ANTERIOR ABDOMINAL WALL
The anterior abdominal wall provides core support to the human torso, confines abdominal viscera, and contributes muscular action for functions such as respiration and elimination. In gynecology, an understanding of the layered structure of the anterior abdominal wall is needed to effectively enter the peritoneal cavity for surgery without neurovascular complications.
Within the skin, the term Langer lines describes the orientation of dermal fibers. In the anterior abdominal wall, they are arranged primarily transversely (Fig. 38-1). As a result, vertical skin incisions sustain more lateral tension and thus in general, develop wider scars compared with transverse skin incisions.
The subcutaneous layer lies deep to the skin. In the anterior abdominal wall, this layer is separated into a superficial, predominantly fatty layer known as Camper fascia and a deeper, more membranous layer known as Scarpa fascia (Fig. 38-2). Camper and Scarpa fasciae are not discrete layers but represent a continuum. If traced caudally, scarpa fascia is continuous with Colles fascia in the perineum.
Clinically, Scarpa fascia is better developed in the lower abdomen and during surgery can be best identified in the lateral portions of a low transverse incision, just superficial to the rectus fascia. In contrast, this fascia is rarely recognized during midline incisions.
The external oblique, internal oblique, and transversus abdominis muscles (flank muscles) all contain a lateral muscular portion and medial fibrous aponeurotic portion. All of their aponeuroses conjoin, and these layers create the rectus sheath (see Fig. 38-2). In the midline, the aponeurotic layers fuse to create the linea alba. In the lower abdomen, transition from the muscular to the aponeurotic component of the external oblique muscle takes place along a vertical line through the anterior superior iliac spine. Transition from muscle to aponeurosis for the internal oblique and transversus abdominis muscles takes place at a more medial site. Accordingly, during low transverse incisions, muscle fibers of the internal oblique muscle are often noted below the aponeurotic layer of the external oblique muscle.
The anatomy of the rectus sheath above and below the arcuate line has significance (see Fig. 38-2). This horizontal line defines the level at which the rectus sheath passes only anterior to the rectus abdominis muscle, and this line typically lies midway between the umbilicus and pubic symphysis. Cephalad to the arcuate line, the rectus sheath lies both anterior and posterior to the rectus abdominis muscle. At this level, the anterior rectus sheath is formed by the aponeurosis of the external oblique muscle and the split aponeurosis of the internal oblique muscle. The posterior rectus sheath is formed by the split aponeurosis of the internal oblique muscle and aponeurosis of the transversus abdominis muscle. Caudad to the arcuate line, all aponeurotic layers pass anterior to the rectus abdominis muscle. Thus, in the lower abdomen, the posterior surface of the rectus abdominis muscle is in direct contact with the transversalis fascia, described next.
Surgically, because the aponeuroses of the internal oblique and transversus abdominis muscles in the lower abdomen fuse, only two layers are identified during low transverse fascial incisions. In contrast, during midline vertical incision, only one fascial layer, namely, the linea alba, is encountered.
Similar to skin fibers, the flank muscle and rectus sheath fibers are oriented primarily transversely. Thus, suture lines placed in a vertical fascial incision must withstand more tension than those in a transverse incision. As a result, vertical fascial incisions are more prone to dehiscence and hernia formation. In addition to incisional hernias, ventral wall hernias are most common along the linea alba. Another type of anterior abdominal wall hernia, the Spiegelian hernia, is rare and forms at the lateral rectus abdominis border, typically at the level of the arcuate line (Fig. 11-8).
This thin fibrous tissue layer lies between the inner surface of the transversus abdominis muscle and preperitoneal fat. Thus, it serves as part of the general fascial layer that lines the abdominal cavity (see Fig. 38-2) (Memon, 1999). Inferiorly, the transversalis fascia blends with the periosteum of the pubic bones.
Surgically, this fascia is best recognized as the layer bluntly or sharply dissected off the anterior surface of the bladder during entry into the abdominal cavity. This is the layer of tissue that is last penetrated to gain extraperitoneal entry into the retropubic space.
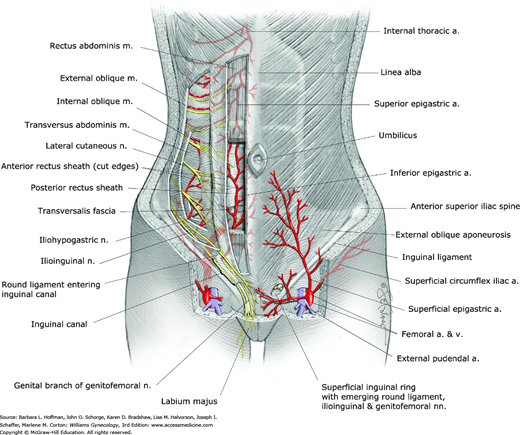
The peritoneum that lines the inner surface of the abdominal walls is termed parietal peritoneum. In the anterior abdominal wall, there are five elevations of parietal peritoneum that are raised by different structures (see Fig. 38-2). All five converge toward the umbilicus and are known as umbilical ligaments.
The single median umbilical ligament is formed by the urachus, an obliterated tube that extends from the apex of the bladder to the umbilicus. In fetal life, the urachus, which is a fibrous remnant of the allantois, extends from the umbilical cord to the urogenital sinus, which gives rise to the bladder. The paired medial umbilical ligaments are formed by the obliterated umbilical arteries that connected the internal iliac arteries to the umbilical cord in fetal life. The paired lateral umbilical ligaments contain the patent inferior epigastric vessels. The initial course of these vessels is just medial to the round ligament as the ligament enters the deep inguinal ring (Fig. 38-3).
Surgically, transection of a patent urachus can result in extravasation of urine into the abdominal cavity. In addition, the differential diagnosis of a midline anterior abdominal wall cyst includes urachal cyst, urachal sinus, and urachal diverticulum.
The umbilical ligaments serve as valuable laparoscopic landmarks. First, the inferior epigastric vessels can be injured during accessory trocar placement (Hurd, 1994; Rahn, 2010). Thus, direct visualization of the lateral umbilical folds can prevent injury to these vessels during laparoscopic port placement. Second, the medial umbilical ligaments, if followed proximally, can guide a surgeon to the internal iliac artery and then to the uterine arteries. The medial umbilical ligament also forms the medial border of the paravesical space, which is developed during radical hysterectomy to isolate the parametrium.
The superficial epigastric, superficial circumflex iliac, and external pudendal arteries arise from the femoral artery just below the inguinal ligament in the femoral triangle, which is bordered by this ligament, the sartorius muscle, and the adductor longus muscle. These vessels supply the skin and subcutaneous layers of the anterior abdominal wall and mons pubis. The superficial epigastric vessels course diagonally toward the umbilicus, similar to the inferior “deep” epigastric vessels.
Surgically, during low transverse skin incision creation, the superficial epigastric vessels can usually be identified halfway between the skin and the rectus fascia, several centimeters from the midline. During laparoscopic procedures in thin patients, these vessels can be identified by transillumination (Chap. 41).
The external pudendal vessels form rich anastomoses with their contralateral equivalents and with other superficial branches. These anastomoses account for the extensive bleeding often encountered with incisions made in the mons pubis area such as for retropubic midurethral sling incisions.
The inferior “deep” epigastric vessels and deep circumflex iliac vessels are branches of the external iliac vessels (see Fig. 38-3). They supply the muscles and fascia of the anterior abdominal wall. The inferior epigastric vessels initially course lateral to, then posterior to the rectus abdominis muscle, which they supply. They then pass anterior to the posterior rectus sheath and course between the sheath and the rectus muscles (see Figs. 38-2 and 38-3). Near the umbilicus, the inferior epigastric vessels anastomose with the superior epigastric artery and veins, which are branches of the internal thoracic vessels.
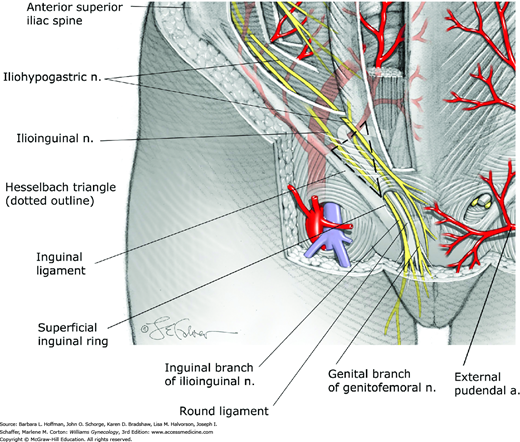
Hesselbach triangle is the region in the anterior abdominal wall bounded inferiorly by the inguinal ligament, medially by the lateral border of the rectus muscles, and laterally by the inferior epigastric vessels (Fig. 38-4). Direct hernias protrude through the abdominal wall within Hesselbach triangle. In contrast, indirect hernias protrude into the deep inguinal ring lying lateral to this triangle.
Surgically, low transverse abdominal incisions that extend beyond the lateral margins of the rectus muscles can lead to inferior epigastric vessel laceration with severe hemorrhage or anterior abdominal wall hematoma formation. These vessels should be identified and ligated when performing a Maylard incision. As another surgical landmark, the deep circumflex iliac vein serves as the caudal border during pelvic lymph node dissection.
The anterior abdominal wall is innervated by the abdominal extensions of the intercostal nerves (T7-11), the subcostal nerve (T12), and the iliohypogastric and the ilioinguinal nerves (L1) (see Fig. 38-3). The T10 dermatome approximates the level of the umbilicus. Of these, the iliohypogastric nerve provides sensation to the skin over the suprapubic area. The ilioinguinal nerve supplies the skin of the lower abdominal wall and upper portion of the labia majora and medial portion of the thigh through its inguinal branch (see Fig. 38-4). At a site 2 to 3 cm medial to the anterior superior iliac spine, these two nerves pierce through the internal oblique muscle and course superficial to it and toward the midline (Whiteside, 2003).
Clinically, the ilioinguinal and iliohypogastric nerves can be entrapped during closure of low transverse incisions, especially if incisions extend beyond the lateral borders of the rectus abdominis muscle. They may also be wounded by lower abdominal insertion of accessory trocars. The risk of iliohypogastric and ilioinguinal nerve injury can be minimized if lateral trocars are placed superior to the anterior superior iliac spines and if low transverse fascial incisions are not extended beyond the lateral borders of the rectus muscle (Rahn, 2010).
BONY PELVIS
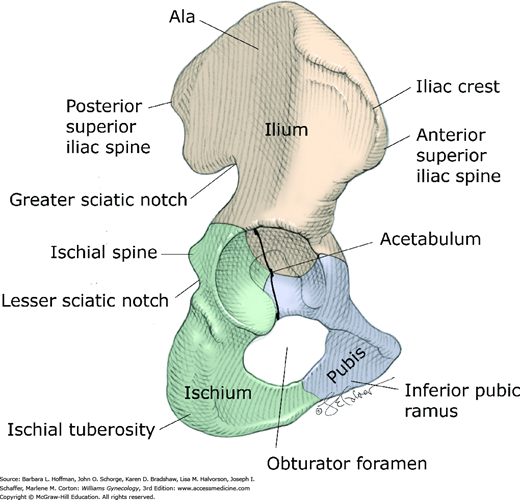
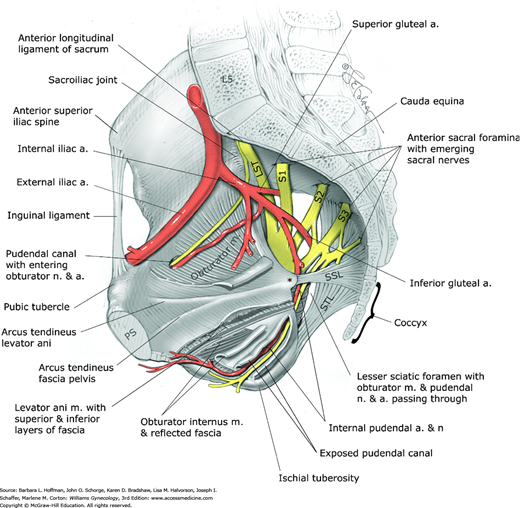
The sacrum; the coccyx; and two hip bones, termed the innominate bones form the bony pelvis (Fig. 38-5). The innominate bones consist of the ilium, ischium, and pubis, which fuse at the acetabulum, a cup-shaped structure that articulates with the femoral head. The ilium articulates with the sacrum posteriorly at the sacroiliac joint, and the pubic bones articulate with each other anteriorly at the symphysis pubis. The sacroiliac joint is a synovial joint that connects the articular surfaces of the sacrum and ilium. This joint and its ligaments contribute significantly to the stability of the bony pelvis. The symphysis pubis is a cartilaginous joint, which connects the articular surfaces of the pubic bones by way of a fibrocartilaginous disc. The ischial spines are clinically important bony prominences that project posteromedially from the medial surface of the ischium approximately at the level of the fifth sacral vertebra (S5).
The posterior, lateral, and inferior walls of the pelvis have several openings through which many important structures pass. The large obturator foramen between the ischium and pubis is filled almost completely by the obturator membrane. In the superior portion of this membrane, a small aperture known as the obturator canal allows passage of the obturator neurovascular bundle into the medial (adductor) compartment of the thigh (Fig. 38-6).
FIGURE 38-6
Bones, ligaments, and openings of the pelvic walls and associated structures. Note the obturator internus muscle extending below the levator ani muscle and then exiting through the lesser sciatic foramen to insert into the lateral femoral trochanter. Ischial spine is marked by an asterisk. L5 = fifth lumbar vertebra; LST = lumbosacral trunk; PS = pubic symphysis; S1–S3 = first through third sacral nerves; SSL = sacrospinous ligament; STL = sacrotuberous ligament.
The posterolateral walls of the pelvis are not covered by bone. Instead, two important accessory ligaments, the sacrospinous and sacrotuberous ligaments, divide the greater and lesser sciatic notches of the ischium into the greater sciatic foramen and lesser sciatic foramen. Through the greater sciatic foramen, the piriformis muscle, internal pudendal and superior and inferior gluteal vessels, sciatic and pudendal nerve, and other branches of the sacral nerve plexus pass in close proximity to the ischial spines. Surgically, this anatomy is critical to avoid neurovascular injury during sacrospinous fixation procedures and when administering pudendal nerve blockade (Roshanravan, 2007).
The internal pudendal vessels, pudendal nerve, and obturator internus tendon pass through the lesser sciatic foramen. Posteriorly, four pairs of pelvic sacral foramina allow passage of the anterior divisions of the first four sacral nerves and lateral sacral arteries and veins.
The ligaments of the pelvis vary in composition and function. They range from connective tissue structures that support the bony pelvis and pelvic organs to smooth muscle and loose areolar tissue that add no significant support. The sacrospinous, sacrotuberous, and anterior longitudinal ligaments of the sacrum consist of dense connective tissue that joins bony structures and contributes significantly to bony pelvis stability (see Fig. 38-6).
The round and broad ligaments consist of smooth muscle and loose areolar tissue, respectively. Although they connect the uterus and adnexa to the pelvic walls, they do not contribute to the support of these organs. In contrast, the cardinal and uterosacral ligaments do aid pelvic organ support and are discussed later.
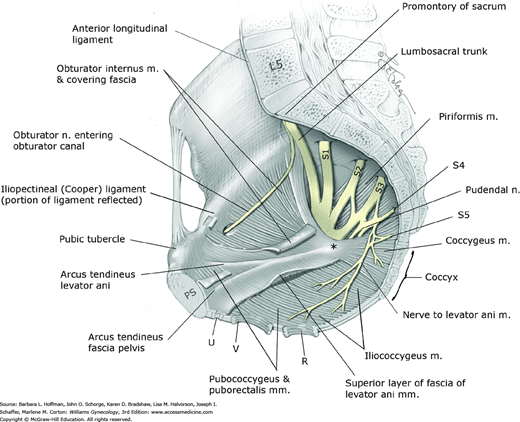
Clinically, the sacrospinous and anterior longitudinal ligament serve as suture fixation sites in suspensory procedures used to correct pelvic organ prolapse. The iliopectineal ligament, also termed Cooper ligament, is a thickening in the pubic bone periosteum, which is often used to anchor sutures in retropubic bladder neck suspension procedures (Fig. 38-7).
PELVIC WALL MUSCLES AND FASCIA
The posterior, lateral, and inferior walls of the pelvis are partially covered by striated muscles and their investing layers of fasciae (see Fig. 38-7). The piriformis muscle arises from the anterior and lateral surface of the sacrum and partially fills the posterolateral pelvic walls. It exits the pelvis through the greater sciatic foramen, attaches to the greater trochanter of the femur, and functions as an external or lateral hip rotator. Clinically, stretch injury to the piriformis muscle may cause persistent hip pain that can be confused with other hip or pelvic pathology.
The obturator internus muscle partially fills the sidewalls of the pelvis. This muscle arises from the pelvic surfaces of the ilium and ischium and from the obturator membrane. It exits the pelvis through the lesser sciatic foramen, attaches to the greater trochanter of the femur, and also functions as an external hip rotator.
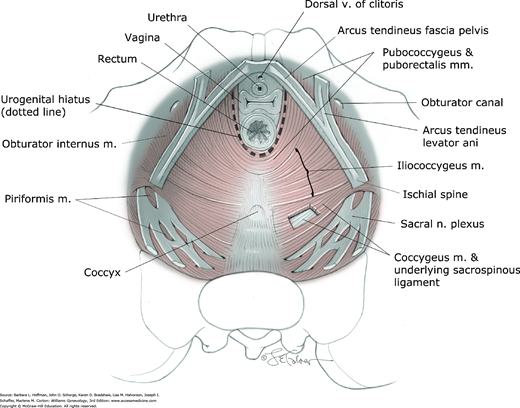
The fascia that invests striated muscles is termed parietal fascia. Pelvic parietal fascia provides muscle attachment to the bony pelvis and serves as anchoring points for visceral fascia, also termed endopelvic fascia. The arcus tendineus levator ani is a condensation of parietal fascia covering the medial surface of the obturator internus muscle (see Fig. 38-7 and Fig. 38-8). This structure serves as the point of origin for parts of the very important levator ani muscle. Also shown is the arcus tendineus fascia pelvis, a condensation of parietal fascia covering the medial aspects of the obturator internus and levator ani muscles. It serves as the lateral point of attachment of the anterior vaginal wall.
PELVIC FLOOR
The muscles that span the pelvic floor are collectively known as the pelvic diaphragm (see Figs. 38-7, 38-8, and Fig. 38-9). This diaphragm consists of the levator ani and coccygeus muscles, along with their superior and inferior investing fascial layers. Inferior to the pelvic diaphragm, the perineal membrane and perineal body also contribute to the pelvic floor. The urogenital hiatus is the U-shaped opening in the pelvic floor muscles through which the urethra, vagina, and rectum pass.
These are the most important muscles in the pelvic floor and provide critical pelvic organ support (see Figs. 38-7 through 38-9). Physiologically, normal levator ani muscles maintain a constant state of contraction, thus providing a stable floor, which supports the weight of the abdominopelvic contents against intraabdominal forces.
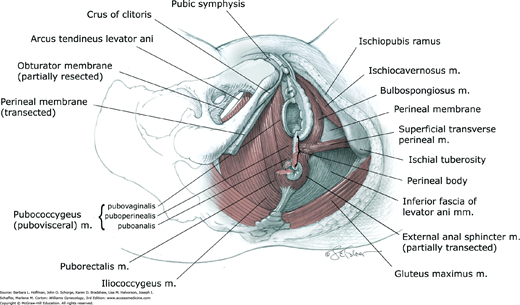
The levator ani muscle is a complex unit, which consists of several muscle components with different origins and insertions and therefore different functions. The pubococcygeus, puborectalis, and iliococcygeus muscles are the three components. Of these, the pubococcygeus muscle is further divided into the pubovaginalis, puboperinealis, and puboanalis muscles according to their fiber attachments. Due to the significant attachments of the pubococcygeus muscle to the walls of the pelvic viscera, the term pubovisceral muscle is frequently used (Kerney, 2004; Lawson, 1974).
The anterior ends of the pubococcygeus (pubovisceral muscle) arise on either side from the inner surface of the pubic bone. The pubovaginalis refers to the medial fibers that attach to the lateral walls of the vagina (see Fig. 38-9). Although there are no direct attachments of the levator ani muscles to the urethra in females, those fibers of the muscle that attach to the vagina are responsible for elevating the urethra during a pelvic muscle contraction and hence may contribute to urinary continence (DeLancey, 1990). The puboperinealis refers to the fibers that attach to the perineal body and draw this structure toward the pubic symphysis. The puboanalis refers to the fibers that attach to the anus at the intersphincteric groove between the internal and external anal sphincters. These fibers elevate the anus and, along with the rest of the pubococcygeus and puborectalis fibers, keep the urogenital hiatus narrowed (see Fig. 38-8).
The puborectalis represents the medial and inferior fibers of the levator ani muscle that arise on either side from the pubic bone and form a U-shaped sling behind the anorectal junction (Figs. 38-8 through 38-10). The action of the puborectalis draws the anorectal junction toward the pubis, contributing to the anorectal angle. This muscle is considered part of the anal sphincter complex and may contribute to fecal continence (Chap. 25).
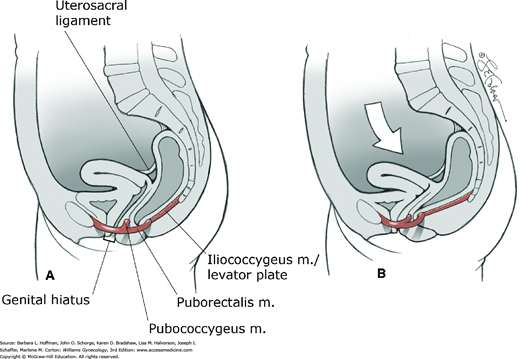
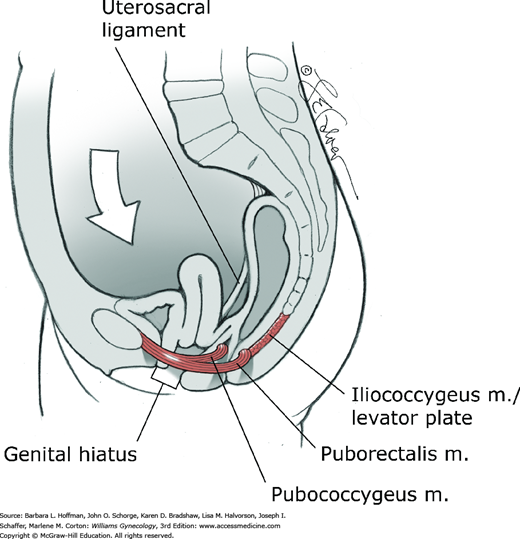
This muscle is the most posterior and thinnest part of the levator ani muscle and has a primarily supportive role. It arises laterally from the arcus tendineus levator ani and the ischial spines (see Figs. 38-7 through 38-10). Muscle fibers from one side join those from the opposite side in the midline between the anus and the coccyx. This meeting line is termed the iliococcygeal or anococcygeal raphe. In addition to the iliococcygeus muscle, some fibers of the pubococcygeus muscle pass behind the rectum and attach to the coccyx. These muscle fibers course cephalad or deep to the iliococcygeus muscle and may also contribute to the anococcygeal raphe. The levator plate is the clinical term used to describe the anococcygeal raphe (see Fig. 38-10). This portion of the levator muscles forms a supportive shelf on which the rectum, upper vagina, and uterus rest.
The levator plate in women with normal support has a mean angle of 44 degrees relative to a horizontal reference line during Valsalva, although earlier studies suggested no elevation (Berglas, 1953; Hsu, 2006). During Valsalva, women with prolapse have a statistically greater levator plate angle compared with controls. This larger angle moderately correlates with larger levator hiatus length and greater displacement of the perineal body in women with prolapse compared with controls.
With this in mind, one theory suggests that levator plate support prevents excessive tension or stretching of the connective tissue pelvic ligaments and fasciae (Paramore, 1908). Neuromuscular injury to the levator muscles may lead to eventual sagging or vertical inclination of the levator plate and opening of the urogenital hiatus. Consequently, the vaginal axis becomes more vertical, and the cervix is oriented over the opened hiatus (Fig. 38-11). The mechanical effect of this change is to increase strain on connective tissues that support the pelvic viscera. Increased urogenital hiatus size has been shown to correlate with increased prolapse severity (DeLancey, 1998).
The pelvic diaphragm muscles are primarily innervated by direct somatic efferents from the second through the fifth sacral nerve roots (S2-5) (see Fig. 38-7) (Barber, 2002; Roshanravan, 2007).
Traditionally, a dual innervation has been described. The pelvic or superior surface of the muscles is supplied by direct efferents from S2-5, collectively known as the nerve to the levator ani muscle. The perineal or inferior surface is supplied by pudendal nerve branches. This latter relationship has been challenged, and investigators suggest that the pudendal nerve does not contribute to levator muscle innervation (Barber, 2002). Pudendal branches do, however, innervate parts of the striated urethral sphincter and external anal sphincter muscles. Such separate innervation may explain why some women develop pelvic organ prolapse and others develop urinary or fecal incontinence (Heit, 1996).
Subperitoneal perivascular connective tissue and loose areolar tissue are found throughout the pelvis. This tissue connects the pelvic viscera to the pelvic walls and is termed visceral or endopelvic “fascia.” Recall that visceral fascia differs from parietal fascia, which invests most striated muscles (Table 38-1). Visceral fascia is intimately associated with the walls of the viscera and cannot be dissected in the same way that parietal fascia can be separated from its skeletal muscle.
| Type of Fascia | ||
| Characteristic | Visceral or Endopelvic | Parietal |
| Histologic | Loose arrangements of collagen, elastin, and adipose tissue | Organized collagen arrangements |
| Function | Allows expansion and contraction of invested structures | Provides muscle attachment to bones |
| Supportive role | Condensations lend some support to invested organs; encases neurovascular structures | Invests muscles to provide pelvic floor stability and function |
| Tensile strength | Elastic | Rigid |
Condensations of visceral connective tissue that have assumed special supportive roles have been given different names. Some examples include the cardinal and uterosacral ligaments and the vesicovaginal and rectovaginal fascia. These are described further in later sections.
PELVIC BLOOD SUPPLY
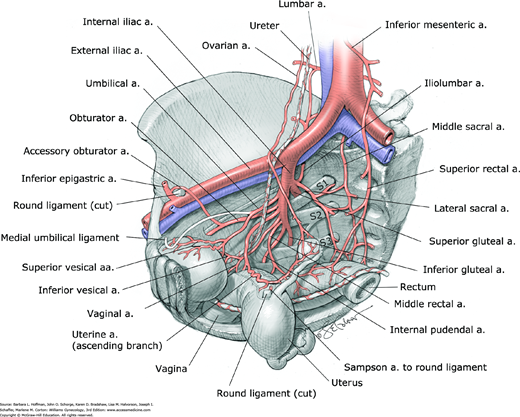
The pelvic organs are supplied by the visceral branches of the internal iliac (hypogastric) artery and by direct branches from the abdominal aorta (Fig. 38-12). Clinically, the internal iliac artery can be separated into an anterior and posterior division in the area of the greater sciatic foramen (see Fig. 38-6). Each division has three parietal branches that supply nonvisceral structures. The iliolumbar, lateral sacral, and superior gluteal arteries are the three parietal branches of the posterior division. The internal pudendal, obturator, and inferior gluteal arteries are parietal branches that most commonly arise from the anterior division. The remaining branches of the anterior division supply pelvic viscera (bladder, uterus, vagina, and rectum). These include the uterine, vaginal, and middle rectal arteries and the superior vesical arteries. The superior vesical arteries commonly arise from the patent part of the umbilical arteries (Table 38-2). Internal iliac branches that supply the inferior and middle portions of the bladder are present in women, but their origin is highly variable. The middle rectal arteries are generally very small-caliber vessels but may be absent. They usually contribute to posterior vaginal wall blood supply.
| Internal Iliac Artery a | |||
| Anterior Division | Posterior Division | ||
| Parietal Branches | Visceral Branches | Parietal Branches | Visceral Branches |
| Obturator Internal pudendal Inferior gluteal | Superior vesical (from patent segment of umbilical) Uterine Vaginal Middle rectal Middle & inferior vesicals (±) | Iliolumbar Lateral sacral Superior gluteal | None |
The two most important direct branches of the aorta that contribute to pelvic organ blood supply are the superior rectal and ovarian arteries. The superior rectal artery, which is the terminal branch of the inferior mesenteric artery, anastomoses with the middle rectal arteries, thus contributing blood supply to the rectum and vagina. The ovarian arteries arise directly from the aorta just inferior to the renal vessels and anastomose with the ascending branch of the uterine artery. These anastomoses contribute to the blood supply of the uterus and adnexa. Other important anastomoses between the aorta and internal iliac arteries include anastomoses between the middle sacral and lateral sacral arteries and anastomoses between the lumbar and iliolumbar arteries.
PELVIC INNERVATION
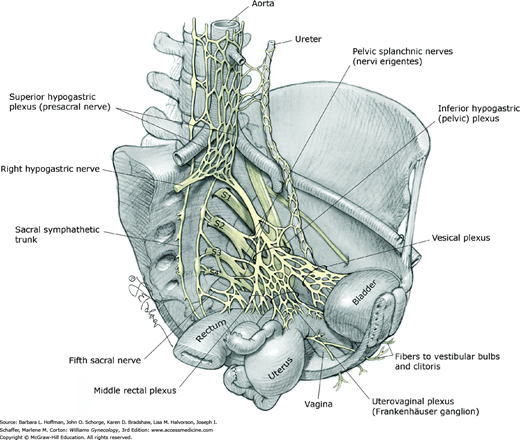
Nerve supply to the visceral structures in the pelvis (bladder, urethra, vagina, uterus, adnexa, and rectum) arises from the autonomic nervous system. The two most important components of this system in the pelvis include the superior and inferior hypogastric plexuses. The superior hypogastric plexus, also known as the presacral nerve, is an extension of the aortic plexus found below the aortic bifurcation (Fig. 38-13). This plexus primarily contains sympathetic fibers and sensory afferent fibers from the uterus.
The superior hypogastric plexus terminates by dividing into the hypogastric nerves. These nerves join parasympathetic efferents from the second through the fourth sacral nerve roots (pelvic splanchnic nerves) to form the inferior hypogastric plexus, also known as the pelvic plexus. In addition, the inferior hypogastric plexus generally receives contributions from the sacral sympathetic trunk.
With variability, fibers of the inferior hypogastric plexus accompany the branches of the internal iliac artery to the pelvic viscera. Accordingly, they are divided into three portions: the vesical, uterovaginal (Frankenhäuser ganglion), and middle rectal plexuses. Extensions of the inferior hypogastric plexus reach the perineum along the vagina and urethra to innervate the clitoris and vestibular bulbs.
Clinically, the sensory afferent fibers contained within the superior hypogastric plexus are targeted in presacral neurectomy, a surgical procedure performed to treat central pelvic pain (Chap. 11). Although visceral and sexual dysfunction has been reported following complete interruption of the superior hypogastric plexus, contributions from the sacral sympathetic trunk may offset interruption of this sympathetic component to the inferior hypogastric plexus. Injury to the branches of the inferior hypogastric plexus during cancer debulking or other extensive pelvic surgeries can lead to varying degrees of voiding, sexual, and defecatory dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree