INTRODUCTION
Each year, more than 30 million surgical procedures are performed. During these, nearly 1 million patients suffer a postoperative complication (Mangano, 2004). As surgeons, gynecologists assume responsibility for assessing a patient’s clinical status to identify modifiable risk factors and prevent perioperative morbidity. However, clinicians should also be prepared to diagnose and manage such complications if they arise.
PREOPERATIVE PATIENT EVALUATION
A properly performed preoperative evaluation serves three important functions. It uncovers comorbidities that require further evaluation and improvement to avert perioperative complications. Second, evaluation allows effective use of operating room resources (Roizen, 2000). Finally, the surgeon is able to anticipate potential problems and devise an appropriate perioperative plan (Johnson, 2008).
In many cases, a thorough history and physical examination averts the need for medical consultation. However, if a poorly controlled or previously undiagnosed disease is discovered, consultation with an internist can be beneficial. Preoperative internal medicine consultation does not provide “medical clearance” but rather offers a risk assessment of a woman’s current medical state. For consultation, a summary of the surgical illness is provided, and clear questions are posed to the consultant (Fleisher, 2009; Goldman, 1983). In addition, a complete history and physical examination and prior medical records that report already completed diagnostic testing should be available to the consulting physician. This can prevent unnecessary surgical delays and cost from redundant testing.
PULMONARY EVALUATION
Common postoperative pulmonary morbidities include atelectasis, pneumonia, and exacerbation of chronic lung diseases. Incidences of such complications following surgery are estimated to be between 20 and 70 percent (Bernstein, 2008; Brooks-Brunn, 1997; Qaseem, 2006).
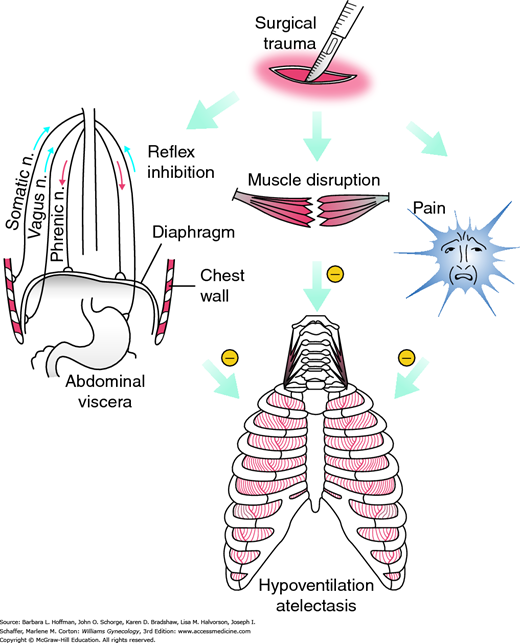
Risks for pulmonary complications fall into one of two major categories: procedure-related and patient-related. Of procedure-related risks, upper abdominal incisions as they approach the diaphragm can worsen pulmonary function through three mechanisms, shown in Figure 39-1. Resulting poor diaphragmatic movement can produce persistent declines in vital capacity and in functional residual capacity. These predispose to atelectasis (Warner, 2000). Surgery duration is another procedure-associated factor. Operations in which patients receive general anesthesia for longer than 3 hours are associated with nearly double the rate of postoperative pulmonary complications. Finally, emergency surgery remains a significant independent risk. Although these factors are largely unmodifiable, an appreciation of their sequelae should prompt increased postoperative vigilance.
FIGURE 39-1
Surgical factors producing respiratory muscle dysfunction. These factors can reduce lung volumes and produce hypoventilation and atelectasis. (Reproduced with permission from Warner DO: Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology 2000 May;92(5):1467–1472.)
Of patient-associated factors, age plays a role. Individuals older than 60 years are at increased risk for developing postoperative pulmonary complications. After patients are stratified for comorbidities, those between 60 and 69 years have a twofold increased risk. In those older than 70 years, risk rises threefold (Qaseem, 2006). Importantly, for at-risk patients, baseline cognition should be documented and postoperative sensorium monitored, as changes may be an early indicator of pulmonary function compromise.
Smoking, specifically a greater than 20-pack-year smoking history, confers a high incidence of postoperative pulmonary complications. Fortunately, this risk can be reduced with smoking abstinence before surgery. Preoperative cessation for at least 6 to 8 weeks offers significant improvement in lung function and reversal of smoking-related immune impairment (Akrawi, 1997; Buist, 1976). Other short-term benefits may be related to reduced nicotine and carboxyhemoglobin levels, improved mucociliary function, decreased upper airway hypersensitivity, and improved wound healing (Møller, 2002; Nakagawa, 2001). Patients with a 6-month or longer history of smoking cessation have complication risks similar to those who have never smoked. Moreover, patients often see surgery as an opportunity for positive change (Shi, 2010). Brief interventions offered in close proximity to surgery may have small benefit on long-term smoking behavior (Thomsen, 2014). Education alone may prompt successful behavior modification. For others, agents to assist with smoking cessation can be found in Table 1-4,.
In chronic obstructive pulmonary disease (COPD), inflammatory mediators may account for the intra- and extrapulmonary complications observed in affected patients (Maddali, 2008). Simple COPD optimization may not reduce the incidence of postoperative pulmonary complications. However, postoperative physiotherapy and incentive spirometry with inspiratory muscle training reduce complication rates (Agostini, 2010).
Obesity can decreases chest wall compliance and functional residual capacity and predispose patients with a body mass index (BMI) ≥30 kg/m2 to intra- and postoperative atelectasis (Agostini, 2010; Zerah, 1993). Eichenberger and colleagues (2002) observed that pulmonary changes in these patients may persist for more than 24 hours and require aggressive postoperative lung expansion. Moreover, in obese patients undergoing laparoscopy, these pulmonary parameters are further compromised by increased intraabdominal pressures from pneumoperitoneum, as described in Chapter 41.
Asthma, if well-controlled, is not a risk factor for postoperative pulmonary complications. Warner and coworkers (1996) reported that rates of bronchospasm were less than 2 percent in asthmatic patients.
Elements in a pulmonary review of systems that may serve as harbingers of underlying disease include poor exercise tolerance, chronic cough, and otherwise unexplained dyspnea (Smetana, 1999). Examination findings of decreased breath sounds, dullness to percussion, rales, wheezes, rhonchi, and a prolonged expiratory phase can carry a nearly sixfold increase in pulmonary complications (Straus, 2000).
In general, pulmonary function tests (PFTs) offer little information during preoperative pulmonary assessment of patients undergoing nonthoracic procedures. Outside of diagnosing COPD, PFTs are not superior to a thorough history and physical examination (Johnson, 2008; Qaseem, 2006). However, if the etiology of pulmonary symptoms remains unclear after clinical examination, then PFTs may provide information to alter perioperative management.
Chest radiography is not routinely obtained preoperatively. Compared with a clinical history and physical examination, preoperative chest radiographs rarely provide evidence to modify therapy (Archer, 1993). The American College of Radiology (2011) recommends that patients with new or exacerbated cardiopulmonary symptoms or those older than 70 years with chronic cardiopulmonary disease as suitable candidates for imaging. Although not exhaustive, conditions for which radiography may be reasonable include acute or chronic cardiovascular or pulmonary disease, cancer, American Society of Anesthesiologist (ASA) status >3, heavy smoking, immunosuppression, recent chest radiation therapy, and recent emigration from areas with endemic pulmonary disease.
The National Veterans Administration Surgical Quality Improvement Program reported that serum albumin levels less than 3.5 g/dL were significantly associated with increased perioperative pulmonary morbidity and mortality rates (Arozullah, 2000; Lee, 2009). For each 1 mg/dL decline in serum albumin concentration, the odds of mortality are increased by 137 percent and morbidity by 89 percent (Vincent, 2004). Serum albumin’s association with morbidity and mortality may be due to confounding comorbidity, and thus it is a marker of malnutrition and disease (Goldwasser, 1997). Although a serum albumin level is not routinely recommended for gynecologic procedures, the information may be predictive in the elderly or in those with multiple comorbidities. Moreover, serum blood urea nitrogen (BUN) levels greater than 21 mg/dL similarly correlate with increased morbidity and mortality rates, but not to the same degree as serum albumin levels.
The ASA Classification was created to help predict perioperative mortality rates. It also serves to assess risks for cardiovascular and pulmonary complications (Wolters, 1996). Table 39-1 summarizes the ASA categories and associated rates of postoperative pulmonary complications (Qaseem, 2006).
| ASA Class | Class Definition | Rates of PPCs by Class (%) |
| I | Normally healthy patient | 1.2 |
| II | With mild systemic disease | 5.4 |
| III | With systemic disease that is not incapacitating | 11.4 |
| IV | With an incapacitating systemic disease that is a constant threat to life | 10.9 |
| V | Moribund patient who is not expected to survive for 24 hours with or without operation | NA |
Techniques aimed at reducing anticipated postoperative decreases in lung volume can be simple and include deep breathing exercises, incentive spirometry, and early ambulation. In conscious and cooperative patients, deep breathing effectively improves lung compliance and gas distribution (Chumillas, 1998; Ferris, 1960; Thomas, 1994). With these exercises, a woman is asked to take five sequential deep breaths every hour while awake and hold each for 5 seconds. An incentive spirometer can be added to provide direct visual feedback of her efforts. Last, early ambulation can enhance lung expansion and provide some protection against venous thromboembolism. Meyers and associates (1975) demonstrated an increase in functional residual lung capacity of up to 20 percent by simply maintaining an upright posture. Alternatively, formal respiratory physiotherapy may include chest physical therapy in the form of percussion, clapping, or vibration; intermittent positive-pressure breathing (IPPB); and continuous positive airway pressure (CPAP).
The simple and the more formal prophylactic methods are all effective in preventing postoperative pulmonary morbidity, and no method is superior to another. Thomas and colleagues (1994) performed a metaanalysis to compare incentive spirometry (IS), IPPB, and deep-breathing exercises (DBE). In comparison with no therapy, IS and DBE are superior in preventing postoperative pulmonary complications, and greater than 50-percent reductions were observed. In addition, no significant differences were noted comparing IS to DBE, IS to IPPB, and DBE to IPPB (Thomas, 1994). However, chest physical therapy, IPPB, and CPAP are more expensive and labor intensive (Pasquina, 2006). Accordingly, these methods are typically reserved for patients who are unable to perform simpler effort-dependent therapies.
Postoperatively, nasogastric tubes (NGTs) are often placed for gastric decompression. However, nasogastric intubation bypasses normal upper and lower respiratory tract mucosal defenses and exposes patients to risks for nosocomial sinusitis and pneumonia. Routine use of NGT after surgery is associated with increased cases of pneumonia, atelectasis, and aspiration compared with selective use (Cheatham, 1995). Indications for selective use might include symptomatic abdominal distention or postoperative nausea and vomiting from suspected ileus. Accordingly, the choice to implement NGT drainage is balanced against respiratory risks.
CARDIAC EVALUATION
Coronary artery disease (CAD) is a leading cause of death in developed countries and contributes significantly to perioperative cardiac complication rates in patients undergoing procedures (Stepp, 2005; Williams, 2009). Accordingly, much of cardiac risk assessment focuses on CAD and is described on page 828.
For congestive heart failure (CHF), a cardiologist may employ strategies to maximize hemodynamic function, such as preoperative coronary revascularization or perioperative medical therapy. With CHF, diuretics are commonly used. However, perioperatively, restraining their use will usually avoid intraoperative hypovolemia and related hypotension. But, if fluid resuscitation is needed, it ideally is gradual and limited to avoid volume overload.
Arrhythmias are usually symptoms of underlying cardiopulmonary disease or electrolyte abnormalities. Accordingly, preoperative management focuses on correcting the primary process. However, if pacemakers and implantable cardioverter-defibrillators (ICDs) are required for arrhythmia treatment prior to surgery, they are typically placed for the same indications as in nonoperative circumstances (Gregoratos, 2002). For those with pacemakers in place, electrosurgery can create electromagnetic interference even during noncardiac surgical and endoscopic procedures. Although encountered less frequently with newer devices, such interference can lead to pacing failure or complete system malfunction (Cheng, 2008). Thus, current guidelines recommend that all systems be evaluated by an appropriately trained physician before and after any invasive procedure (Fleisher, 2009). In addition, as discussed in Chapter 40, intraoperative efforts strive to minimize the chance for electromagnetic interference from electrosurgery. Practices include selecting bipolar electrosurgery if possible, using short intermittent bursts of electric current at the lowest possible energy levels, maximizing the distance between the current source and cardiac device, and placing the grounding pad in a position to minimize current flow toward the device.
Hypertension is not predictive of perioperative cardiac events and should not postpone surgery (Goldman, 1979; Weksler, 2003). Exceptions for elective procedures might include systolic blood pressures >180 mm Hg and diastolic blood pressures >110 mm Hg. If possible, to lower postoperative cardiac complications related to hypertension, blood pressure is lowered several months prior to an anticipated procedure (Fleisher, 2002). Preoperatively, patients on angiotensin-converting enzyme inhibitors and angiotensin-receptor antagonists have their morning dose held to reduce the risk of immediate postinduction hypotension (Comfere, 2005). In all patients with hypertension, avoiding hypo- or hypertension intraoperatively with careful postoperative monitoring is recommended. Importantly, intravascular volume expansion, pain, and agitation may exacerbate postoperative hypertension.
Valvular heart disease is a less frequently encountered cardiac comorbidity. Of these, aortic stenosis carries the highest independent factor for perioperative complications (Kertai, 2004). For other lesions, the degree of heart failure and associated cardiac arrhythmias are the best indicators of risk. If cardiac sounds are suggestive of valvular disease, echocardiography will assist in defining the abnormality. Importantly, endocarditis prophylaxis for valvular lesions during gastrointestinal (GI) or genitourinary (GU) procedures is no longer recommended by the American Heart Association (Nishimura, 2014). The transient enterococcal bacteremia caused by these procedures has not been irrefutably correlated to infective endocarditis.
As with pulmonary disease, history and physical examination can effectively identify or characterize cardiac disease. One questioning strategy is outlined in Figure 39-2. During physical examination, surgeons observe for dependent edema or jugular venous distention, whereas chest palpation searches for the point of maximum impulse and possible thrills. Auscultation of carotid arteries should exclude bruits, and listening at cardiac points investigates cardiac rate, regularity, and extra heart sounds.
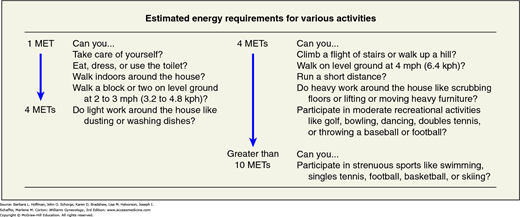
FIGURE 39-2
Questions used to assess functional capacity. METs are used in the algorithm in Figure 39-3. CAD = coronary artery disease; kph = kilometers per hour; MET = metabolic equivalent; mph = miles per hour. (Reproduced with permission from Fleisher LA, Beckman JA, Brown KA, et al: 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009 Nov 24;120(21):e169–e276.)
Of preoperative cardiac tests, 12-lead electrocardiogram (ECG) and chest radiograph are commonly considered. According to the American College of Cardiology and the American Heart Association (ACC/AHA), ECG is reasonable for patients with known coronary heart disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, or other significant structural heart disease. ECG is not recommended for those undergoing low-risk surgeries. For asymptomatic patients without known coronary heart disease, ECG may be considered, but again is not useful in those undergoing low-risk procedures (Fleisher, 2014). Indications for preoperative chest radiography are limited and discussed on page 826. Other testing is usually ordered by a consulting cardiologist and often directed by guidelines discussed next.
Preoperative guidelines have been developed by several groups to help predict perioperative cardiac complications and direct perioperative care. The two most prominent are: (1) those jointly developed by the ACC/AHA and (2) the Revised Cardiac Risk Index (RCRI) (Fleisher, 2014; Lee, 1999).
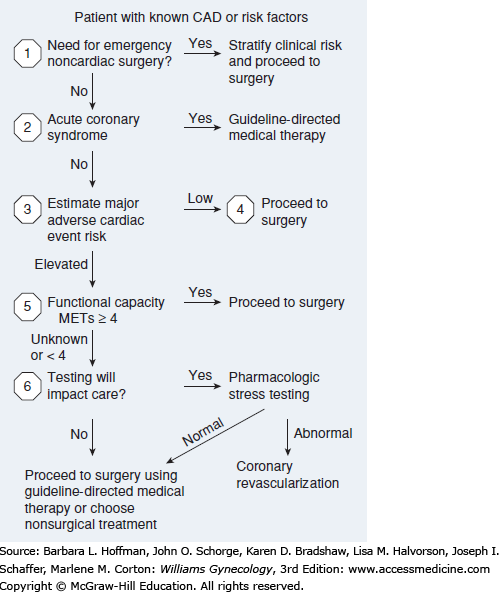
Of the two, ACC/AHA guidelines provide a stepwise strategy to assess three major considerations—clinical predictors, surgery-specific risk, and functional capacity—to ascertain the need for cardiac testing (Fig. 39-3). In general, for gynecologic surgery, cardiac complication risks are greatest with major emergency procedures and operations associated with large intravascular fluid shifts. In contrast, lowest risks are found with planned, brief endoscopic procedures.
FIGURE 39-3
Stepwise approach to perioperative cardiac assessment in those with coronary artery disease (CAD). MET = metabolic equivalent. (Adapted with permission from Fleisher LA, Fleischmann KE, Auerbach AD, et al: 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol 2014 Dec 9;64(22):e77–137.)
The Revised Cardiac Risk Index is an easy assessment of clinical predictors. It has been tested extensively and offers accurate estimates of cardiac risk (Lee, 1999). The major difference between the RCRI and the ACC/AHA guidelines is the incorporation of exercise capacity in the ACC/AHA tool. Creators of the RCRI suggest that cardiac risk may be overestimated by a patient’s noncardiac limitations in exercise function, such as musculoskeletal pain. Thus, these investigators place greater emphasis on cardiac and vascular disease markers.
Preoperative beta-blocker use to reduce in-hospital mortality rates were advocated as a result of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of studies. Unfortunately, fictitious databases and errant methods led to a discounting of these results (Erasmus Medical Centre, 2012). Subsequently, Bouri and colleagues (2014) conducted a metaanalysis of the available secure data from randomized controlled trials assessing the value of perioperative beta-blockade. They found the use of beta-blockers caused a statistically significant increase in all-cause mortality rates by 27 percent. Despite a reduction in nonfatal myocardial infarctions, rates of stroke and hypotension increased significantly. These new findings challenge the current AHA guidelines recommending beta-blockade in targeted high-risk patients and even suggest that they may benefit the least (Poldermans, 2009).
Diagnostic cardiac catheterization is considered in high-risk cardiac patients if noninvasive stress testing suggests advanced disease. In such cases, revascularization through coronary artery bypass grafting (CABG) or percutaneous coronary interventions offer comparable benefits perioperatively (Hassan, 2001).
This has been shown to be an independent risk factor for congestive heart failure (Kannel, 1987). A study by Silverberg and associates (2001) found that correction of even mild anemia (Hgb <12.5 percent) offered significant improvements in cardiac function. Iron therapy is not a substitution for appropriate cardiac disease treatment, but extrapolated data suggest that maintaining a hemoglobin level above 10 percent is important and reduces perioperative morbidity and mortality rates for those with cardiac disease.
HEPATIC EVALUATION
The rising incidence of hepatic disease has similarly increased the number of patients with hepatic dysfunction. Perioperative care must address this impairment, as the liver plays a central role in drug metabolism; synthesis of proteins, glucose, and coagulation factors; and excretion of endogenous compounds.
Patients with suspected disease are queried regarding family histories of jaundice or anemia, recent travel history, exposure to alcohol or other hepatotoxins, and medication use (Suman, 2006). Physical findings include jaundice, scleral icterus, spider angiomas, ascites, hepatomegaly, asterixis, and cachexia.
If underlying liver disease is known or suspected, hepatic function is assessed. In addition, prothrombin time (PT), partial thromboplastin time (PTT), serum albumin level, and a serum chemistry panel are valuable adjuncts.
Of liver diseases, acute and chronic hepatitis are commonly encountered. With acute hepatitis, regardless of the cause, high associated perioperative mortality rates have been documented by multiple investigators. For this reason, primary management involves supportive care and delay of elective surgery until the acute process has subsided (Patel, 1999). In those with chronic hepatitis, hepatic dysfunction is variable. Compensated disease carries a low risk of perioperative complications (Sirinek, 1987). However, in patients with cirrhosis, the Child-Pugh score is a useful tool to predict survival after abdominal surgery. Clinical measures include serum total bilirubin and albumin levels, international normalized ratio (INR) values, and severity of associated ascites and encephalopathy. Approximate mortality risks based on Child-Pugh class are class A—10 percent; class B—30 percent; and class C—70 percent (Mansour, 1997).
RENAL EVALUATION
The kidney is involved with metabolic waste excretion, erythropoietin production, and fluid and electrolyte balance. Accordingly, patients with known renal insufficiency typically have serum electrolytes, renal function, and complete blood count (CBC) evaluated prior to surgery. Chronic anemia due to renal insufficiency will typically require preoperative administration of erythropoietin or perioperative transfusion depending on the procedure planned and degree of anemia. Dialysis patients require intensive pre- and postoperative surveillance for signs of electrolyte abnormalities and fluid overload. Ideally, these patients’ volume status and electrolytes (potassium in particular) are optimized by performing dialysis the day prior to surgery. Additionally, further renal insult is averted by avoiding nephrotoxic agents. Pharmacokinetic consultation may be warranted to adjust other medication dosages as serum levels in these patients may be unpredictable postoperatively.
HEMATOLOGIC EVALUATION
This is frequently encountered in preoperative gynecologic surgery evaluation. In the absence of a clear etiology, evaluation serves to potentially correct reversible causes. Queries focus on signs of symptomatic anemia such as fatigue, dyspnea with exertion, and palpitations. Inquiry also seeks to identify risk factors for underlying cardiovascular disease as anemia is less well tolerated in these individuals. The physical examination incorporates thorough pelvic and rectal examination, stool guaiac screening, and urinalysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree