INTRODUCTION
Gestational trophoblastic disease (GTD) refers to a spectrum of interrelated but histologically distinct tumors originating from the placenta (Table 37-1). These diseases are characterized by a reliable tumor marker, which is the β-subunit of human chorionic gonadotropin (β-hCG), and have varied tendencies for local invasion and spread.
Gestational trophoblastic neoplasia (GTN) refers to the subset of GTD that develops malignant sequelae. These tumors require formal staging and typically respond favorably to chemotherapy. Most commonly, GTN develops after a molar pregnancy but may follow any gestation. The prognosis for most GTN cases is excellent, and patients are routinely cured, even with widespread metastases. The outlook for preservation of fertility and for successful subsequent pregnancy outcomes is equally bright (Vargas, 2014; Wong, 2014). Accordingly, although GTD is uncommon, because the opportunity for cure is great, clinicians should be familiar with its presentation, diagnosis, and management.
EPIDEMIOLOGY AND RISK FACTORS
The incidence of GTD has remained fairly constant at approximately 1 to 2 per 1000 deliveries in North America and Europe (Drake, 2006; Loukovaara, 2005; Lybol, 2011). Although historically higher incidence rates have been reported in parts of Asia, some of this disparity may reflect discrepancies between population-based and hospital-based data collection (Chong, 1999; Kim, 2004; Matsui, 2003). Improved socioeconomic conditions and dietary changes may be partly responsible as well. That said, certain Southeast Asian populations as well as Hispanics and Native Americans living in the United States do have increased incidences (Drake, 2006; Smith, 2003; Tham, 2003).
Maternal age at the upper and lower extremes carries a higher risk of GTD (Altman, 2008; Loukovaara, 2005). This association is much greater for complete moles, whereas the risk of partial molar pregnancy varies relatively little with age. Moreover, compared with the risk in those aged 15 years or younger, the degree of risk is much greater for women 45 years (1 percent) or older (17 percent at age 50) (Savage, 2010; Sebire, 2002a). One explanation relates to ova from older women having higher rates of abnormal fertilization. Similarly, older paternal age has been associated with increased risk (La Vecchia, 1984; Parazzini, 1986).
A history of prior unsuccessful pregnancies also increases the risk of GTD. For example, previous spontaneous abortion at least doubles the risk of molar pregnancy (Parazzini, 1991). More significantly, a personal history of GTD increases the risk of developing a molar gestation in a subsequent pregnancy at least 10-fold. The frequency in a subsequent conception is approximately 1 percent, and most cases mirror the same type of mole as the preceding pregnancy (Garrett, 2008; Sebire, 2003). Furthermore, following two episodes of molar pregnancy, 23 percent of later conceptions result in another molar gestation (Berkowitz, 1998). For this reason, women with a prior history of GTD should undergo first-trimester sonographic examination in subsequent pregnancies. Familial molar pregnancies, however, are rare (Fallahian, 2003).
Of other risk factors, combination oral contraceptive (COC) pill use has been associated with an increased risk of GTD. Specifically, prior COC use approximately doubles the risk, and longer duration of use also correlates positively with risk (Palmer, 1999; Parazzini, 2002). Moreover, women who used COCs during the cycle in which they conceived have a higher risk in some but not all studies (Costa, 2006; Palmer, 1999). Many of these associations, however, are weak and could be explained by confounding factors other than causality (Parazzini, 2002).
Some epidemiologic characteristics differ markedly between complete and partial moles. For example, vitamin A deficiency and low dietary intake of carotene are associated only with an increased risk of complete moles (Berkowitz, 1985, 1995; Parazzini, 1988). Partial moles have been linked to higher educational levels, smoking, irregular menstrual cycles, and obstetric histories in which only male infants are among the prior live births (Berkowitz, 1995; Parazzini, 1986).
HYDATIDIFORM MOLE (MOLAR PREGNANCY)
Hydatidiform moles are abnormal pregnancies characterized histologically by aberrant changes within the placenta. Classically, the chorionic villi in these placenta show varying degrees of trophoblast proliferation and edema of the stroma within villi (Fig. 37-1). Hydatidiform moles are categorized as either complete hydatidiform moles or partial hydatidiform moles (Table 37-2). Chromosomal abnormalities play an integral role in hydatidiform mole development.
| Feature | Complete Mole | Partial Mole |
| Karyotype | 46,XX or 46,XY | 69,XXX or 69,XXY |
| Pathology | ||
| Fetus/embryo Villous edema Trophoblastic proliferation p57Kip2 immunostaining | Absent Diffuse Can be marked Negative | Present Focal Focal and minimal Positive |
| Clinical presentation | ||
| Typical diagnosis Postmolar malignant sequelae | Molar gestation 15% | Missed abortion 4–6% |
FIGURE 37-1
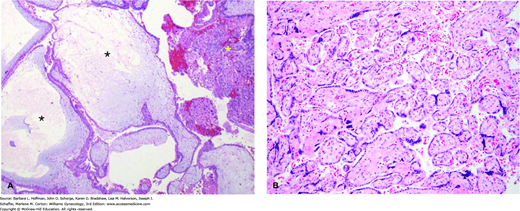
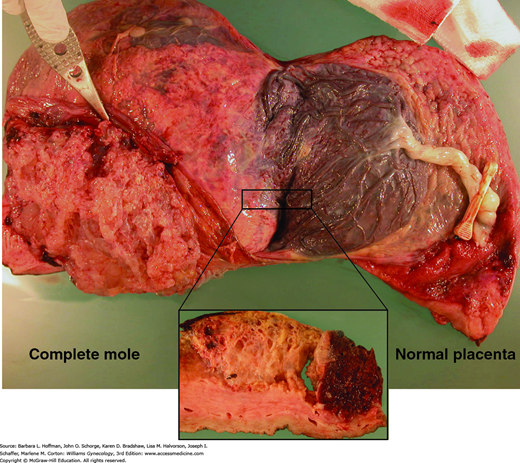
A. Complete hydatidiform mole. These moles classically have swollen enlarged villi, some of which show cistern formation, that is, central cavitation within the large villi (black asterisks). Seen diffusely throughout the placenta, these villous changes create the vesicles noted grossly in complete moles (see Fig. 37-3). Complete moles also typically show trophoblastic proliferation (yellow asterisk), which may be focal or widespread. (Used with permission from Dr. Erika Fong.) B. Normal term placenta showing smaller, nonedematous villi and absence of trophoblastic proliferation. (Used with permission from Dr. Kelley Carrick.)
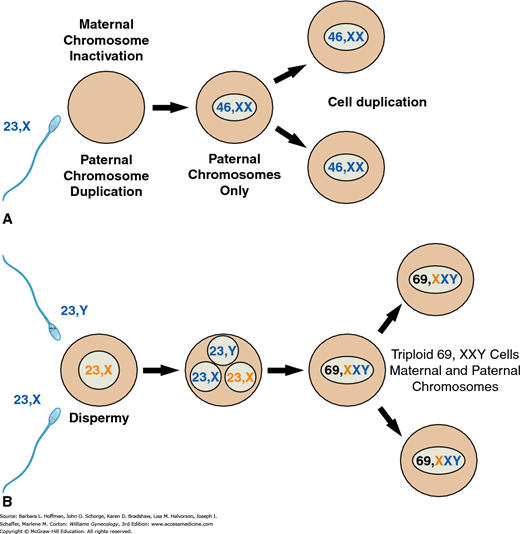
These molar pregnancies differ from partial moles with regard to their karyotype, their histologic appearance, and their clinical presentation. First, complete moles typically have a diploid karyotype, and 85 to 90 percent of cases are 46,XX. The chromosomes, however, in these pregnancies are entirely of paternal origin, and thus, the diploid set is described as diandric. Specifically, complete moles are formed by androgenesis, in which the ovum is fertilized by a haploid sperm that then duplicates its own chromosomes after meiosis (Fig. 37-2) (Fan, 2002; Kajii, 1977). The ovum fails to contribute chromosomes. Most of these moles are 46,XX, but dispermic fertilization of a single ovum, that is, simultaneous fertilization by two sperm, can produce a 46,XY karyotype (Lawler, 1987). Although nuclear DNA is entirely paternal, mitochondrial DNA remains maternal in origin (Azuma, 1991).
FIGURE 37-2
A. A 46,XX complete mole may be formed if a 23,X-bearing haploid sperm penetrates a 23,X-containing haploid egg whose genes have become “inactive.” Paternal chromosomes then duplicate to create a 46,XX diploid chromosomal complement solely of paternal origin. Alternatively, this same type of inactivated egg can be fertilized independently by two sperm, either 23,X- or 23,Y-bearing, to create a 46,XX or 46,XY chromosomal complement, again of paternal origin only. B. Partial moles may be formed if two sperm, either 23,X- or 23,Y-bearing, both fertilize a 23,X-containing haploid egg, whose genes have not been inactivated. The resulting fertilized egg is triploid. Alternatively, a similar haploid egg may be fertilized by an unreduced diploid 46,XY sperm.
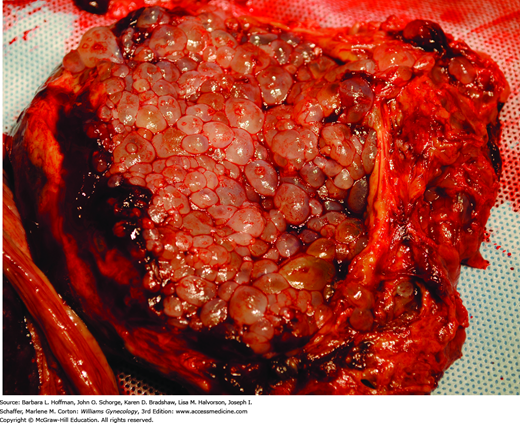
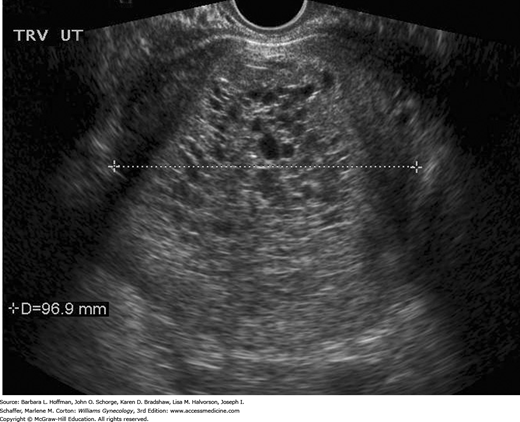
Microscopically, complete moles display enlarged, edematous villi and abnormal trophoblastic proliferation. These changes diffusely involve the entire placenta (see Fig. 37-1). Macroscopically, these changes transform the chorionic villi into clusters of vesicles with variable dimensions. Indeed, the name hydatidiform mole literally stems from this “bunch of grapes” appearance. In these pregnancies, no fetal tissue or amnion is produced. As a result, this mass of placental tissue completely fills the endometrial cavity (Fig. 37-3).
Clinically, the presentation of a complete mole has changed considerably. In the 1960s and 1970s, more than half of affected patients had anemia and uterine sizes in excess of that predicted for their gestational age. In addition, hyperemesis gravidarum, preeclampsia, and theca-lutein cysts developed in approximately one quarter of women (Soto-Wright, 1995). As described in Chapter 9, theca-lutein cysts develop with prolonged exposure to luteinizing hormone (LH) or β-hCG (Fig. 37-4). These cysts range in size from 3 to 20 cm, and most regress with falling β-hCG titers after molar evacuation. If such cysts are present, and especially if they are bilateral, the risk of postmolar GTN is increased.
Complete moles, however, infrequently present today with these traditional signs and symptoms (Mangili, 2008). As a result of β-hCG testing and sonography, the mean gestational age at evacuation currently approximates 12 weeks, compared with 16 to 17 weeks in the 1960s and 1970s (Drake, 2006; Soto-Wright, 1995). A large proportion of patients are asymptomatic at diagnosis (Joneborg, 2014). For the remainder, vaginal bleeding remains the most common presenting symptom, and β-hCG levels are often greater than expected. One quarter of women will present with uterine size greater than dates, but the incidence of anemia is less than 10 percent. Moreover, hyperemesis gravidarum, preeclampsia, and symptomatic theca-lutein cysts are now rare (Soto-Wright, 1995). Currently, these sequelae typically develop chiefly in patients without early prenatal care who present with a more advanced gestational age and markedly elevated serum β-hCG levels. Last, plasma thyroxine levels are often increased in women with complete moles, but clinical hyperthyroidism is infrequent. In these circumstances, serum free thyroxine levels are elevated as a consequence of the thyrotropin-like effect of β-hCG (Chap. 15).
These moles differ from complete hydatidiform moles clinically, genetically, and histologically. The degree and extent of trophoblastic proliferation and villous edema are decreased compared with those of complete moles. Moreover, most partial moles contain fetal tissue and amnion, in addition to placental tissues.
As a result, patients with partial moles typically present with signs and symptoms of an incomplete or missed abortion. Many women will have vaginal bleeding. However, because trophoblastic proliferation is slight and only focal, uterine enlargement in excess of gestational age is uncommon. Similarly, preeclampsia, theca-lutein cysts, hyperthyroidism, or other dramatic clinical features are rare. Preevacuation β-hCG levels are typically much lower than those for complete moles and often do not exceed 100,000 mIU/mL. For this reason, partial moles are often not identified until after a histologic review of a curettage specimen.
Partial moles have a triploid karyotype (69,XXX, 69,XXY, or less commonly 69,XYY) that is composed of one maternal and two paternal haploid sets of chromosomes (see Fig. 37-2) (Lawler, 1991). The coexisting fetus present with a partial mole is nonviable and typically has multiple malformations with abnormal growth (Jauniaux, 1999).
In reproductive-aged women with vaginal bleeding, diagnoses may include gynecologic causes of bleeding and complications of first-trimester pregnancy. The trophoblast of molar pregnancies produce β-hCG, and elevated hormone levels reflect their proliferation. Accordingly, initial urine or serum β-hCG measurement and transvaginal sonography are invaluable in guiding evaluation. Because of these, first-trimester diagnosis of hydatidiform mole is now common.
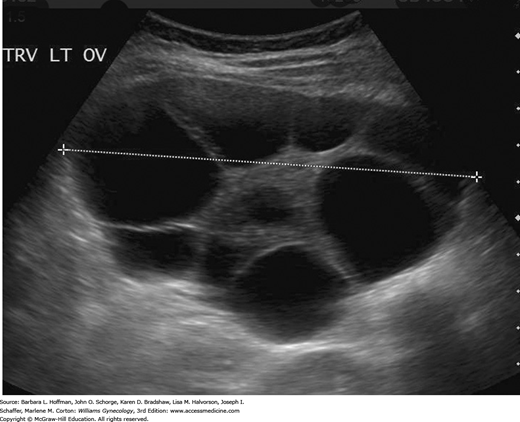
Although β-hCG levels are helpful, the diagnosis of molar pregnancy is more frequently found sonographically. Most first-trimester complete moles demonstrate a complex, echogenic, intrauterine mass containing many small cystic spaces, which reflect swollen chorionic villi. Fetal tissues and amnionic sac are absent (Fig. 37-5) (Benson, 2000). In contrast, sonographic features of a partial molar pregnancy include a thickened, hydropic placenta with a concomitant fetus (Zhou, 2005).
However, there are diagnostic limitations. For example, β-hCG levels in early molar pregnancies may not always be elevated in the first trimester (Lazarus, 1999). Moreover, sonography can lead to a false-negative diagnosis if performed at very early gestational ages, before the chorionic villi have attained their characteristic vesicular pattern. Studies show that only 20 to 30 percent of patients may have sonographic evidence to indicate a partial mole (Johns, 2005; Lindholm, 1999; Sebire, 2001). Consequently, diagnosis in early gestations is usually difficult. Often, the diagnosis commonly is not made until after histologic review of the abortal specimen. In unclear cases with a live fetus and a desired pregnancy, fetal karyotyping to identify a triploid fetal chromosomal pattern can clarify the diagnosis and management.
The histopathologic changes typical of hydatidiform moles are listed in Table 37-2. But, in early pregnancy, it may be difficult to distinguish among these and a hydropic abortus. Hydropic abortuses are pregnancies formed by the traditional union of one haploid egg and one haploid sperm but are pregnancies that have failed. Their placentas display hydropic degeneration, in which villi are edematous and swollen, and thus mimic some villous features of hydatidiform moles (Fig. 37-6). Although no single criterion distinguishes these three, complete moles generally have two prominent features: (1) trophoblastic proliferation and (2) hydropic villi. In gestations younger than 10 weeks, however, hydropic villi may not be apparent, and molar stroma may still be vascular (Paradinas, 1997). As a result, identification of early complete moles must rely on more subtle histologic abnormalities, supplemented by immunohistochemical and molecular diagnostic techniques. Partial moles are optimally diagnosed when three or four major diagnostic criteria are demonstrated: (1) two populations of villi, (2) enlarged, irregular, dysmorphic villi (with trophoblast inclusions), (3) enlarged, cavitated villi (≥3 to 4 mm), and (4) syncytiotrophoblast hyperplasia/atypia (Chew, 2000). Good diagnostic reproducibility can still be achieved in most circumstances using these histologic distinctions of complete and partial mole.
FIGURE 37-6
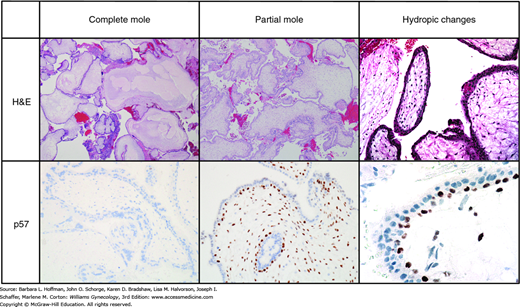
Composite diagram of differences among normal hydropic abortuses and partial or complete hydatidiform moles. The first row shows typical appearances after hematoxylin and eosin (H&E) staining. The second row shows results after p57 staining. p57 is a nuclear protein whose gene product is produced only in tissues containing a maternal allele. After immunostaining for p57, note the positive (brown) staining in the villi of the partial hydatidiform mole and normal hydropic abortus. This contrasts to the absent staining for p57 in the complete mole (only blue counterstain is seen). (Used with permission from Drs. Kelley Carrick and Raheela Ashfaq.)
Histopathologic evaluation can be enhanced by immunohistochemical staining for p57 expression and by molecular genotyping. p57KIP2 is a nuclear protein whose gene is paternally imprinted and maternally expressed. This means that the gene product is produced only in tissues containing a maternal allele. Because complete moles contain only paternal genes, the p57KIP2 protein is absent in complete moles, and tissues do not pick up this stain (Merchant, 2005). In contrast, this nuclear protein is strongly expressed in normal placentas, in spontaneous pregnancy losses with hydropic degeneration, and in partial hydatidiform moles (Castrillon, 2001). Accordingly, immunostaining for p57KIP2 is an effective means to isolate complete mole from the diagnostic list. For distinction of a partial mole from a nonmolar hydropic abortus, both of which express p57, molecular genotyping can be used. Molecular genotyping determines the parental source of polymorphic alleles. Thereby, it can distinguish among a diploid diandric genome (complete mole), a triploid diandric-monogynic genome (partial mole), or biparental diploidy (nonmolar abortus) (Ronnett, 2011).
Suction curettage is the preferred method of evacuation regardless of uterine size in patients who wish to remain fertile (American College of Obstetricians and Gynecologists, 2014; Tidy, 2000). Nulliparous women are not given prostanoids to ripen the cervix since these drugs can induce uterine contractions and might increase the risk of trophoblastic embolization to the pulmonary vasculature (Seckl, 2010). Hysterectomy is rarely recommended unless the patient wishes surgical sterilization or is approaching menopause (Elias, 2010). Symptomatic theca-lutein ovarian cysts are an unusual finding and tend to regress after molar evacuation. In extreme cases, these may be aspirated, but oophorectomy is not performed except when torsion leads to extensive ovarian infarction (Mungan, 1996).
Prior to surgery, patients are evaluated for associated medical complications. Fortunately, thyroid storm from untreated hyperthyroidism, respiratory insufficiency from trophoblastic emboli, and other severe coexisting conditions are rare. Because of the tremendous vascularity of these placentas, blood products should be available prior to the evacuation of larger moles, and adequate infusion lines established.
At the beginning of the evacuation, the cervix is dilated to admit a 10- to 12-mm plastic suction curette. As aspiration of molar tissues ensues, intravenous oxytocin is given. At our institution, 20 units of synthetic oxytocin (Pitocin) are mixed with 1 L of crystalloid and infused at rates to achieve uterine contraction. In some cases, intraoperative sonography may be indicated to help reduce the risk of uterine perforation and assist in confirming complete evacuation. Finally, a thorough, gentle curettage is performed.
Following curettage, because of the possibility of partial mole and its attendant fetal tissue, Rh immune globulin is given to nonsensitized Rh D-negative women. Rh immune globulin, however, may be withheld if the diagnosis of complete mole is certain (Fung Kee, 2003).
Gestational trophoblastic neoplasia develops after evacuation in 15 percent of patients with complete moles (Golfier, 2007; Wolfberg, 2004). Despite the trend of diagnosing these abnormal pregnancies at earlier gestational ages, this incidence has not declined (Seckl, 2004). Of those women who develop GTN, three quarters have locally invasive molar disease and the remaining one quarter develop metastases. In contrast, GTN develops in only 4 to 6 percent of patients with partial moles following evacuation (Feltmate, 2006; Lavie, 2005). Malignant transformation into metastatic choriocarcinoma does occur after partial mole evacuation, but this is rare (0.1 percent) (Cheung, 2004; Seckl, 2000).
No pathologic or clinical features at presentation accurately predict which patients will ultimately develop GTN. Because of the trophoblastic proliferation that characterizes these neoplasms, serial serum β-hCG levels following molar evacuation can be used to effectively monitor patients for GTN development. Therefore, postmolar surveillance with serial quantitative serum β-hCG levels is the standard. Titers are monitored following uterine evacuation at least every 1 to 2 weeks until they become undetectable.
After undetectable β-hCG levels are achieved, subsequent monthly levels are drawn during 6 months of surveillance for all patients with a molar gestation (Sebire, 2007). However, poor compliance with prolonged monitoring has been reported—especially among indigent women and certain ethnic groups in the United States (Allen, 2003; Massad, 2000). A single blood sample demonstrating an undetectable level of β-hCG following molar evacuation is sufficient to exclude the possibility of progression to GTN in most patients. Thus, some women, especially those with a partial mole, may be safely discharged from routine surveillance once an undetectable value is achieved (Lavie, 2005; Wolfberg, 2004). Shortened surveillance could enable women to attempt a subsequent pregnancy sooner. However, GTN may still rarely develop after an hCG level has returned to normal, potentially leading to increased morbidity (Kerkmeijer, 2007; Sebire, 2007).
When pregnancies occur during the monitoring period, the resulting normal β-hCG production can hinder detection of postmolar progression to GTN (Allen, 2003). But other than complicating the monitoring schedule, these pregnancies fortunately are otherwise uneventful (Tuncer, 1999). To prevent difficulties with interpretation, women are encouraged to use effective contraception until achieving a β-hCG titer <5 mIU/mL or below the individual assay’s threshold. COC use decreases the likelihood of pregnancy compared with less effective barrier contraception and does not increase the risk of GTN (Costa, 2006; Gaffield, 2009). Injectable medroxyprogesterone acetate is particularly useful when poor compliance is anticipated (Massad, 2000). In contrast, intrauterine devices are not inserted until the β-hCG level is undetectable because of the risk of uterine perforation if an invasive mole is present.
At the time of molar evacuation, chemotherapy can be administered to help prevent GTN development in high-risk patients who are unlikely to be compliant or for whom β-hCG surveillance is not available. In clinical practice, however, correctly categorizing a woman as high-risk for GTN development is difficult, as no combination of risk factors is universally accepted. Typical patients have complete moles and multiple risk factors, such as age >40 years, previous history of molar pregnancy, or an excessively high β-hCG titer prior to evacuation. That said, few women are ultimately assigned to this group. Moreover, due to the risks of increased drug resistance, delayed treatment of GTN, and toxic side effects, this practice cannot currently be recommended (American College of Obstetricians and Gynecologists, 2014; Fu, 2012). Prophylactic chemotherapy is not routinely offered in the United States and Europe.
However, a single dose of dactinomycin has been shown to reduce the incidence of postmolar GTN in certain populations. For example, in one randomized trial, 60 Thai women who had high-risk complete moles were assigned to receive either prophylactic dactinomycin or placebo at the time of evacuation (Limpongsanurak, 2001). Adjuvant chemotherapy reduced the incidence of GTN from 50 percent to 14 percent, but toxicity was significant. As a result, prophylactic chemotherapy is generally used only in those countries with limited resources to reliably monitor patients after evacuation (Uberti, 2009).
The true incidence of GTD developing outside the uterine cavity approximates 1.5 per 1 million births (Gillespie, 2004). More than 90 percent of suspected cases will reflect an overdiagnosis of florid extravillous trophoblastic proliferation in the fallopian tube (Burton, 2001; Sebire, 2005b). As with any ectopic pregnancy, initial management usually involves surgical removal of the conceptus and histopathologic evaluation.
At times, a twin pregnancy can consist of a hydatidiform mole and a coexisting fetus. The estimated incidence is 1 per 20,000 to 100,000 pregnancies (Fig. 37-7). Sebire and associates (2002b) described the outcome of 77 twin pregnancies, each composed of a complete mole and a healthy cotwin. Of this group, 24 women chose to have an elective termination, and 53 continued their pregnancies. Twenty-three gestations spontaneously aborted at less than 24 weeks, two were terminated due to severe preeclampsia, and 28 pregnancies lasted at least 24 weeks—resulting in 20 live births. The authors demonstrated that coexisting complete moles and healthy cotwin pregnancies have a high risk of spontaneous abortion, but approximately 40 percent result in live births. The risk of progression to GTN was 16 percent in first-trimester terminations and not significantly higher (21 percent) in women who continued their pregnancies. Because the risk of malignancy is unchanged with advancement of gestational age, pregnancy continuation may be allowed, provided that severe maternal complications are controlled and fetal growth is normal. Importantly, these cases should be distinguished early from a single partial molar pregnancy with its abnormal associated fetus. Fetal karyotyping to confirm a normal fetal chromosomal pattern is also recommended (Marcorelles, 2005; Matsui, 2000).
FIGURE 37-7
Photograph of placentas from a twin pregnancy with one normal twin and with a complete mole. The complete mole (left) shows the characteristic vesicular structure. The placenta on the right appears grossly normal. A transverse section through the border between these two is shown (inset). (Used with permission from Drs. April Bleich and Brian Levenson.)
GESTATIONAL TROPHOBLASTIC NEOPLASIA
This term primarily encompasses pathologic entities that are characterized by aggressive invasion of the endometrium and myometrium by trophoblast cells. Histologic categories include common tumors such as the invasive mole and gestational choriocarcinoma, as well as the rare placental-site trophoblastic tumor and epithelioid trophoblastic tumor. Although these histologic types have been characterized, in most cases of GTN, no tissue is available for pathologic study. Instead, GTN is diagnosed based on elevated β-hCG levels and managed clinically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree