation (Wise, 2007).
Of other factors, estrogen and progesterone hormone treatment in premenopausal women probably has no significant inductive effect on leiomyoma formation. With few exceptions, combination oral contraceptive (COC) pills either lower or have no effect on this risk (Chiaffarino, 1999; Parazzini, 1992).
Smoking alters estrogen metabolism and lowers physiologically active serum estrogen levels (Soldin, 2011). This may explain why women who smoke generally have a lower risk for leiomyoma formation.
As with estrogen, leiomyomas carry a higher progesterone receptor density compared with their surrounding myometrium. Progesterone is considered the critical mitogen for uterine leiomyoma growth and development, and estrogen functions to upregulate and maintain progesterone receptors (Ishikawa, 2010). Thus, cell proliferation, extracellular matrix accumulation, and cell hypertrophy, which all lead to leiomyoma growth, are controlled by progesterone directly and in a permissive role by estrogen.
This relationship is supported by evidence that the antiprogestins mifepristone and ulipristal induce atrophy in most leiomyomas (Donnez, 2012a; Murphy, 1993). Moreover, in women treated with gonadotropin-releasing hormone (GnRH) agonists, leiomyomas typically decrease in size. However, if progestins are given simultaneously with GnRH agents, there is typically increased leiomyoma growth (Carr, 1993).
Clinically, this relationship may be important when prescribing sex steroid hormones. In postmenopausal women, hormone replacement therapy (HRT) has either a stimulatory or no effect on growth (Polatti, 2000; Reed, 2004). Palomba and associates (2002) found that higher doses of medroxyprogesterone acetate (MPA) were associated with leiomyoma growth and recommended using the lowest possible dose of MPA in these patients.
Of other factors associated with myoma development, race and age are notable risks. Myomas are rare in adolescence but increase with age during the reproductive years. In a study by Baird and coworkers (2003), the cumulative incidence by age 50 years was nearly 70 percent in whites and more than 80 percent in African-American women. Lower rates of leiomyomas are linked with pregnancy. Those who have higher parity, have had a more recent pregnancy, and have breastfed all display lower incidences of myoma formation (Terry, 2010). Leiomyomas are more common in African-American women compared with white, Asian, or Hispanic women. Thus, as noted earlier, heredity and specifically gene mutations play a seminal role in myoma development.
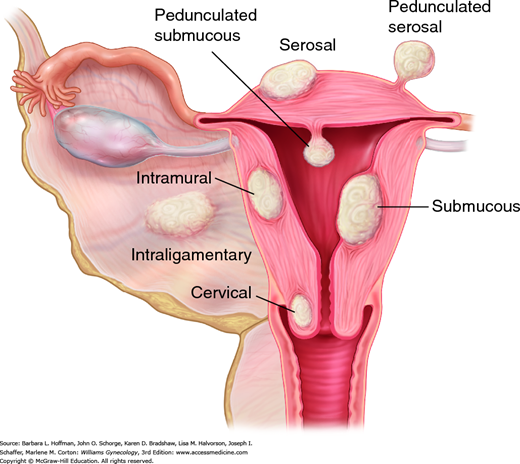
These tumors are classified based on their location and direction of growth (Fig. 9-2). Subserosal leiomyomas originate from myocytes adjacent to the uterine serosa, and their growth is directed outward. When these are attached only by a stalk to their progenitor myometrium, they are called pedunculated leiomyomas. Parasitic leiomyomas are subserosal variants that attach themselves to nearby pelvic structures from which they derive vascular support. These myomas then may or may not detach from the parent myometrium. Intramural leiomyomas are those with growth centered within the uterine walls. Finally, submucous leiomyomas are proximate to the endometrium and grow toward and bulge into the endometrial cavity. For endoscopic resection evaluation, submucous leiomyomas are further classified by their depth of involvement. The European Society of Hysteroscopy and the International Federation of Gynecology and Obstetrics (FIGO) defines leiomyomas as: type 0, if the mass is located entirely within the uterine cavity; type 1, if less than 50 percent is located within the myometrium; and type 2, if greater than 50 percent of the mass is surrounded by myometrium (Wamsteker, 1993). To aid abnormal uterine bleeding research, this numerical classification was expanded by FIGO to similarly assign subclassifying numbers to intramural, subserosal, and parasitic leiomyomas (Munro, 2011).
Of tumors outside the uterine corpus, only about 0.4 percent develop in the cervix (Tiltman, 1998). Leiomyomas have also been found infrequently in the ovary, fallopian tube, broad ligament, vagina, and vulva.
Extrauterine smooth-muscle tumors, which are benign yet infiltrative, may develop in women with concurrent or prior uterine leiomyomas, and this condition is termed leiomyomatosis. In such cases, malignant metastases from a leiomyosarcoma must be excluded.
Intravenous leiomyomatosis invades and extends serpiginously into the uterine veins, other pelvic veins, vena cava, and even cardiac chambers. As such, this rare tumor may lead to classic leiomyoma complaints or to uncharacteristic ones such as right-sided congestive cardiac symptoms. Tumors are usually amenable to resection. Although these are histologically benign, recurrence rates may reach 28 percent (Wang, 2012).
Benign metastasizing leiomyomas derive from morphologically benign uterine leiomyomas that disseminate hematogenously. Lesions have been found most often in the lungs, and less so in lymph nodes, bone, brain, and heart. Classically, these are found in women who have a recent or distant history of pelvic surgery.
Disseminated peritoneal leiomyomatosis (DPL) is benign and appears as multiple small peritoneal nodules on abdominal cavity or abdominal organs surfaces. As such, DPL intraoperatively and radiologically looks like widespread peritoneal metastatic malignancy. DPL is usually found in women of reproductive age, and 70 percent are associated with pregnancy or COC use (Bisceglia, 2014).
Last, with the increased use of electromechanical morcellation during minimally invasive myomectomy or hysterectomy, multiple small peritoneal leiomyomas may be found later after the primary surgery. Secondary implantation of myoma remnants is implicated, and these present similarly to parasitic leiomyomas or DPL (Kho, 2009). A full discussion of this topic is found in Chapter 41.
Treatments for all these benign conditions may involve hysterectomy with oophorectomy, tumor debulking, and agents that lower estrogen and/or progestin levels. Pharmaceutical choices include GnRH agonists, aromatase inhibitors, selective estrogen-receptor modulators, or antiprogestins (Lewis, 2013; Taveira-DaSilva, 2014).
Most women with leiomyomas are asymptomatic. However, affected women may complain of bleeding, pain, pressure, or infertility. In general, symptom risk increases with myoma size and number. Bleeding is common, especially heavy menstrual bleeding (HMB), and dilated endometrial venules are implicated. Dysregulation of local vasoactive growth factors is thought to promote this vasodilatation. When engorged venules are disrupted at the time of menstrual sloughing, bleeding from these markedly dilated vessels overwhelms the usual hemostatic mechanisms (Stewart, 1996). For this reason, subserosal, intramural, and submucous tumors all have a propensity to cause HMB (Wegienka, 2003).
A sufficiently enlarged uterus can cause chronic pressure, urinary frequency, incontinence, or constipation. Rarely, leiomyomas extend laterally to compress a ureter and lead to obstruction and hydronephrosis. Aside from pressure, patient may also note dysmenorrhea, dyspareunia, or noncyclical pelvic pain (Lippman, 2003; Moshesh, 2014).
Acute pelvic pain is a less frequent complaint but is most often seen with a degenerating or prolapsing leiomyoma. Rare tumor complications include torsion of a subserosal pedunculated leiomyoma, acute urinary retention, or deep-vein thromboembolism (Gupta, 2009). With leiomyoma degeneration, tissue necrosis classically causes acute pain, fever, and leukocytosis. This constellation mimics other acute pelvic pain sources. Thus, sonography is typically performed to help identify a cause, and usually a nondescript leiomyoma is found. Computed tomography (CT) may also be obtained, especially if clear interpretation of pelvic anatomy is obscured by multiple large leiomyomas or if appendicitis, nephrolithiasis, diverticulitis, or others are considered. Treatment of myoma degeneration is nonsurgical and includes analgesics and antipyretics as needed. However, broad-spectrum antibiotics are often administered, as differentiating between leiomyoma degeneration and acute endomyometritis can be difficult. In most cases, symptoms improve within 24 to 48 hours. Pain stemming from tumor degeneration classically follows uterine artery embolization (UAE), and treatment with analgesics usually suffices as discussed on page 209.
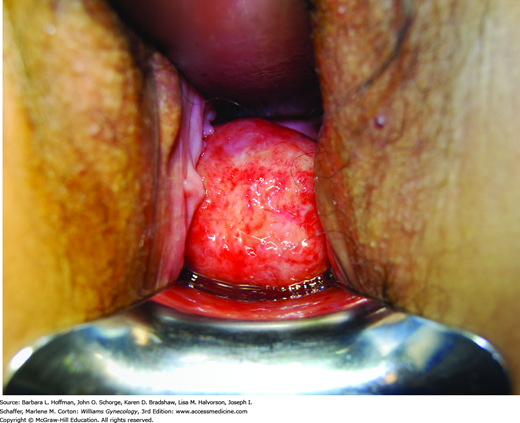
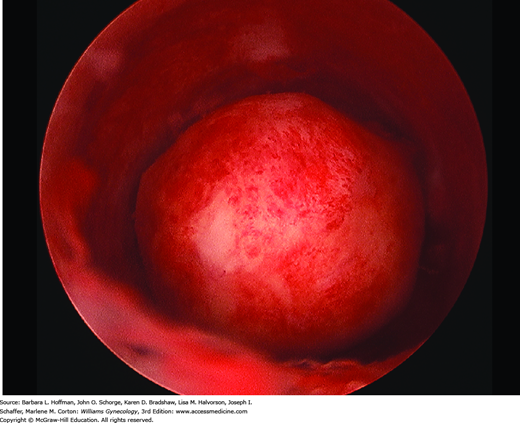
Women with prolapse of a tumor from the endometrial cavity will typically note cramping or acute pain as the tumor stretches the endocervical canal to pass through. Associated bleeding or serosanguinous discharge is common. Visual inspection is usually diagnostic (Fig. 9-3). However, sonography is often performed to evaluate the size and number of other uterine leiomyomas and exclude other possible sources of pain. Surgical treatment involves severing the leiomyoma from its stalk as described in detail in Section 43-11.
Leiomyomas can diminish fertility, but only 1 to 3 percent of infertility cases are due solely to leiomyomas (Buttram, 1981; Donnez, 2002). Their putative effects include occlusion of tubal ostia and disruption of the normal uterine contractions that propel sperm or ova. Distortion of the endometrial cavity may diminish implantation and sperm transport. Importantly, leiomyomas are associated with endometrial inflammation and vascular changes that may disrupt implantation (American Society for Reproductive Medicine, 2008).
Of myomas, subfertility is more closely associated with submucous leiomyomas than with tumors located elsewhere. Improved pregnancy rates following hysteroscopic resection have provided most of the indirect evidence for this link (Casini, 2006; Surrey, 2005). In contrast, evidence does not implicate subserosal tumors. For intramural leiomyomas that do not distort the endometrial cavity, the relationship with subfertility is more tenuous. Several investigators have reported equally good in vitro fertilization (IVF) success rates in women with and without leiomyomas that did not distort the endometrial cavity (Oliveira, 2004; Yan, 2014). Others, however, have reported adverse fertility effects from such intramural leiomyomas (Eldar-Geva, 1998; Hart, 2001). Importantly, the strength of this evidence must be weighed against the morbidity associated with myomectomy. Namely, peritubal or intrauterine adhesions can threaten fertility, and myometrial defects risk uterine rupture during subsequent pregnancies.
Both uterine leiomyoma and spontaneous miscarriage are common, and an association between these has not been shown convincingly. Moreover, there is no conclusive evidence that surgical treatment reduces miscarriage rates (American Society for Reproductive Medicine, 2012b; Pritts, 2009).
Less than 0.5 percent of women with leiomyomas develop myomatous erythrocytosis syndrome. This may result from excessive erythropoietin production by the kidneys or by the leiomyomas themselves (Vlasveld, 2008). In either case, red cell mass returns to normal following hysterectomy.
Leiomyomas occasionally may cause pseudo-Meigs syndrome. Traditionally, Meigs syndrome consists of ascites and pleural effusions that accompany a benign ovarian fibroma. However, any pelvic tumor including large, cystic leiomyomas or other benign ovarian cysts can cause this. The presumed etiology stems from discordancy between the arterial supply and the venous and lymphatic drainage from the leiomyomas. If due to myomas, resolution of ascites and hydrothorax follows hysterectomy or myomectomy.
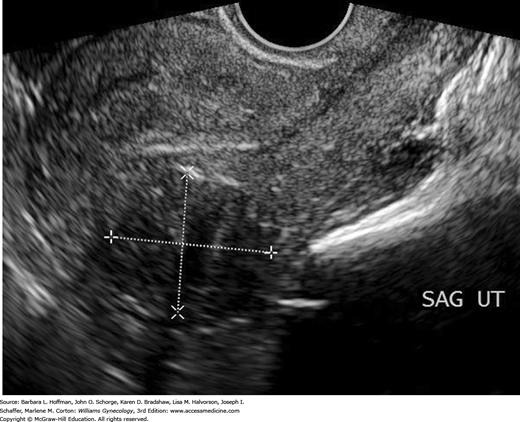
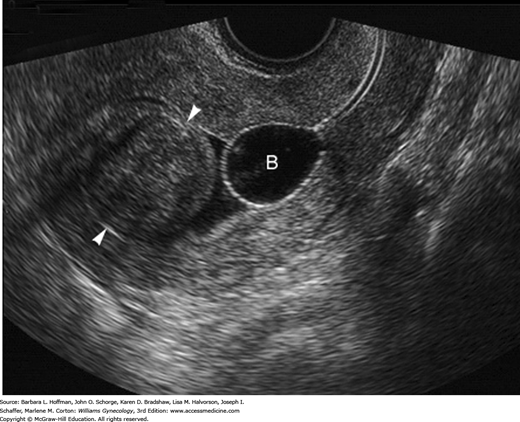
Leiomyomas are often detected by pelvic examination with findings of uterine enlargement, irregular contour, or both. In reproductive-aged women, uterine enlargement prompts determination of a urine or serum β-human chorionic gonadotropin (hCG) level. Sonography is initially done to define pelvic anatomy. Transvaginal sonography (TVS) provides superior resolution, but some uteri are so large that transabdominal sonography is needed to image the entire corpus. The sonographic appearances of leiomyomas vary from hypo- to hyperechoic depending on the ratio of smooth muscle to connective tissue and whether there is degeneration (Fig. 9-4). Calcification and cystic degeneration create the most sonographically distinctive changes. Calcifications appear hyperechoic and commonly rim the tumor or are randomly scattered throughout the mass. Cystic or myxoid degeneration typically fills the leiomyoma with multiple, smooth-walled, round, irregularly sized but generally small hypoechoic or anechoic areas.
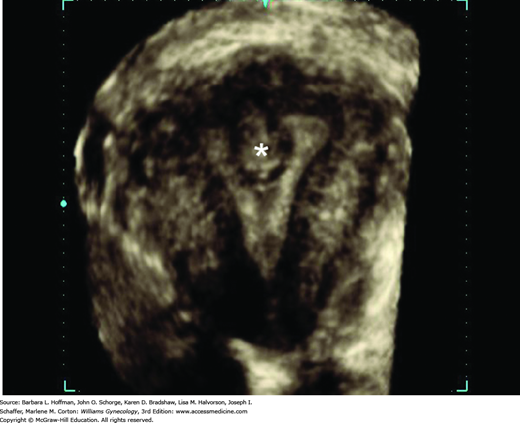
If HMB, dysmenorrhea, or infertility accompanies a pelvic mass, then the endometrial cavity is evaluated for submucous leiomyomas, endometrial polyps, congenital anomalies, or synechiae. With focal lesions such as submucous leiomyomas, the endometrium appears thick or irregular during TVS, and adjunct imaging tools may help clarify anatomy. Of these, saline infusion sonography (SIS) or hysteroscopy may provide additional cavity information (Figs. 9-5 and 9-6), and their advantages are described in Chapters 2 and 8. Also, three-dimensional (3-D) TVS and 3-D SIS can be valuable (Fig. 9-7). Leiomyomas have characteristic vascular patterns that can be identified by color and power Doppler techniques. A peripheral circumferential rim of vascularity from which a few vessels arise to penetrate into the center of the tumor is a classic finding. As such, Doppler imaging can be used to help differentiate an extrauterine leiomyoma from another pelvic mass or a submucous leiomyoma from an endometrial polyp. For the infertile woman, the endometrial cavity can be evaluated with hysterosalpingography (HSG) or hysterosalpingo-contrast sonography HyCoSy (Chap. 2). This offers the advantage to also define tubal patency.
When imaging is limited by body habitus or distorted anatomy, magnetic resonance (MR) imaging may be required. Although used less often for myoma evaluation, this tool allows more accurate assessment of the size, number, and location of leiomyomas, which may help identify appropriate candidates for hysterectomy alternatives such as myomectomy or UAE. Importantly, MR imaging can also aid differentiation of an intramural leiomyoma, which is a suitable indication for myomectomy, from a focal, compact collection of adenomyosis, which is poorly suited for enucleation.
Regardless of their size, asymptomatic leiomyomas usually can be observed and surveilled with an annual pelvic examination. At times, adnexal assessment may be hindered by large uterine size or irregular contour, and adequate uterine and adnexal assessment can both be limited by patient obesity. In these cases, some may choose to add annual sonographic surveillance (Cantuaria, 1998).
Leiomyomas in general are slow-growing. A longitudinal sonography-based study showed the average diameter growth to be only 0.5 cm/yr, although diameter growth greater than 3 cm/yr has been observed (DeWaay, 2002). Moreover, growth rates of leiomyomas within the same patient will vary widely, and some tumors will even spontaneous regress (Peddada, 2008). Therefore, predicting myoma growth or symptom onset is difficult, and watchful waiting may be the best option for an asymptomatic patient.
In the past, most preferred surgical removal of a large, asymptomatic leiomyomatous uterus because of concerns regarding increased later operative morbidity and cancer risks. These concerns have been disproven, and thus otherwise asymptomatic women with large leiomyomas can also be managed expectantly (Parker, 1994). In addition, most infertile women with uterine leiomyomas are initially managed expectantly. For those with symptomatic tumors, conception attempts closely follow surgery, if possible, to limit tumor recurrence before conception.
As discussed in the next section, in some women with symptomatic leiomyomas, long-term medical therapy may be preferred (Table 9-1). In others, medical therapy is used as a short-term preoperative adjunct. Also, because these tumors typically regress postmenopausally, some women choose medical treatment to relieve symptoms in anticipation of menopause.
| Agent | NSAID | COC | DMPA | LNG-IUS | GnRH agonist | Ulipristal a |
| Symptom | ||||||
| Dysmenorrhea | + | + | + | + | + | + |
| Menorrhagia | – | + | + | + | + | + |
| Pelvic pressure | – | – | – | – | + | + |
| Infertility | – | – | – | – | + | – |
Both COCs and progestins have been used to induce endometrial atrophy and to decrease prostaglandin production in women with leiomyomas. Friedman and Thomas (1995) studied 87 women with myomas and reported that women taking low-dose COCs had significantly shorter menses and no evidence of uterine enlargement. Few studies have evaluated DMPA specifically for leiomyoma-related bleeding, and its use is extrapolated from its effects in nonmyomatous uteri. Also, the levonorgestrel-releasing intrauterine system (LNG-IUS), marketed as Mirena, in small studies improves leiomyoma-related HMB (Sayed, 2011; Socolov, 2011). Importantly, tumors that distort the endometrial cavity preclude LNG-IUS use (Bayer, 2014). Also, compared with women without leiomyomas, those with tumors experience higher device expulsion rates (Youm, 2014).
For these reasons, sex steroid contraceptives are a reasonable treatment option for menses-related leiomyoma symptoms. However, because of the unpredictable effects of progestins on leiomyoma growth described earlier, the American College of Obstetricians and Gynecologists (2012a) recommends close monitoring of leiomyoma and uterine size.
As noted, progesterone is considered essential for myoma growth and antiprogestins agents are another potential option. Physiologically, progesterone can bind to either progesterone receptor A (PR-A) or B (PR-B). Of these, PR-A is found in leiomyomas in greater amounts than PR-B (Viville, 1997). Specific agents can competitively bind these receptors. Agents are classified as antiprogestins if they universally prompt antagonist effects. However, agents are termed selective progesterone-receptor modulators (SPRMs) if they exert antiprogestational effects in some tissues but progestational effects in others.
Of antiprogestins, mifepristone, also known as RU-486, diminishes leiomyoma volume by approximately half. Various doses have been used and range from 2.5 to 10 mg given orally daily for 3 to 6 months (Carbonell Esteve, 2008, 2012). Mifepristone therapy, however, has several drawbacks. First, approximately 40 percent of treated women complain of vasomotor symptoms. Second, its antiprogestational effects expose the endometrium to unopposed estrogen. The spectrum of endometrial findings range from simple endometrial hyperplasia to a newer category described as progesterone-receptor modulator-associated endometrial changes (Mutter, 2008). This concern for endometrial stimulation currently limits mifepristone’s use to 3 to 6 months. As a final concern, mifepristone (Mifeprex) is currently Food and Drug Administration (FDA)-approved solely for early pregnancy termination. It is manufactured only as 200-mg tablets, a dose well above that needed for leiomyoma therapy.
Ulipristal acetate is a SPRM that has similar effects to mifepristone. Currently marketed outside the United States, ulipristal acetate (Esmya), given as 5- or 10-mg oral daily doses, controls leiomyoma-related bleeding in 90 percent of patients. It performs comparably with leuprolide acetate (Donnez, 2012a,b). Again, endometrial concerns currently limit its use solely to that of a preoperative adjunct. Other SPRMs are also under investigation, but none are currently commercially available for leiomyoma treatment.
Other sex steroid hormone options include the androgens, danazol and gestrinone, which shrink leiomyoma volume and improve bleeding symptoms (Coutinho, 1989; De Leo, 1999). Unfortunately, their prominent side effects, which include acne and hirsutism, preclude their use as first-line agents.
These compounds are synthetic derivatives of the GnRH decapeptide. They are inactive if taken orally, but intramuscular (IM), subcutaneous, and intranasal preparations are available. Leuprolide acetate (Lupron) is FDA-approved for leiomyoma treatment and is available in a 3.75-mg monthly dose or 11.25-mg 3-month dose, both given IM. Less frequently used GnRH agonists include goserelin (Zoladex), administered as a 3.6-mg monthly or 10.8-mg 3-month subcutaneous depot implant; triptorelin (Trelstar), given as a 3.75-mg monthly or 11.25-mg 3-month IM injection; and nafarelin (Synarel), used in a 200-μg twice-daily nasal spray regimen. These latter three are not specifically FDA-approved for leiomyoma treatment, but their off-label use has been shown effective.
GnRH agonists shrink leiomyomas by targeting the growth effects of estrogen and progesterone. Initially, these agonists stimulate receptors on pituitary gonadotropes to cause a supraphysiological release of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Also called a flare, this phase typically lasts 1 week. With their long-term action, however, agonists downregulate receptors in gonadotropes, thus creating desensitization to further GnRH stimulation. Correspondingly, decreased gonadotropin secretion leads to suppressed estrogen and progesterone levels 1 to 2 weeks after initial GnRH agonist administration (Broekmans, 1996).
Results with GnRH agonist treatment include dramatic decreases in uterine and leiomyoma volume. Most women experience a mean decrease in uterine volume of 40 to 50 percent, and most of this occurs during the first 3 months of therapy. Clinical benefits of reduced leiomyoma volumes include pain relief and diminished HMB, usually amenorrhea. During this time, anemic women are given oral iron therapy to rebuild red cell mass and increase iron stores (Filicori, 1983). GnRH agonist treatment typically is continued for 3 to 6 months. Following their discontinuance, normal menses resume in 4 to 10 weeks. Unfortunately, leiomyoma then regrow, and uterine volumes regain pretreatment sizes within 3 to 4 months (Friedman, 1990). Despite regrowth, Schlaff and coworkers (1989) reported symptom relief for approximately 1 year in half of women given GnRH agonists.
GnRH agonists have significant costs, risks, and side effects. Side effects result from the profound drop in serum estrogen levels, mirror those of menopause, and develop in up to 95 percent of women treated with these drugs (Letterie, 1989). Despite this, less than 10 percent of patients terminate treatment secondary to side effects (Parker, 2007). Importantly, 6 months of agonist therapy can result in a 6-percent loss in trabecular bone, not all of which may be recouped following discontinuation (Scharla, 1990). As a result, these agents alone are not recommended for longer than 6 months of use.
To obviate side effect severity, several medications have been added to GnRH agonist treatment. The goal of this “add-back therapy” is to counter side effects—most importantly vasomotor symptoms and bone loss—without mitigating the shrinking action on uterine and leiomyoma volume. This is made possible by the fact that the estrogen level required to improve vasomotor symptoms and minimize bone loss is below the estrogen threshold that would restimulate leiomyomas growth. Mizutani and associates (1998) found that GnRH agonists suppress leiomyoma cell proliferation and induce cell apoptosis at the fourth week of GnRH agonist therapy. They proposed that add-back therapy be withheld until after this time. Because of these and other observations, add-back therapy is typically begun 1 to 3 months following GnRH agonist initiation.
Add-back therapy traditionally includes estrogen combined with a progestin, and those studied have generally been low-dose preparations equivalent to menopausal HRT. An oral regimen of MPA, 10 mg (days 16–25), combined with equine estrogen, 0.3 to 0.625 mg (days 1–25), or a continuous daily regimen of MPA 2.5 mg and equine estrogen 0.3 mg to 0.625 mg may be used.
Add-back therapy with selective estrogen-receptor modulators (SERMs), such as tibolone and raloxifene, has also been shown to prevent bone loss. Advantages of SERMs include the ability to begin them concurrently with GnRH agonist treatment without negating the agonist effects of leiomyoma shrinkage. Unfortunately, a high percentage of women complain of vasomotor symptoms while taking SERMs (Palomba, 1998, 2004). Raloxifene is associated with greater venous thromboembolism risks (Goldstein, 2009).
Because of the limitations of GnRH agonist therapy, the American College of Obstetricians and Gynecologists (2014a) recommends that it not be used longer than 6 months without add-back therapy. Short-term, preoperative GnRH agonist use offers several advantages. Their use decreases HMB and may allow correction of anemia. Decreased uterine size as a result of treatment may allow a less complicated or extensive surgical procedure. For example, hysterectomy or myomectomy may be performed through a smaller laparotomy incision or by vaginal or minimally invasive surgery (MIS) approaches. This advantage may be less robust for GnRH agonist use prior to hysteroscopic myomectomy and is discussed in that section of the atlas.
In contrast to agonists, GnRH antagonists are available. Two agents in this class, cetrorelix and ganirelix, are currently FDA-approved for infertility use in women undergoing controlled ovarian hyperstimulation. However, a limitation of these drugs is that they are daily injectables. Also, a depot form of cetrorelix did not provide adequate or consistent suppression of estrogen production or leiomyoma growth (Felberbaum, 1998). A new agent, elagolix, is a nonpeptide oral GnRH antagonist that is currently being evaluated for both endometriosis and leiomyoma treatment (Diamond, 2014).
Tranexamic acid (TXA) is an antifibrinolytic agent described fully in Chapter 8. Studies have not evaluated TXA specifically for myoma-related HMB, but subgroup analysis does provide some support for its use for myoma-related bleeding (Eder, 2013).
The benefits of NSAIDs for leiomyoma-related bleeding are less clear, and the few studies have conflicting results (Anteby, 1985; Mäkäräinen, 1986; Ylikorkala, 1986). Thus, although NSAIDs are potentially helpful for myoma-related dysmenorrhea, available data do not support their use as sole agents for leiomyoma-related HMB.
Because aromatase levels are higher with myomas, aromatase inhibitors (AIs) for leiomyoma treatment seem logical. However, only a few small studies have evaluated short-term use of the oral nonsteroid AIs, letrozole and anastrozole (Parsanezhad, 2010; Varelas, 2007). As with GnRH agonists, the induced profound systemic hypoestrogenism leads to menopausal symptoms and potential bone loss. Moreover, their use is associated with increased FSH release, which could cause multiple follicular cyst formation. Larger prospective studies are needed to define their clinical role.
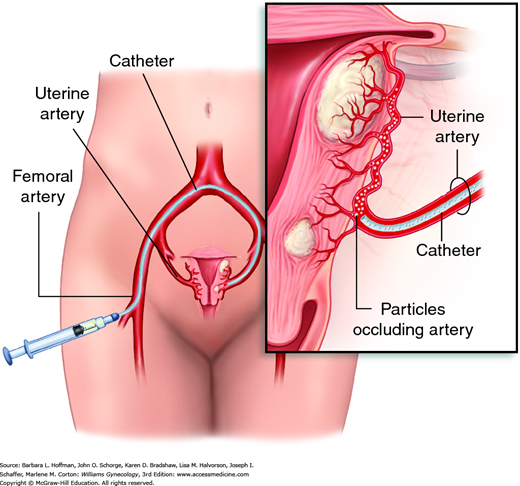
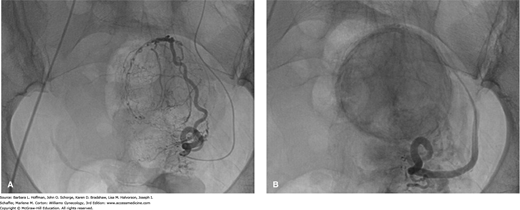
This is an is an angiographic interventional procedure that delivers polyvinyl alcohol microspheres or other synthetic particulate emboli into both uterine arteries. Uterine blood flow is thereby obstructed, producing ischemia and necrosis. Because vessels serving leiomyomas have a larger caliber, these microspheres are preferentially directed to the tumors, sparing the surrounding myometrium.
During UAE, an angiographic catheter is placed in one femoral artery and advanced under fluoroscopic guidance to sequentially catheterize both uterine arteries (Figs. 9-8 and 9-9). Failure to embolize both uterine arteries allows existing collateral circulation between the two uterine arteries to sustain leiomyoma blood flow and is associated with a significantly lower success rates (Bratby, 2008).
FIGURE 9-9
Fluoroscopic images obtained during uterine artery embolization (UAE). A. Before embolization, the leiomyoma can be identified by its numerous, hypertrophied, tortuous arteries wrapping around its periphery and extending within it. B. After embolization, most of the blood vessels are occluded by particles and appear truncated. Leiomyomas are again easily visualized and appear dark and smudged as the contrast/particle mixture stagnates within the tumor. (Used with permission from Dr. Samuel C. Chao.)
UAE is a management option for women with documented uterine leiomyomas who have significant symptoms despite medical management and who might otherwise be considered a candidate for hysterectomy or myomectomy. Based on current evidence and discussed later, women who have not completed childbearing may be better served by myomectomy (Gupta, 2012; Mara, 2008). Other patient limitations are listed in Table 9-2, and many are associated with altered vascular anatomy. This is a reason why GnRH agonists are not recommended prior to UAE. In addition, pedunculated submucous tumors are not suitable as these tumors can infarct and slough. Pedunculated subserosal tumors were previously excluded for similar reasons. But based on additional data, the Society of Interventional Radiology removed this caveat (Dariushnia, 2014).
| Absolute Pregnancy Active uterine or adnexal infection Suspected reproductive tract malignancy a | |
| Relative | Reason |
| Coagulopathy Renal impairment Severe contrast allergy Desire for future fertility Uterine size >20–24 weeks Prior salpingectomy or SO Prior pelvic radiation Large hydrosalpinx GnRH agonist use | Bleeding complications Renal effects of contrast Allergic reaction Difficult to embolize Altered arterial anatomy Altered arterial anatomy Increased infection risk Narrows vascularity |
Prior to UAE, a woman undergoes a thorough evaluation by her gynecologist. Components include current cervical cancer screening and negative testing for Neisseria gonorrhoeae and Chlamydia trachomatis. Endometrial biopsy is completed in those with endometrial cancer risk factors (Chap. 8). Complete blood count, creatinine level, prothrombin time (PT), and partial thromboplastin time (PTT) are also obtained.
Following UAE, pain management typically requires a 24- to 48-hour hospital admission. After discharge, most patients have pain controlled with NSAIDs and have a rapid return to daily activities. However, as a result of leiomyoma necrosis, approximately 10 percent of patients develop significant postprocedural symptoms and require hospital readmission (Hehenkamp, 2005, 2006). The postembolization syndrome, seen in approximately 25 percent of cases, usually lasts 2 to 7 days and is classically marked by pelvic pain, nausea, low-grade fever, mild white blood cell count elevation, and malaise (Edwards, 2007). Symptom intensity varies, and management includes supportive care and analgesia. Because symptoms stem from myoma necrosis, antibiotics are not typically required but may be administered if infectious endomyometritis is an alternative diagnosis.
Embolization is effective for leiomyoma-related symptoms. Several randomized controlled trials have shown high rates of patient satisfaction and symptom improvement (Edwards, 2007; Hehenkamp, 2008). Compared with hysterectomy, UAE is associated with shorter hospitalization, reduced 24-hour pain scores, and earlier return to daily activities. UAE also compares favorably with myomectomy for symptom relief (Goodwin, 2006; Manyonda, 2012). However, some patients do not achieve adequate improvement. Namely, long-term surveillance reveals that approximately 26 to 37 percent of UAE-treated patients will require a subsequent procedure, which in many cases is hysterectomy (Moss, 2011; Van der Kooij, 2010).
There are several complications associated with UAE. Leiomyoma tissue passage is common and likely is seen only with leiomyomas that initially have contact with the endometrial surface. Necrotic leiomyomas that pass into the vagina usually can be removed in the office. Those that do not pass spontaneously from the uterine cavity or that remain firmly attached to the uterine wall may require dilatation and evacuation (Spies, 2002). Groin hematoma and prolonged vaginal discharge are other frequent complications. Brief amenorrhea and associated transiently elevated FSH levels may last a few menstrual cycles after UAE. Permanent amenorrhea, however, develops occasionally, and more often in older reproductive-aged patients (Hehenkamp, 2007). This complication likely results from concurrent embolization of the ovaries via anastomoses between the uterine and ovarian arteries. Rarely, embolization may incite necrosis in surrounding tissues such as the uterus, adnexa, bladder, and soft tissues.
Pregnancy subsequent to UAE can pose complications. Although the number of evaluable pregnancies is small, consistent problems include increased rates of miscarriage, postpartum hemorrhage, and cesarean delivery (Homer, 2010). Other complications, noted by some but not all studies, are higher rates of preterm delivery, fetal malpresentation, fetal growth restriction, and abnormal placentation (Goldberg, 2004; Pron, 2005; Walker, 2006).
In sum, UAE has typically low major complication rates and high symptom-relief scores. However, these are balanced against the need for ultimate reintervention in a significant number of women.
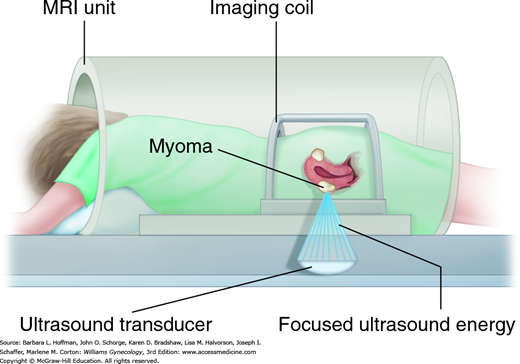
This is also called MR-guided high intensity focused ultrasound (MR-HIFU). With this FDA-approved intervention, ultrasound energy is focused to heat and incite coagulative necrosis in selected myomas (Fig. 9-10). Concurrent MR imaging enables precise targeting and provides real-time tissue temperature feedback to limit surrounding thermal injury. During sessions lasting 2 to 3 hours, a patient lies prone within the MR imaging unit, and the bladder is continuously drained. Manufacturer contraindications are general contraindications to MR imaging, pregnancy, and energy-path obstructions such as abdominal wall scars, bowel, or foreign bodies. Other study exclusions have included future fertility desires, current pelvic infection, other uterine pathology, menopause, myoma size >10 cm, and uterine size >24 weeks. Also, myomas with poor perfusion characteristics, pedunculated serosal or submucous myomas, or those near vital structures increase failure rates or injury risks. Moreover, each session has limits on total myoma volume treated and on time, which may leave some myomas untreated.
Advantageously, MRgFUS is noninvasive, requires only conscious sedation, and is associated with rapid recovery and return to daily activities. In early prospective studies, MRgFUS improves quality-of-life scores and is well tolerated (Hindley, 2004). Complications have included skin burns, adjacent tissue injury, and venous thromboembolism. Notably, similar to UAE, symptoms relief wanes with time, and ≥12 months following MRgFUS, 8 to 24 percent of women seek alternative procedures for their symptoms, including hysterectomy (Machtinger, 2012; Okada, 2009). Compared with UAE in one small nonrandomized study, MRgFUS showed superior symptom improvement at 5 years (Froeling, 2013). Results from an ongoing randomized controlled trial comparing these two are awaited.
For women with persistent symptoms despite conservative therapy, surgery is necessary for many with myomas. Options include hysterectomy, myomectomy, endometrial ablation, and myolysis. Of these, hysterectomy is the definitive and most common surgery. In 2007, nearly 540,000 hysterectomies were performed, and 43 percent of cases had a diagnosis of leiomyoma (Wechter, 2011). Hysterectomy is effective for myoma symptoms, and a study of 418 women undergoing hysterectomy found satisfaction rates greater than 90 percent (Carlson, 1994). There were marked improvements in pelvic pain, urinary symptoms, fatigue, psychological symptoms, and sexual dysfunction. However, benefits are balanced against the risks of major surgery.
Hysterectomy can be performed vaginally, abdominally, or laparoscopically depending on patient and uterine factors. With hysterectomy, removal of the ovaries may or may not be desired. Prophylactic salpingectomy to lower ovarian cancer risk is another consideration. The decision making for each is presented fully in Section 43-12.
This uterus-preserving surgery excises myomas and is considered for women who desire fertility preservation or who decline hysterectomy. This can be performed hysteroscopically, laparoscopically, or via laparotomy. In general, predominantly intracavitary myomas are resected hysteroscopically, whereas subserosal or intramural myomas require laparotomy or laparoscopy for excision.
Hysteroscopic resection is an incisionless, day-surgery procedure that affords quick recovery. Resection is most effective with type 0 and type 1 tumors, and other surgical evaluation aspects are discussed in Chapter 44 (1040). For myoma-related HMB, long-term effectiveness ranges from 85 to 90 percent (Derman, 1991; Emanuel, 1999). Infertility is improved following removal, as previously described on page 205. However, despite its advantages, hysteroscopic resection is possible for only a small subset of myomas.
For women with subserosal or intramural myomas, surgeons must use a laparotomic or laparoscopic approach to enucleate tumors buried in the muscular uterine walls and then reconstruct normal anatomy. As such, surgical complexity and subsequent risks are increased. This type of myomectomy usually improves pain and bleeding. For example, HMB improves in approximately 70 to 80 percent of patients (Buttram, 1981; Olufowobi, 2004).
When selecting a surgical approach for subserosal or intramural myomas, several factors are weighed. Laparoscopic leiomyoma resection yields successful outcomes and recurrence rates comparable to those for laparotomy (Rossetti, 2001). Advantageously, shorter hospital stays and less febrile morbidity, blood loss, adhesion formation, and pain are found with laparoscopic resection compared with laparotomy (Mais, 1996; Takeuchi, 2002). However, limitations to a laparoscopic approach include myoma size, number, and location, and laparoscopic surgical skills, especially multilayer suturing of the leiomyoma beds following enucleation. In general, large intramural and multiple myomas require higher skill levels. Also, seeding the abdominal cavity with myomatous implants is a concern with intraabdominal morcellation, and tissue extraction options are described in Chapter 41. To overcome some of these limits, minilaparotomy techniques may be selected. However, as with larger laparotomy incisions, minilaparotomy is faster than laparoscopic myomectomy but still underperforms laparoscopy regarding patient pain scores, hospital stay, and blood loss (Alessandri, 2006; Palomba, 2007). Also, robot-assisted myomectomy has been described. In general, this offers similar MIS advantages but longer operating times. Moreover, due to poor tactile feedback from robotic instruments, myomas may be missed and lead to higher recurrence rates (Griffin, 2013).
In sum, for those considering myomectomy, hysteroscopic resection is preferred when possible. For remaining cases, abdominal approach selection varies depending on myoma characteristics and surgeon skill. That said, MIS offers decreased postoperative pain and comparable complication rates, although long-term durability data are limited.
In women not seeking pregnancy, risk and benefits aid the decision between myomectomy and hysterectomy. Again, for intracavitary lesions, hysteroscopic resection is preferred. For intramural or subserosal lesions, open myomectomy compared with open hysterectomy yields similar blood loss, intraoperative injuries, and febrile morbidity (Iverson, 1996; Sawin, 2000). However, if laparoscopic approaches are examined, one study showed laparoscopic myomectomy resulted in greater blood loss, higher rates of transfusion and conversion to laparotomy, but lower risks of bladder injury compared with laparoscopic hysterectomy (Odejinmi, 2015).
Moreover, with all myomectomy approaches, symptom relief may be incomplete and prompt additional interventions. Also, myomas can redevelop. Specifically, recurrence rates following myomectomy range from 40 to 50 percent (Acien, 1996; Fedele, 1995). Last, compared with hysterectomy, myomectomy leads to a greater risk for postoperative intraabdominal adhesions (Stricker, 1994).
There are several tissue-destructive modalities that cause endometrial ablation, and they are discussed in Section 44-15. These techniques are effective for women with abnormal uterine bleeding from endometrial dysfunction (AUB-E). But when used as a sole technique for myoma-related bleeding, the failure rate approaches 40 percent (Goldfarb, 1999
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree