DEFINITIONS
Abnormal uterine bleeding (AUB) may display several patterns, and descriptive terms have been updated to standardize nomenclature (Munro, 2011). For example, heavy menstrual bleeding (HMB) (formerly menorrhagia) defines prolonged or heavy cyclic menstruation. Objectively, menses lasting longer than 7 days or exceeding 80 mL of blood loss are determining values. The term intermenstrual bleeding replaces metrorrhagia. Frequently, women may complain of both patterns. The term breakthrough bleeding is a more informal term for intermenstrual bleeding that accompanies hormone administration. In some women, there is diminished flow or shortening of menses, hypomenorrhea. Women normally menstruate every 28 days ± 7 days. Cycles with intervals longer than 35 days describe a state of oligomenorrhea. The term withdrawal bleeding refers to the predictable bleeding that results from an abrupt decline in progesterone levels. Finally, postcoital bleeding is that prompted by vaginal intercourse.
Assessing HMB in a clinical setting has its limitations. First, patient perception of blood loss and objective measurement often fail to correlate (Chimbira, 1980b). As a result, objective methods to assess blood loss have been investigated. Hallberg and associates (1966) describe a technique to extract hemoglobin from sanitary napkins using sodium hydroxide. Hemoglobin is converted to hematin and can be measured spectrophotometrically. Although used in research, this approach in a clinical setting has obvious constraints.
Other tools used to estimate menstrual blood loss include hemoglobin and hematocrit evaluation. Hemoglobin concentrations below 12 g/dL increase the chance of identifying women with HMB. A normal level, however, does not exclude HMB, as many women with clinically significant bleeding have normal values.
Another method involves estimating the number and type of pads or tampons used by a woman during menses. Warner and colleagues (2004) found positive correlations between objective HMB and passing clots more than 1 inch in diameter and changing pads more frequently than every 3 hours. Attempts to standardize this type of evaluation have lead to development of the pictorial blood assessment chart (PBAC) (Fig. 8-1). With a scoring sheet, patients are asked to record daily the number of sanitary products that are lightly, moderately, or completely saturated. Scores are assigned as follows: 1 point for each lightly stained tampon, 5 if moderately saturated, and 10 if completely soaked. Pads are similarly given ascending scores of 1, 5, and 20, respectively. Small clots score 1 point, whereas large clots score 5. Points are then tallied for each day. Totals more than 100 points per menstrual cycle correlate with greater than 80 mL objective blood loss (Higham, 1990).
Menstrual calendars are also frequently used to evaluate abnormal bleeding and its patterns. With this, patients are asked to record dates and blood flow quality throughout the month. These calendars can be used to aid diagnosis and to document improvement during medical treatment.
INCIDENCE
Abnormal uterine bleeding is common, and etiologies include anatomic changes, hormonal dysfunction, infection, system disease, medications, and pregnancy complications (Table 8-1). As a result, AUB may affect females of all ages. Factors that influence incidence most greatly are age and reproductive status.
| Structural Uterine—leiomyoma, adenomyosis, endometrial polyp, endometrial hyperplasia or cancer, uterine sarcoma, AVM Cervix—endocervical polyp, dysplasia, or cancer Vagina—cancer Fallopian tube—cancer Ovary—sex cord-stromal tumors Atrophic vaginal, cervical, or endometrial epithelia Partial outflow obstruction—congenital müllerian defect, Asherman syndrome Intrinsic endometrial |
| Anovulation Immature HPO axis or aging ovarian follicles Hypothyroidism Hyperprolactinemia—pituitary or hypothalamic disorder Androgen excess—PCOS, CAH, Cushing syndrome/disease Premature ovarian failure |
| Pregnancy Implantation, abortion, ectopic pregnancy, GTD |
| Exogenous IUD, foreign body, trauma Medications—sex steroids, anticoagulants, hyperprolactinemia inducing |
| Infection STD, TB, chronic endometritis, postabortal or postpartum infection |
| Systemic abnormalities Coagulopathies, hepatic or chronic renal failure, hyperthyroidism, obesity |
Prior to menarche, bleeding is investigated as an abnormal finding. In children, the vagina, rather than the uterus, is more frequently involved. Vulvovaginitis is often the cause, but dermatologic conditions, neoplasms, and trauma by accident, abuse, or foreign body are others. In addition to vaginal sources, urethra bleeding may originate from urethral prolapse or infection. True uterine bleeding usually results from increased estrogen levels, and precocious puberty, accidental exogenous ingestion, and ovarian neoplasm are considered. These are each discussed further in Chapter 14.
In adolescence, AUB results from anovulation and coagulation defects at disproportionately higher rates compared with older reproductive-aged women (Ahuja, 2010). In contrast, benign or malignant neoplastic growths are less frequent. Pregnancy, sexually transmitted diseases, and sexual abuse are also considered in this population.
Following adolescence, the hypothalamic-pituitary-ovarian (HPO) axis matures, and anovulatory uterine bleeding is encountered less often. With increased sexual activity, rates of bleeding related to pregnancy and sexually transmitted disease rise. The incidences of bleeding from leiomyomas and endometrial polyps also increase with age.
During the perimenopause, as with perimenarchal girls, anovulatory uterine bleeding from HPO axis dysfunction is a more frequent finding (Chap. 21). In contrast, the incidences of bleeding related to pregnancy and sexually transmitted disease decline. With aging, risks of benign and malignant neoplastic growth increase.
After menopause, bleeding typically can be traced to a benign origin such as endometrial or vaginal atrophy or polyps. Even so, malignant neoplasms, especially endometrial carcinoma, are found more often in this age group. Less commonly, estrogen-producing ovarian carcinoma may cause endometrial hyperplasia with uterine bleeding. Similarly, ulcerative vulvar, vaginal, or cervical neoplasms can be sources. And rarely, serosanguinous discharge from a fallopian tube cancer may appear as uterine bleeding. Thus, bleeding in this demographic usually prompts evaluation to exclude these cancers.
PATHOPHYSIOLOGY
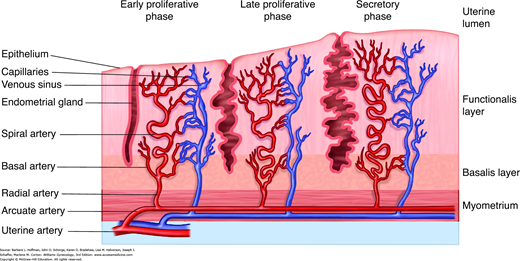
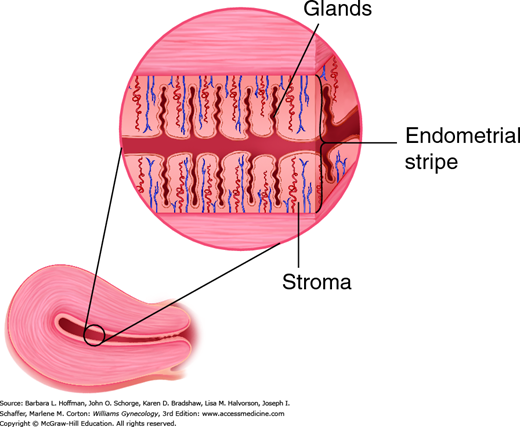
The endometrium consists of two distinct zones, the functionalis layer and the basalis layer (Fig. 8-2). The basalis layer lies in direct contact with the myometrium, is beneath the functionalis, and is less hormonally responsive. The basalis serves as a reservoir for regeneration of the functionalis layer following menses. In contrast, the functionalis layer lines the uterine cavity, undergoes dramatic change throughout the menstrual cycle, and ultimately sloughs during menstruation. Histologically, the functionalis has a surface epithelium and underlying subepithelial capillary plexus. Beneath these are organized stroma, glands, and interspersed leukocytes.
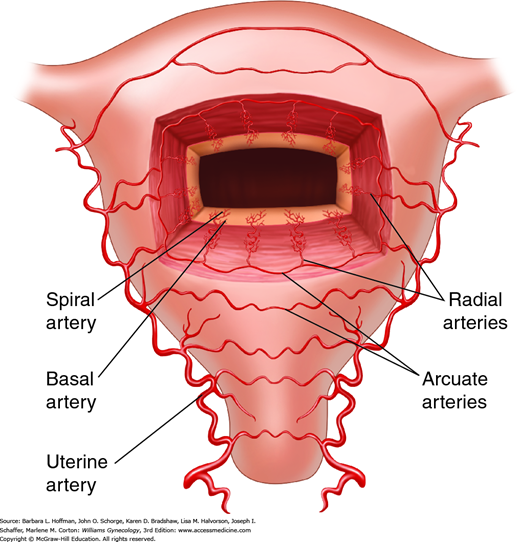
Blood reaches the uterus via the uterine and ovarian arteries. From these, the arcuate arteries arise to supply the myometrium. These in turn branch into the radial arteries, which extend toward the endometrium at right angles from the arcuate arteries (Fig. 8-3). At the endometrium-myometrium junction, the radial arteries bifurcate to create the basal and spiral arteries. The basal arteries serve the basalis layer of the endometrium and are relatively insensitive to hormonal changes. The spiral arteries stretch to supply the functionalis layer and end in a subepithelial capillary plexus.
At the end of each menstrual cycle, progesterone levels drop and lead to release of lytic matrix metalloproteinases (MMP). These enzymes break down the stroma and vascular architecture of the functionalis layer. Subsequent bleeding and sloughing of this layer constitute menstruation (Jabbour, 2006). Initially, platelet aggregation and thrombi control blood loss. In addition, the remaining endometrial arteries, under the influence of mediators, vasoconstrict to limit further bleeding (Ferenczy, 2003).
DIAGNOSIS
With AUB, the diagnostic goal is exclusion of pregnancy or cancer and identification of the underlying pathology to allow optimal treatment. During initial evaluation of abnormal bleeding, a thorough menstrual history is collected. Topics typically include age at menarche, date of last menstrual period, birth control method, and the timing and amount of bleeding. Associated symptoms such as fever, fatigue, bulk symptoms, tissue passage, or pain can also direct evaluation. Importantly, medications are reviewed, as abnormal bleeding can accompany use of nonsteroidal antiinflammatory drugs (NSAIDs), anticoagulants, and agents associated with hyperprolactinemia (Table 12-2). Less robust evidence implicates herbal supplements such as ginseng, garlic, ginkgo, don quai, and St. John wort (Cordier, 2012).
Most gynecologic disorders do not consistently display a specific bleeding pattern, and patients may complain of HMB or intermenstrual bleeding or both. Thus, the pattern for a particular woman may be of limited value in diagnosing the underlying bleeding cause but can be used to assess improvement with treatment.
Of pain symptoms, dysmenorrhea often accompanies abnormal bleeding caused by structural abnormalities, infections, and pregnancy complications. This seems intuitive because of the role of prostaglandins in both HMB and dysmenorrhea. Painful intercourse and noncyclic pain are less frequent in women with AUB and usually suggest a structural or infectious source.
Following a historical inventory, physical examination attempts to identify findings that may suggest an etiology. Moreover, the site of uterine bleeding is confirmed, because vaginal, rectal, or urethral bleeding can present similarly. This is more difficult if there is no active bleeding, and urinalysis or stool guaiac evaluation may be helpful adjuncts.
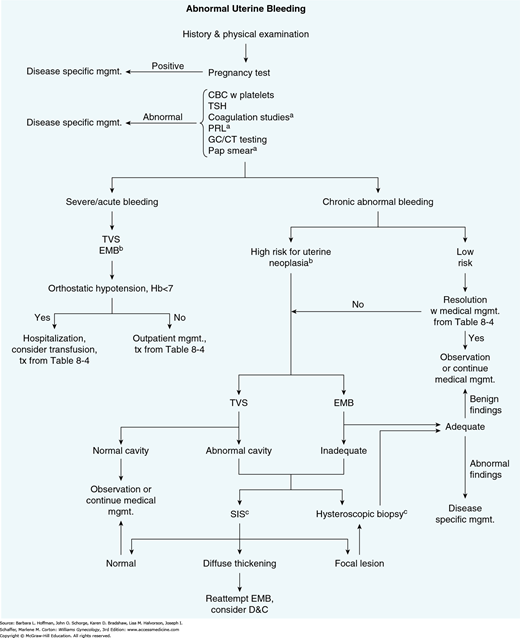
To complement physical findings, blood tests, cervical cytology, sonography (with or without saline infusion), endometrial biopsy, and hysteroscopy are used primarily (Fig. 8-4). In many cases following history and physical examination, these tools may not be required or may be individually selected based on patient variables, suspected diagnosis, available resources, and/or provider training. Test suitability for a given patient is discussed next.
FIGURE 8-4
Diagnostic algorithm to identify endometrial pathology in patients with abnormal uterine bleeding.
aStudy obtained as indicated by patient history.
bPatients with chronic anovulation, obesity, ≥45 years of age, tamoxifen use, or other risks for endometrial cancer.
cBoth comparable in sensitivity and specificity. Either or both may be selected depending on patient characteristics and physician preference.
CBC = complete blood count; D&C = dilatation and curettage; GC/CT = Neisseria gonorrhoeae and Chlamydia trachomatis; EMB = endometrial biopsy; Hb = hemoglobin level; mgmt. = management; PRL = prolactin level; SIS = saline infusion sonography; TSH = thyroid-stimulating hormone level; TVS = transvaginal sonography; tx = treatment.
Miscarriage, ectopic pregnancies, and hydatidiform moles may cause life-threatening hemorrhage. Pregnancy complications are quickly excluded with determination of urine or serum β-human chorionic gonadotropin (hCG) levels. This is typically obtained on all reproductive-aged women with a uterus.
Additionally, in women with AUB, a complete blood count (CBC) will identify anemia and the degree of blood loss. With chronic loss, erythrocyte indices will reflect a microcytic, hypochromic anemia and show decreases in mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). Moreover, in women with classic iron-deficiency anemia from chronic blood loss, an elevated platelet count may be seen. In those for whom the cause of anemia is unclear, those with profound anemia, or in those who fail to improve with oral iron therapy, iron studies are often indicated. Specifically, iron-deficiency anemia produces low serum ferritin and low serum iron levels but an elevated total iron-binding capacity. As discussed further on page 192, screening for disordered hemostasis is considered in women and adolescents with HMB and no other obvious cause.
Cervicitis often causes intermenstrual or postcoital spotting. Accordingly, microscopic examination of a saline preparation of cervical secretions or “wet prep” can be informative. With mucopurulent discharge, sheets of neutrophils (>30 per high-power field) and red blood cells are typical. With trichomoniasis, motile trichomonads are also found. Cervicitis-related bleeding is frequently reproduced during sampling from an inflamed cervix with a friable epithelium.
The association between mucopurulent cervicitis and cervical infection with Chlamydia trachomatis and Neisseria gonorrhoeae is well established (Brunham, 1984). Thus, the Centers for Disease Control and Prevention (CDC) (2015) recommend testing for both when mucopurulent cervicitis is found. Moreover, even without frank discharge, these organisms can cause endometritis (Eckert, 2004). Thus, bleeding or spotting alone may merit screening for these two in at-risk populations listed in Table 1-1. Last, herpes simplex virus (HSV) may manifest as diffuse erosive and hemorrhagic ectocervical lesions (Paavonen, 1988). In patients with such findings who lack a known HSV history, directed culture or serologic testing can be obtained.
Both cervical and endometrial cancers can bleed, and evidence for these tumors may be detected during diagnostic Pap smear evaluation. The most frequent abnormal cytologic results involve squamous cell pathology and may reflect cervicitis, intraepithelial neoplasia, or cancer. Less commonly, atypical glandular or endometrial cells are found. Thus, depending on the cytologic results, colposcopy, endocervical curettage, and/or endometrial biopsy may be indicated as discussed in Chapter 29. Moreover, at times, a visibly suspicious vaginal or cervical lesion may bleed and warrant direct biopsy with Tischler forceps.
In women with AUB, sampling and histologic evaluation of the endometrium may identify infection or neoplastic lesions such as endometrial hyperplasia or cancer.
AUB is noted in 80 to 90 percent of women with endometrial cancer. The incidence and risk of this cancer increases with age, and most affected women are postmenopausal (National Cancer Institute, 2014). Thus, in postmenopausal women, the need to exclude cancer intensifies, and endometrial biopsy is typically indicated. Of premenopausal women with endometrial neoplasia, most are obese or have chronic anovulation or both. Thus, women with AUB in these two groups also warrant exclusion of endometrial cancer. Specifically, the American College of Obstetricians and Gynecologists (2012) recommends endometrial assessment in any woman older than 45 years with AUB, and in those younger than 45 years with a history of unopposed estrogen exposure such as seen in obesity or polycystic ovarian syndrome (PCOS), failed medical management, and persistent AUB.
For years, dilatation and curettage (D & C) was used for endometrial sampling. However, because of associated surgical risks, expense, postoperative pain, and need for operative anesthesia, other suitable substitutes were evaluated. In addition, investigators have demonstrated incomplete sampling and missed pathology even with D & C (Grimes, 1982; Stock, 1975).
Initial office techniques used metal curettes. Endometrial samples that are removed with these curettes show significant positive correlation with histologic results obtained from hysterectomy specimens (Stovall, 1989). Thus, they are deemed adequate sampling methods. However, disadvantages include patient discomfort and rare procedural complications such as uterine perforation and infection.
To minimize these, flexible plastic samplers have been evaluated for endometrial biopsy. Advantageously, samples from these catheters have comparable histologic findings with tissues obtained by D & C, hysterectomy, or stiff metal curette (Stovall, 1991). Moreover, they afford greater patient comfort.
Prior to performing endometrial biopsy, pregnancy is excluded in women of reproductive age. With Pipelle insertion, patients frequently note cramping, which can be allayed by a preprocedural NSAID. For some, slow transcervical intrauterine instillation of 5 mL of 2-percent lidocaine using an 18-gauge angiocatheter can lower perceived pain scores (Kosus, 2014).
After patient education and consent, a speculum is placed, and the cervix is cleansed with an antibacterial solution, such as povidone-iodine solution. In many cases, a single-tooth tenaculum is needed to stabilize the cervix and permit passage of the Pipelle through the cervical os and into the endometrial cavity. When placing the tenaculum on the anterior cervical lip, closing the clamp slowly can decrease discomfort. Some evidence also supports topical anesthetic use. Examples are 10-percent lidocaine spray immediately prior or 5-percent lidocaine/prilocaine cream (EMLA cream) 10 minutes before tenaculum placement (Davies, 1997; Zullo, 1999).
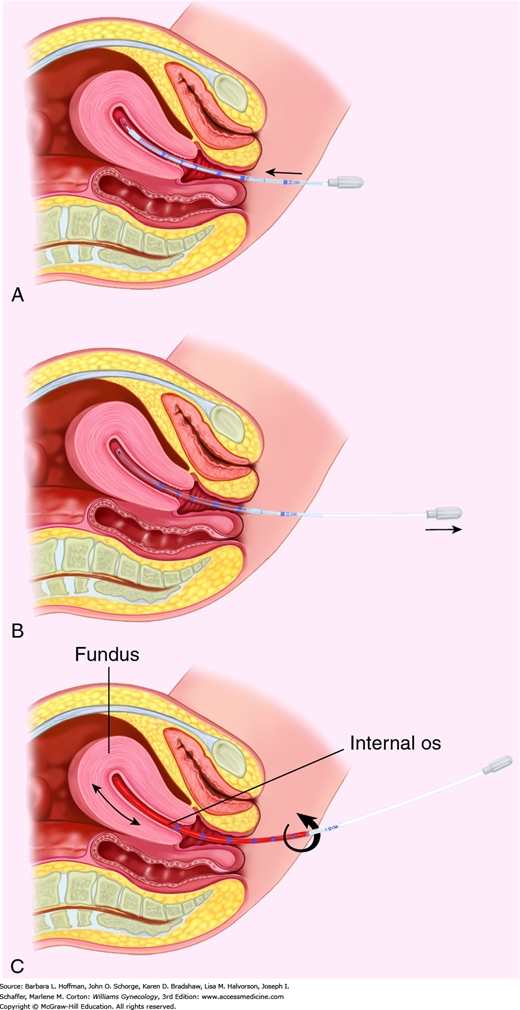
With sampling, the Pipelle is directed toward the fundus until resistance is met (Fig. 8-5). Markings on the Pipelle allow measurement of uterine depth, and this value is recorded in the procedure note. The inner Pipelle stilette is then retracted to create suction within the cylinder. Several times, the Pipelle is withdrawn to the level of the internal cervical os and advanced back to the fundus. The device is gently turned during its advance and retraction to allow thorough sampling of all endometrial surfaces. Uncommonly, a vagal response can follow Pipelle insertion. In this instance, the procedure is terminated, and patient support is provided.
FIGURE 8-5
Steps of endometrial biopsy. A. During biopsy, the Pipelle is inserted through the cervical os and directed to the uterine fundus. B. The stilette of the Pipelle is retracted to create suction within the cylinder. C. Several times, the Pipelle is withdrawn to the level of the internal cervical os and advanced back to the fundus. The Pipelle is gently turned during its advance and retraction to allow thorough sampling of all endometrial surfaces.
Despite its advantages, there are limitations to endometrial sampling with the Pipelle device. First, a tissue sample that is inadequate for histologic evaluation, such as from endometrial atrophy, or an inability to pass the catheter into the endometrial cavity is encountered in up to 28 percent of biopsy attempts (Smith-Bindman, 1998). Cervical stenosis and large submucous leiomyomas are classic obstructions. An incomplete evaluation often necessitates further investigation with D & C, transvaginal sonography with or without saline infusion, or diagnostic hysteroscopy (Emanuel, 1995). Second, endometrial biopsy has a cancer-detection failure rate of 0.9 percent. Thus, a positive histologic result is accurate to diagnose cancer, but a negative result does not definitively exclude it. Therefore, if an endometrial biopsy with normal tissue is obtained, but abnormal bleeding continues despite conservative treatment or if the suspicion of endometrial cancer is high, then further diagnostic efforts are warranted. Finally, endometrial sampling is associated with a greater percentage of false-negative results with focal pathology such as endometrial polyps. In a study of 639 women evaluated by diagnostic office hysteroscopy and endometrial biopsy, Svirsky and colleagues (2008) found that the sensitivity of endometrial sampling for detection of endometrial polyps and submucosal fibroids was only 8.4 and 1.4 percent, respectively. Because of these limitations with endometrial sampling, investigators have evaluated sonography, hysteroscopy, or both to replace or complement endometrial sampling.
With improved resolution, this technology is chosen by many instead of endometrial biopsy as a first-line tool to assess AUB. Advantageously, it allows assessment of both the myometrium and the endometrium. Thus, if AUB stems from myometrial pathology such as leiomyomas, sonography offers anatomic information that is not afforded by hysteroscopy or endometrial biopsy. In addition, transvaginal sonography (TVS) compared with these other two typically offers greater patient comfort and suitable detection of postmenopausal endometrial hyperplasia and cancer (Karlsson, 1995; Van den Bosch, 2008). That said, no tool, including TVS, is recommended for routine endometrial cancer screening in asymptomatic women (Breijer, 2012).
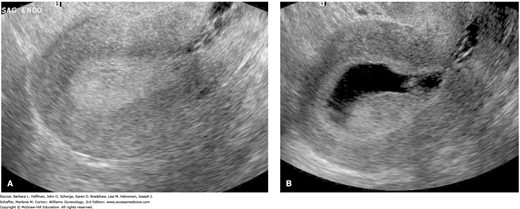
When the endometrium is imaged in a sagittal view, opposed endometrial surfaces appear as a hyperechoic endometrial stripe down the center of the uterine body (Fig. 8-6 and Fig. 2-16). In postmenopausal women, this endometrial thickness has been correlated with endometrial cancer risk. Although endometrial thickness varies among patients, ranges have been established. Granberg and coworkers (1991) found thickness measurements of 3.4 ± 1.2 mm in postmenopausal women with atrophic endometrium, 9.7 ± 2.5 mm in those with endometrial hyperplasia, and 18.2 ± 6.2 mm in those with endometrial cancer. Subsequent investigations have similarly focused on endometrial thickness as it relates to hyperplasia and cancer risks in postmenopausal women. For endometrial cancer, negative predictive values >99 percent have been reported using a measurement of ≤4 mm (Karlsson, 1995; Tsuda, 1997). Use of hormone replacement therapy (HRT) does not appear to affect the threshold used (Smith-Bindman, 1998). In those using cyclic HRT, completing TVS on day 4 or 5 following cycle bleeding is recommended (Goldstein, 2001). For postmenopausal women, an endometrial thickness >4 mm typically requires additional evaluation with saline infusion sonography (SIS), hysteroscopy, or endometrial biopsy.
Consensus, however, is lacking regarding the asymptomatic postmenopausal women in whom a thick endometrium is found. The American College of Obstetricians and Gynecologists (2013d) notes that this finding need not routinely prompt evaluation but that further testing is directed by coexistent patient risks. Focal lesions are common in this subgroup and thus may favor SIS or hysteroscopy if additional evaluation is indicated (Schmidt, 2009).
Researchers have also attempted to create endometrial thickness guidelines for premenopausal women. Merz and colleagues (1996) found that the normal endometrial thickness in premenopausal women did not exceed 4 mm on day 4 of the menstrual cycle, nor did it measure more than 8 mm by day 8. However, endometrial thicknesses can vary considerably among premenopausal women, and evidence-based abnormal thresholds that have been proposed range from ≥4 mm to >16 mm (Breitkopf, 2004; Goldstein, 1997; Shi, 2008). Thus, a consensus for endometrial thickness guidelines has not been established for this group. At our institution, no additional evaluation is recommended for a normal-appearing endometrium measuring ≤10 mm in a premenopausal female experiencing AUB if she has no other risk factors to prompt further testing. Risk factors for endometrial carcinoma include extended AUB, chronic anovulation, diabetes mellitus, obesity, and tamoxifen use.
Qualities other than endometrial thickness are also considered because textural changes may indicate pathology. Punctate cystic areas within the endometrium may indicate a polyp. Conversely, hypoechoic masses that distort the endometrium and originate from the inner layer of myometrium most likely are submucous leiomyomas. Although there are no specific sonographic findings that are characteristic of endometrial cancer, some findings have been linked with greater frequency (Fig. 33-3). For example, intermingled hypo- and hyperechoic areas within the endometrium may indicate malignancy. Endometrial cavity fluid collections and an irregular endometrial-myometrial junction have also been implicated. Thus, with these findings, even with a normal endometrial stripe width in postmenopausal patients, endometrial biopsy or hysteroscopy with biopsy is considered to exclude malignancy (Sheikh, 2000).
Although these criteria can safely reduce endometrial biopsy rates for many patients, others consider false-negative rates as too high with this strategy for evaluation of postmenopausal women (Timmermans, 2010). Some advocate hysteroscopy with direct biopsy or D & C to evaluate postmenopausal bleeding (Litta, 2005; Tabor, 2002). In other patient populations, the 4-mm guideline may also be inappropriate. For example, van Doorn and coworkers (2004) reported decreased diagnostic accuracy in diabetic or obese women, and they recommend consideration of endometrial sampling.
A major limitation of TVS is its higher false-negative rate for diagnosing focal intrauterine pathology. This results in part from the physical inability of TVS to clearly assess the endometrium when there is concurrent uterine pathology such as leiomyomas or polyps. In these cases, SIS or hysteroscopy may be informative.
This simple, minimally invasive, and effective sonographic procedure can be used to evaluate the myometrium, endometrium, and endometrial cavity (Chap. 2). Also known as sonohysterography or hysterosonography, SIS allows identification of common masses associated with AUB such as endometrial polyps, submucous leiomyomas, and intracavitary blood clots. These masses frequently create nondescript distortion or thickening of the endometrial lining when imaged with TVS. Thus, compared with TVS, SIS typically permits superior detection of intracavitary masses and differentiation of lesions as being endometrial, submucous, or intramural (Fig. 8-7). In addition, Moschos and colleagues (2009) describe a method of endometrial biopsy during SIS using a sonography-guided Pipelle. Although not yet widely used, this technique enables directed histologic sampling of endometrial pathology and has proved superior to blind endometrial biopsy in providing a diagnosis for AUB in peri- and postmenopausal women.
SIS has also been compared with hysteroscopy to detect uterine cavitary focal lesions. De Kroon and coworkers (2003) performed a metaanalysis of 24 studies and reported SIS to equal the diagnostic accuracy of hysteroscopy. Importantly, neither hysteroscopy nor SIS can reliably discriminate between benign and malignant focal lesions. Thus, because of the malignant potential of many focal lesions, biopsy or excision of most structural lesions, when identified, is recommended for those with risk factors. For this, operative hysteroscopy is typically used.
SIS has other limitations. First, it is cycle dependent and best performed in the proliferative phase to minimize false-negative and false-positive results. For example, focal lesions may be concealed in a thick, secretory endometrium. Also, the amount of endometrial tissue that can develop during the normal secretory phase can be mistaken for a small polyp or focal hyperplasia. Second, SIS usually has more patient discomfort than TVS, and approximately 5 percent of examinations cannot be completed because of cervical stenosis or patient discomfort. As expected, stenosis is more prevalent in postmenopausal women, and the incompletion rate mirrors that of diagnostic hysteroscopy.
Although accurate for identifying focal lesions, SIS may not add to the value of TVS for evaluation of diffuse lesions such as hyperplasia and cancer. Therefore, in postmenopausal women with AUB, and in whom the exclusion of cancer is more relevant than evaluating focal intracavitary lesions, SIS alone as an initial diagnostic tool may not have advantages over TVS.
In selected instances, other imaging modalities can provide information beyond that obtained from TVS and SIS. Of these, color and pulsed Doppler, by demonstrating vascularity, may better highlight suspected focal abnormalities (Bennett, 2011). Similarly, 3-dimensional (3-D) sonography and 3-D SIS are most helpful to clarify focal lesions (Benacerraf, 2008; Makris, 2007). With power Doppler, finding multiple irregularly branching vessels may suggest malignancy (Opolskiene, 2007). 3-D power Doppler has been employed to differentiate malignant and benign endometrium, but its value is still undefined (Alcazar, 2009; Opolskiene, 2010). Last, although preferred to computed tomography (CT), magnetic resonance (MR) imaging is rarely needed for AUB evaluation but can display endometrium in cases in which sonographic views are obstructed.
With this procedure, an endoscope, usually 3 to 5 mm in diameter, is inserted into the endometrial cavity as explained in detail in Section 44-12. The uterine cavity is then distended with saline or another medium for visualization. In addition to inspection, biopsy of the endometrium allows histologic diagnosis of abnormal areas and has been shown to be a safe and accurate means of identifying pathology. Also, focal lesions can be diagnosed and completely removed in the same session. In fact, many studies examining the accuracy of TVS or SIS for intracavitary pathology evaluation use hysteroscopy as the “gold standard” for comparison.
The main advantage of hysteroscopy is detection of intracavitary lesions such as leiomyomas and polyps that might be missed using TVS or endometrial sampling (Tahir, 1999). It also permits simultaneous removal of many lesions once identified. Thus, some advocate hysteroscopy as the primary tool for AUB diagnosis. However, the invasiveness and cost of hysteroscopy is balanced against improved diagnostic efficiency. Moreover, although accurate for identifying endometrial cancer, hysteroscopy is less accurate for endometrial hyperplasia. Accordingly, some recommend endometrial biopsy or endometrial curettage in conjunction with hysteroscopy (Ben-Yehuda, 1998; Clark, 2002).
Hysteroscopy has other limitations. Cervical stenosis will sometimes block successful introduction of the endoscope, and heavy bleeding may obscure and hinder an adequate examination. Hysteroscopy is more expensive and technically challenging than TVS or SIS. Costs can be lower with office hysteroscopy rather than that in an operative suite. However, patient discomfort may limit complete examination during some office procedures. Use of a smaller diameter or flexible hysteroscope may diminish this procedural pain (Cicinelli, 2003). In either arena, associated infection and uterine perforation have been reported, but their incidences are low (Bradley, 2002; Vercellini, 1997). Last, peritoneal seeding with malignant cells may take place during hysteroscopy via retrograde flow through the fallopian tubes in some women subsequently diagnosed with endometrial cancer (Bradley, 2004; Zerbe, 2000). Despite the risk of peritoneal contamination by cancer cells with hysteroscopy, patient prognosis overall does not appear to be worsened (Cicinelli, 2010; Polyzos, 2010). The American College of Obstetricians and Gynecologists (2011) considers hysteroscopy acceptable for AUB evaluation in those without advanced-stage uterine or cervical cancer.
There is no one clear sequence to the use of endometrial biopsy, TVS, SIS, and hysteroscopy when evaluating AUB. None of these will distinguish all anatomic lesions with high sensitivity and specificity. That said, TVS for several reasons is a logical first step. It is well tolerated, is cost-effective, and requires relatively minimal technical skill. Additionally, it can reliably determine stripe thickness and whether a lesion is myometrial or endometrial. Once potential anatomic lesions have been identified, subsequent evaluation requires individualization. If endometrial hyperplasia or cancer is suspected, then endometrial biopsy may offer advantages. Alternatively, possible focal lesions may be best investigated with either hysteroscopy or SIS. Ultimately, the diagnostic goal is to identify and treat pathology and specifically to exclude endometrial carcinoma. Thus, selection of appropriate tests depends on their accuracy in characterizing the most likely anatomic lesions.
ETIOLOGY CLASSIFICATION
Causes of AUB are numerous and summarized by the acronym PALM-COEIN (Munro, 2011). In this International Federation of Gynecology and Obstetrics (FIGO) classification system, letters reflect Polyp, Adenomyosis, Leiomyoma, Malignancy and hyperplasia, Coagulopathy, Ovulatory disorders, Endometrial dysfunction, Iatrogenic, and those Not yet classified. Pregnancy is not considered in this system, but AUB is encountered in 15 to 20 percent of pregnancies (Everett, 1997; Weiss, 2004). Although frequently no reason is found, bleeding may reflect early abortion, ectopic pregnancy, cervical infection, hydatidiform mole, cervical eversion, or polyp. Detailed discussions of bleeding associated with these are found in Chapters 6, 7, and 37.
STRUCTURAL ABNORMALITIES
Structural abnormalities are frequent causes of abnormal bleeding, and of these, leiomyomas are by far the most common. Myomas, adenomyosis, and isthmoceles are presented in Chapter 9. Uterine and cervical neoplasms are discussed in Chapters 30, 33, and 34. As described in Chapter 18, partially obstructive congenital reproductive tract anomalies may at times cause chronic intermenstrual bleeding. Endometrial and endocervical structural abnormalities such as polyps and arteriovenous malformations are described here.
These soft, fleshy intrauterine growths are composed of endometrial glands, fibrous stroma, and surface epithelium. Polyps are common, and their prevalence in the general population approximates 8 percent (Dreisler, 2009a). Moreover, in those with AUB, rates range from 10 to 30 percent (Bakour, 2000; Goldstein, 1997). Intact polyps may be single or multiple, measure from a few millimeters to several centimeters, and be sessile or pedunculated (Fig. 8-8). Estrogen and progesterone have been implicated in their growth, and higher receptor levels are noted within polyps compared with adjacent normal endometrium (Leão, 2013). These hormones elongate endometrial glands, stromal tissue, and spiral arteries, leading to the characteristic polypoid appearance. Others suggest local immune disturbances contribute to polyp formation and to associated AUB and infertility (Al-Jefout, 2009; Kitaya, 2012).
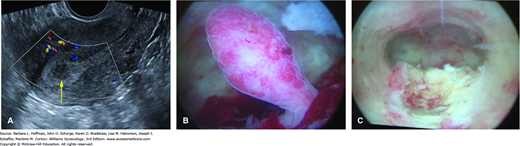
FIGURE 8-8
Endometrial polyp. A. Sagittal image of a uterus using transvaginal sonography with color Doppler. The yellow arrow points to the polyp, which is multicystic and hypoechoic compared with the surrounding endometrium. B. Hysteroscopic image of same polyp. C. Endometrial cavity following polyp resection. (Used with permission from Drs. David Rogers and Hilary Myears.)
Patient risk factors include increasing age, obesity, and tamoxifen use (Reslova, 1999). Although some studies suggest an association between hormone replacement therapy and polyp formation, others do not (Bakour, 2002; Dreisler, 2009a; Maia, 2004; Oguz, 2005). Use of oral contraceptive pills appear to be protective (Dreisler, 2009b). Similarly, for women taking tamoxifen, the levonorgestrel-releasing intrauterine system (LNG-IUS) was investigated and shown to lower endometrial polyp formation rates, but its ultimate effects on breast cancer recurrence are incompletely defined and a concern (Wong, 2013).
Women with polyps may have no complaints, and polyps are identified during imaging for other indications (Goldstein, 2002). More frequently, heavy cyclic or intermenstrual bleeding is an associated symptom. Bleeding may stem from surface epithelium breaks associated with chronic inflammation and vascular fragility or from apical ischemic tissue necrosis (Ferenczy, 2003). Infertility has been linked indirectly with endometrial polyps. For example, small studies have shown increased pregnancy rates and fewer early pregnancy losses in infertile women following hysteroscopic excision (Pérez-Medina, 2005; Preutthipan, 2005). The exact mechanisms related to infertility are unknown, although local inflammation may play a role as noted earlier. Also, polyps found near the tubal ostia may hinder ostium function and block sperm migration (Shokeir, 2004; Yanaihara, 2008). Accordingly, many advocate polyp removal in infertile women.
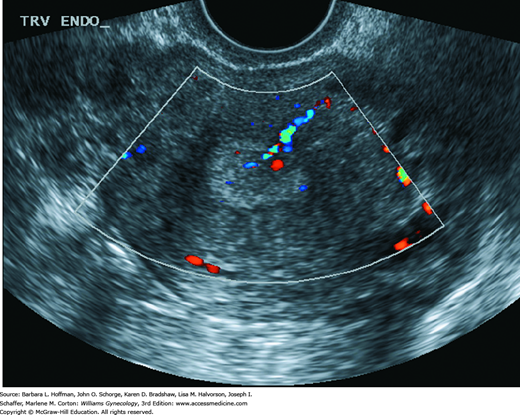
The main diagnostic tools for endometrial polyp evaluation include TVS with applied color Doppler, SIS, and hysteroscopy. Endometrial biopsy may identify polyps but has less diagnostic sensitivity. In premenopausal women, TVS is best performed prior to day 10 of the cycle to lower the risk of false-positive and false-negative findings. With TVS, an endometrial polyp may appear as a nonspecific endometrial thickening or as a round or elongated hyperechoic focal mass within the endometrial cavity. Sonolucent cystic spaces corresponding to dilated endometrial glands are seen within some polyps (Nalaboff, 2001). TVS can be augmented with color or power Doppler. Endometrial polyps typically have only one arterial feeding vessel, whereas submucous leiomyomas generally received blood flow from several vessels arising from the inner myometrium (Fig. 8-9) (Cil, 2010; Fleischer, 2003).
SIS and hysteroscopy are both accurate in identifying endometrial polyps (Soares, 2000). With SIS, polyps appear as echogenic, smooth, intracavitary masses with either broad bases or thin stalks and are outlined by fluid (see Fig. 8-9B). Hysteroscopy identifies nearly all cases of endometrial polyps (see Fig. 8-8). Another advantage of hysteroscopy is the ability to identify and remove the polyp concurrently.
The Pap smear is an ineffective tool to identify polyps. However, it occasionally incidentally leads to their identification. For example, 5 percent of postmenopausal women with benign endometrial cells identified on Pap smear are found to have endometrial polyps (Karim, 2002). Moreover, in postmenopausal women with atypical glandular cells of undetermined significance (AGUS), endometrial polyps were the most frequent underlying pathology found (Obenson, 2000).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree