INTRODUCTION
In the United States, ovarian cancer accounts for more deaths than all other gynecologic malignancies combined. Worldwide each year, more than 225,000 women are diagnosed, and 140,000 women die from this disease (Jemal, 2011). Of these, epithelial ovarian carcinomas make up 90 to 95 percent of all cases, including the more indolent low-malignant-potential (borderline) tumors (Quirk, 2005). The remainder includes germ cell and sex cord-stromal tumors, which are described in Chapter 36. Due to the similarities of primary peritoneal carcinomas and fallopian tube cancers, they are included within this section for simplicity.
Approximately one quarter of patients will have stage I disease and an excellent long-term survival rate. However, there are no effective screening tests for ovarian cancer and few notable early symptoms. As a result, two thirds of patients have advanced disease when they are diagnosed. Aggressive debulking surgery, followed by platinum-based chemotherapy, usually results in clinical remission. However, up to 80 percent of these women will develop a relapse that eventually leads to disease progression and death.
EPIDEMIOLOGY AND RISK FACTORS
One in 78 American women (1.3 percent) will develop ovarian cancer during her lifetime. Because the incidence has slowly declined since the early 1990s, ovarian cancer is now the ninth leading cause of cancer in women. In 2015, 21, 290 new cases and 14,180 deaths are expected, and ovarian cancer remains the fifth leading cause of cancer-related death (Siegel, 2015). Overall, the average age at diagnosis is in the early 60s.
Numerous reproductive, environmental, and genetic risk factors have been associated with ovarian cancer (Table 35-1). The most important is a family history of breast or ovarian cancer, and approximately 10 percent of patients have an inherited genetic predisposition. For the other 90 percent with no identifiable genetic link for their ovarian cancer, most risks are related to a pattern of uninterrupted ovulatory cycles during the reproductive years (Pelucchi, 2007). Repeated stimulation of the ovarian surface epithelium is hypothesized to lead to malignant transformation (Schildkraut, 1997).
|
Nulliparity is associated with long periods of repetitive ovulation, and patients without children have double the risk of developing ovarian cancer (Purdie, 2003). Among nulliparous women, those with a history of infertility have an even higher risk. Although the reasons are unclear, it is more likely to be an inherent ovarian predisposition rather than an iatrogenic effect of ovulation-inducing drugs. For example, women treated for infertility who successfully achieve a live birth do not have an increased risk of ovarian cancer (Rossing, 2004). In general, risks decrease with each live birth, eventually plateauing in women delivering five times (Hinkula, 2006). One theory suggests that pregnancy may induce premalignant ovarian cell shedding (Rostgaard, 2003).
Early menarche and late menopause are also associated risks. In contrast, breastfeeding has a protective effect, perhaps by prolonging amenorrhea (Yen, 2003). Presumably by also preventing ovulation, long-term combination oral contraceptive use reduces the risk of ovarian cancer by 50 percent. The duration of protection lasts up to 25 years after the last use (Riman, 2002). In contrast, hormone replacement therapy after menopause has an elevated associated risk (Lacey, 2006; Mørch, 2009).
White women have the highest incidence of ovarian cancer among all racial and ethnic groups (Quirk, 2005). Compared with that of black and Hispanic women, the risk is elevated by 30 to 40 percent (Goodman, 2003). Although exact reasons are unknown, racial discrepancies in parity and rates of gynecologic surgery may account for some of the differences.
Tubal ligation and hysterectomy are each associated with a substantial reduction in risk (Rice, 2014). Theoretically, any gynecologic procedure that precludes irritants from reaching the ovaries via ascension from the lower genital tract might plausibly exert a similar protective effect. In turn, women who regularly use perineal talc may possibly have an elevated risk (Gertig, 2000; Houghton, 2014; Rosenblatt, 2011).
Age is another risk, and the overall incidence of ovarian cancer rises with age up to the mid-70s and then declines slightly among women beyond 80 years (Goodman, 2003). In general, aging allows an extended period to accumulate random genetic alterations within the ovarian surface epithelium.
Women residing in North America, Northern Europe, or any industrialized Western country have a higher ovarian cancer risk. Globally, the incidence varies greatly, but developing countries and Japan have the lowest rates (Jemal, 2011). Regional dietary habits may be partly responsible (Kiani, 2006). For example, consumption of foods low in fat but high in fiber, carotene, and vitamins appears protective (Zhang, 2004).
A family history of ovarian cancer in a first-degree relative, that is, a mother, daughter, or sister, triples a woman’s lifetime risk. The risks further escalate with two or more afflicted first-degree relatives, or with other individuals with premenopausal breast cancer. If a family history is mainly composed of colon cancer, clinicians may consider Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC). Patients with this syndrome have a high lifetime risk of colon cancer (85 percent) and ovarian cancer (10 to 12 percent). Because the predominant gynecologic malignancy is endometrial cancer (40 to 60 percent lifetime risk), HNPCC is described in more detail in Chapter 33.
More than 90 percent of inherited ovarian cancers result from germline mutations in the BRCA1 or BRCA2 genes. Thus, any patient with a personal history of epithelial ovarian cancer or breast cancer in certain circumstances, or from a family with a known deleterious mutation, should undergo testing (Table 35-2) (Daly, 2014).
|
Typically, a patient is referred to a certified genetic counselor, and a comprehensive pedigree is constructed first. Then, risk assessment is performed using one of several validated population models. These include the BRCAPRO and Tyrer-Cuzick programs, which are available, respectively, at: http://www4.utsouthwestern.edu/breasthealth/cagene/default.asp, and by contacting the International Breast Cancer Intervention Study (IBIS) at ibis@cancer.org.uk. These models and their associated software allow quantification of an individual’s risk for carrying a germline deleterious mutation of the BRCA1 and BRCA2 genes (Euhus, 2002; James, 2006; Parmigiani, 2007). However, assessment of family history, even by a validated model, cannot effectively target testing to a high-risk ovarian cancer patient population, which strongly supports the recommendation to offer BRCA1/BRCA2 genetic testing to all patients with high-grade serous ovarian cancer regardless of family history (Daniels, 2014; Norquist, 2013).
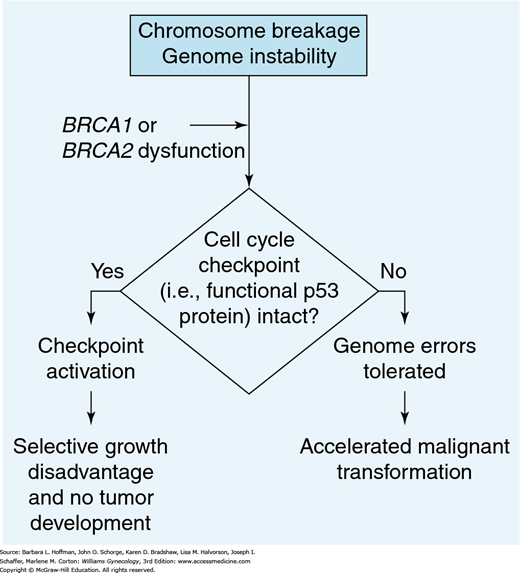
These are two tumor-suppressor genes, whose protein products are BRCA1 and BRCA2. These two proteins interact with recombination/DNA repair proteins to preserve intact chromosomal structure. Mutations of BRCA1 and BRCA2 genes lead to BRCA1 and BRCA2 protein dysfunction, which results in genetic instability and subjects cells to a higher risk of malignant transformation (Fig. 35-1) (Deng, 2006; Scully, 2000).
FIGURE 35-1
Diagram describing the role of the BRCA mutation in tumor development. Cells with damaged DNA are frequently blocked at checkpoints along the cell cycle and thereby prohibited from moving to the mitotic phase. If these checkpoints are nonfunctional, then these genomic errors may be tolerated and lead to malignant transformation. (Reproduced with permission from Scully R, Livingston DM: In search of the tumour-suppressor functions of BRCA1 and BRCA2, Nature 2000 Nov 23;408(6811):429–432.)
The BRCA1 gene is located on chromosome 17q21. Patients with a proven mutation have a dramatically elevated risk of developing ovarian cancer (39 to 46 percent). BRCA2 is located on chromosome 13q12 and in general is less likely to lead to ovarian cancer (12 to 20 percent). The estimated lifetime risk of breast cancer with a BRCA1 or BRCA2 mutation is 65 to 74 percent (American College of Obstetricians and Gynecologists, 2013; Chen, 2006; Risch, 2006). Both genes are inherited in an autosomal dominant fashion, but with variable penetrance. In essence, a carrier has a 50:50 chance of passing the gene to a son or daughter, but it is uncertain whether an individual with the gene mutation will actually develop breast or ovarian cancer. As a result, manifestations of BRCA1 or BRCA2 mutations can appear to skip generations.
Ideally, genetic testing identifies women with deleterious BRCA1 and BRCA2 mutations, leads to intervention with prophylactic surgery, and thereby prevents ovarian cancer. Three distinct results are possible with this testing. A “positive” test suggests the presence of a deleterious mutation. The most common are the three “Jewish founder” mutations: 185delAG or 5382insC in BRCA1 and 6174delT in BRCA2. Each of these “frameshift” mutations significantly alters the downstream amino acid sequence, resulting in alteration of the BRCA1 or BRCA2 tumor suppressor protein. As suggested, these three specific mutations are thought to have originated from within the Ashkenazi population thousands of years ago. Although Jewish founder mutations are most common, any frameshift mutation within the BRCA genes may result in a deleterious predisposition to developing breast and ovarian cancer.
Second, “variants of uncertain clinical significance” may actually be pathogenic (true mutations) or just polymorphisms (normal variants found in at least 1 percent of alleles in the general population). These unclassified variants are common, representing approximately one third of BRCA1 test results and half of those for BRCA2. Most are missense mutations, which result in a single amino acid change in the protein, without a frameshift. Given the prognostic uncertainty and high rate of reclassification, individualized counseling and directing efforts toward surveillance, chemoprevention, or salpingectomy are recommended (Garcia, 2014).
The third potential and most reassuring genetic test result is “negative.” However, due to the large size of the BRCA1 and BRCA2 genes, the false-negative rate is 5 to 10 percent. To capture additional, otherwise undetected mutations, reflex testing of large genomic rearrangements is available for high-risk patients (Palma, 2008).
PREVENTION
In addition to genetic testing, other screening strategies for ovarian cancer have been evaluated. However, despite enormous effort, there is no proof that routine screening with serum markers, sonography, or pelvic examinations decreases mortality rates (American College of Obstetricians and Gynecologists, 2013; Morgan, 2014; Schorge, 2010a). Hundreds of possible markers have been identified, yet no test currently available approaches sufficient levels of accuracy (American College of Obstetricians and Gynecologists, 2011).
For the most part, screening strategies are directed at BRCA1 or BRCA2 carriers, in addition to women with a strong family history of breast and ovarian cancer. Most commonly, cancer antigen 125 (CA125) level measurements and/or transvaginal sonography have been tested, albeit with marginal success. Thus, in BRCA1 or BRCA2 mutation carriers who do not wish to undergo prophylactic surgery, a screening strategy that combines thorough pelvic examination, transvaginal sonographic evaluation, and CA125 blood testing may be offered (American College of Obstetricians and Gynecologists, 2013).
CA125 is a glycoprotein that is not produced by normal ovarian epithelium but that may be produced by both benign and malignant ovarian tumors. CA125 is synthesized within affected ovarian epithelial cells and often secreted into cysts. In benign tumors, excess antigen is released into and may accumulate within cyst fluid. Hypothetically, abnormal tissue architecture associated with malignant tumors allows antigen release into the vascular circulation (Verheijen, 1999).
Alone, CA125 is not a useful marker for detecting ovarian cancer. However, a more sensitive Risk of Ovarian Cancer Algorithm (ROCA) has been developed and is based on the slope of serial CA125 measurements drawn at regular intervals (Skates, 2003). If a ROCA score exceeds a 1-percent risk of having ovarian cancer, patients then undergo transvaginal sonography to determine whether additional intervention is warranted. This strategy is currently being studied in a prospective, international trial of 2605 high-risk women who initially chose to undergo either risk-reducing salpingo-oophorectomy or screening alone (Greene, 2008).
Because no sufficiently accurate early detection tests are currently available, routine screening for women at average risk is not recommended (Moyer, 2012). For example, in the United States’ prospective Prostate, Lung, Colorectal and Ovarian (PLCO) Trial of screening versus usual care, 34,261 women without prior oophorectomy were randomly assigned to annual CA125 level measurement and transvaginal sonographic examination. Of those with an abnormal screen, approximately 1 percent had invasive ovarian cancer, demonstrating a relatively low predictive value of both tests (Buys, 2005, 2011; Partridge, 2009).
To evaluate the efficacy, cost, morbidity, compliance, and acceptability of ROCA-based CA125 screening and study-directed sonography, a randomized trial of 202,638 patients was conducted. In The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), asymptomatic, average-risk postmenopausal women aged 50 to 74 years were randomly assigned to no treatment, to annual CA125 screening with transvaginal sonography as a second-line test if indicated by ROCA interpretation, or to annual screening with transvaginal sonography. The ROCA-directed approach demonstrated a 35-percent positive-predictive value, more than 10 times higher than annual sonography (3 percent). Although in this study ROCA-directed sonography was shown to be feasible, the results of ongoing screening to determine whether there is any meaningful effect on mortality rates will be available in 2015 (Menon, 2009, 2014). Despite most major professional and government groups recommending against it, approximately a third of U.S. physicians continue to order CA125 or sonography to screen for ovarian cancer (Baldwin, 2012).
To identify a more accurate screening test for early ovarian cancer detection, various potential biomarkers have been described. Dozens have been evaluated alone and in combination with CA125 (Cramer, 2011; Yurkovetsky, 2010).
One example, based on a preliminary study published in 2002, suggested that proteomics may help detect early-stage ovarian cancer (Petricoin, 2002). By profiling the patterns of thousands of proteins with a high degree of sensitivity and specificity, it was hoped that an accurate test, such as OvaCheck, would reliably distinguish those with early ovarian cancer from unaffected women.
Another entry, the OvaSure blood test, has also generated enthusiasm. Based on the simultaneous evaluation of six analytes (leptin, osteopontin, insulin-like growth factor-II, macrophage inhibitory factor, and CA125), it was reported to yield high sensitivity and specificity for ovarian cancer (Mor, 2005; Visintin, 2008).
Importantly, prospective clinical trials must be designed and completed before any of these new diagnostic tests can be offered outside of a trial. Unfortunately, neither proteomics nor any other screening strategy is currently near implementation into routine clinical practice.
In general, pelvic examination only occasionally detects ovarian cancer, generally when the disease is already in advanced stages. In asymptomatic women, there is no evidence that it lowers mortality or morbidity rates as a screening test (Bloomfield, 2014). As a result, bimanual examination was not even included as a screening modality in either the PLCO or UKCTOCS trials.
Oral contraceptive use is associated with a 50-percent decreased risk of developing ovarian cancer. However, there is a short-term increased risk of developing breast cancer and cervical cancer that should be considered when counseling patients (International Collaboration of Epidemiological Studies of Cervical Cancer, 2006, 2007; National Cancer Institute, 2014a).
The only proven way to directly prevent ovarian cancer is surgical removal. In BRCA1 or BRCA2 carriers, prophylactic bilateral salpingo-oophorectomy (BSO) may be performed either upon completion of childbearing or by age 40 years (American College of Obstetricians and Gynecologists, 2013, 2014). In these patients, the procedure is approximately 90-percent effective in preventing epithelial ovarian cancer (Kauff, 2002; Rebbeck, 2002). Prophylactic BSO reduces the risk of developing breast cancer by 50 percent (Rebbeck, 2002). Predictably, the protective effect is strongest among premenopausal women (Kramer, 2005). In women with HNPCC, the ovarian cancer risk reduction approaches 100 percent (Schmeler, 2006). Yet, significant adverse consequences accompany premature menopause. Moreover, recent studies suggest that a substantial proportion of “ovarian cancers” in high-risk women actually arise from precursor lesions located in the distal fallopian tube. Thus, prophylactic salpingectomy followed later by postmenopausal oophorectomy may be a safe alternative (Holman, 2014; Kwon, 2013; Perets, 2013). Surgical excision ideally removes the entire tube from fimbria to uterotubal junction, but the interstitial portion within the myometrium remains.
The term prophylactic implies that the tubes and ovaries are normal at the time of removal. However, approximately 4 to 5 percent of BRCA mutation carriers undergoing prophylactic BSO will have an otherwise undetected, often microscopic, cancer at the time of surgery (Sherman, 2014). In fact, the distal fallopian tube seems to be the dominant site of origin for occult malignancies detected during risk-reducing surgery (Callahan, 2007). To account for this possibility, cytologic washings, peritoneal biopsies, and an omental sample may be routinely collected during surgery. When submitting the final surgical specimen, the pathology requisition should clearly state that the BSO was performed for a prophylactic indication. In these cases, the ovaries and tubes, especially the fimbria, undergo more intensive scrutiny and are serially microsectioned to identify occult disease. Using a rigorous operative and pathologic protocol such as this can significantly increase the detection rate of occult tubal or ovarian malignancy in BRCA mutation carriers (Powell, 2005). Typically, the excision, washings, and biopsies can all be completed by laparoscopic surgery.
Prophylactic BSO in young women will induce premature menopause and its associated effects of vasomotor and urogenital symptoms, decline in sexual interest, and osteoporosis (National Cancer Institute, 2014a). Estrogen replacement therapy is commonly used to alleviate these symptoms but may be less effective than is often assumed (Madalinska, 2006). Overall, mainly due to the favorable impact in reducing cancer worries, prophylactic BSO does not adversely affect quality of life (Madalinska, 2005).
In women with the HNPCC syndrome, hysterectomy is mandatory when performing prophylactic BSO because of coexisting endometrial cancer risks. In BRCA mutation carriers, it should not be recommended routinely (Vyarvelska, 2014). Few reports have suggested a meaningful association between BRCA mutations and an increased risk of endometrial cancer. Mainly, these develop in patients taking tamoxifen for breast cancer treatment or breast cancer chemoprevention (Beiner, 2007).
In low-risk patients who are not BRCA carriers, risk-reducing salpingectomy is now also considered in those undergoing hysterectomy or permanent sterilization, in hopes of preventing pelvic serous cancers (Creinin, 2014; Lessard-Anderson, 2014; McAlpine, 2014; Morelli, 2013). This consideration has been endorsed by both the Society of Gynecologic Oncology (2013) and the American College of Obstetricians and Gynecologists (2015). Pathologic specimen processing in low-risk women includes representative sections of the tube, any suspicious lesions, and entire sectioning of the fimbriae. Neither organization specifies pelvic washing collection in this low-risk population.
LOW-MALIGNANT-POTENTIAL TUMORS
Ten to 15 percent of epithelial ovarian cancers have histologic and biologic features that are intermediate between clearly benign cysts and frankly invasive carcinomas. In general, these low-malignant-potential (LMP) tumors, also termed borderline tumors, are associated with risk factors that are similar to those for epithelial ovarian cancer (Huusom, 2006). Typically, they are not considered part of any of the hereditary breast-ovarian cancer syndromes. Although LMP tumors may develop at any age, on average, patients are in their mid-40s, which is 15 years younger than women with invasive ovarian carcinoma. For various reasons, their diagnosis and optimal management are frequently problematic.
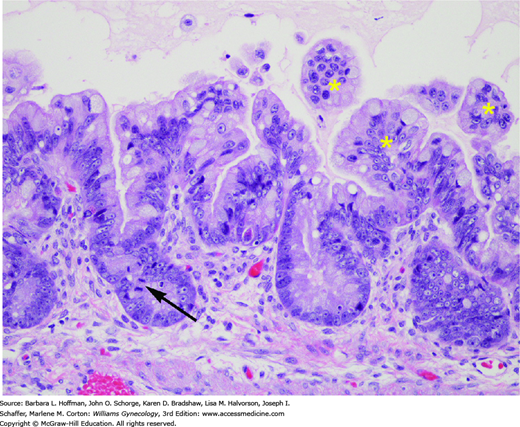
Histologically, LMP tumors are distinguished from benign cysts by having at least two of the following features: nuclear atypia, epithelial stratification, microscopic papillary projections, cellular pleomorphism, or mitotic activity (Fig. 35-2). Unlike invasive carcinomas, LMP tumors lack stromal invasion. However, up to 10 percent of LMP tumors will exhibit areas of microinvasion, defined as foci measuring <3 mm in diameter and forming <5 percent of the tumor (Buttin, 2002). Due to the subtle nature of many of these findings, it is challenging to diagnose an LMP tumor with certainty based on frozen section specimen analysis.
FIGURE 35-2
Mucinous borderline tumor. These tumors are distinguished from benign mucinous cystadenomas by the presence of epithelial proliferation and nuclear atypia. This example of a mucinous borderline tumor has mild to moderate nuclear atypia as evidenced by limited nuclear pleomorphism and visible nucleoli. A mitotic figure is also seen (arrow). Epithelial proliferation is indicated by epithelial tufts (asterisks), which are unsupported by fibrovascular cores. (Used with permission from Dr. Kelley Carrick.)
Ovarian LMP tumors present similar to other adnexal masses. Patients may have pelvic pain, distention, or increasing abdominal girth. Alternatively, an asymptomatic mass may be palpated during routine pelvic examination. These tumors are occasionally detected as an incidental finding during routine obstetric sonographic examination or at the time of cesarean delivery.
As with other ovarian tumors, size varies widely. Preoperatively, there is no pathognomonic sonographic appearance, and serum CA125 levels are nonspecific. Depending on the clinical setting, computed tomography (CT) scanning may be indicated to exclude ascites or omental caking, which would suggest a more typical ovarian cancer. Regardless, any woman with a suspicious adnexal mass should have it removed.
Surgery is the cornerstone management for LMP tumors. The operative plan will vary, depending on circumstances, and patients are carefully counseled beforehand. All women should be prepared for complete ovarian cancer surgical staging or debulking, if necessary. In many cases, a laparoscopic approach is appropriate. If laparotomy is planned, then a vertical incision is selected to allow access to the upper abdomen and paraaortic nodes, if needed, for cancer staging.
During surgery, peritoneal washings are immediately collected upon entrance into the abdomen, followed by exploration. The ovarian mass is removed intact and submitted for pathologic consultation and frozen section evaluation. However, it is almost impossible to know with certainty whether a patient has a benign adnexal mass, LMP tumor, or invasive ovarian cancer until final histologic slides have been reviewed (Houck, 2000; Tempfer, 2007). Accordingly, in those with LMP diagnosed intraoperatively, premenopausal women who have not completed childbearing may undergo fertility-sparing surgery with preservation of the uterus and contralateral ovary (Park, 2009; Zanetta, 2001). This is a reasonable approach even if the final diagnosis shows invasive stage I cancer (Schilder, 2002). Alternatively, postmenopausal women should undergo hysterectomy with BSO.
Limited staging biopsies of the peritoneum and omentum are considered, although they rarely contain microscopic foci of metastatic LMP unless the tissues appear abnormal (Kristensen, 2014). Additionally, the appendix is also examined and potentially removed, especially if the tumor has mucinous histology (Timofeev, 2010). In the absence of enlarged nodes or a frozen section suggestive of frankly invasive disease, routine pelvic and paraaortic lymph node dissection may not be necessary (Rao, 2004).
LMP tumors are staged with the same FIGO criteria used for invasive ovarian cancer. For the most part, surgical staging has limited value in altering the prognosis of those with LMP tumors unless invasive cancer is ultimately diagnosed (Wingo, 2006). Although 97 percent of gynecologic oncologists advocate comprehensive surgical staging of LMP tumors, in current practice it is performed in only 12 percent of patients (Lin, 1999; Menzin, 2000). This disparity stems from the fact that often the diagnosis is not suspected intraoperatively, no frozen section is requested or it is inaccurate, and a clinician is alerted only when the final pathology report has been completed. In this circumstance, consultation with a gynecologic oncologist is recommended, but comprehensive surgical restaging is not necessarily required if the tumor appears confined to a single ovary (Zapardiel, 2010). However, if a cystectomy has been performed, the risk of residual disease should prompt a discussion regarding removal of the entire adnexa with washings and limited staging (Poncelet, 2006).
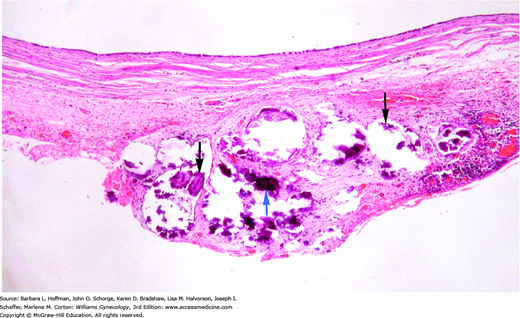
For patients with stage II-IV disease, usually demonstrated by noninvasive implants (Fig. 35-3) or nodal metastases, the utility of adjuvant chemotherapy is speculative (Shih, 2010; Sutton, 1991). The most worrisome finding is invasive peritoneal implants. In general, these patients are treated like those with typical epithelial ovarian carcinoma, including debulking and postoperative chemotherapy (Leary, 2014).
FIGURE 35-3
Noninvasive implant
from patient with ovarian serous borderline tumor. A noninvasive implant does not have destructive invasion of the underlying tissue. In this noninvasive implant, proliferative serous-type epithelium (black arrows) and psammoma bodies (blue arrow) typical of serous proliferations appear to adhere to the peritoneal tissue, but do not invade it. Psammoma bodies are fragmented in this tissue, as calcified material often shatters when sectioned if not decalcified prior to sectioning. (Used with permission from Dr. Raheela Ashfaq.)
The prognosis is excellent for patients with ovarian LMP tumors. Five-year survival rates range from 96 to 99 percent for stages I-III, whereas it reaches 77 percent for stage IV disease (Trimble, 2002). Overall, more than 80 percent have stage I disease, and if treated by hysterectomy and BSO, stage I tumors rarely, if ever, recur (du Bois, 2013). In fact, such women have an overall survival similar to the general population (Hannibal, 2014). Fertility-sparing surgery is associated with up to a 15-percent risk of relapse, usually in the contralateral ovary, but remains highly curable by reoperation and resection (Park, 2009; Rao, 2005).
Approximately 15 percent of LMP tumors have stage II and III disease, almost invariably of serous histology. Stage IV ovarian LMP tumors account for fewer than 5 percent of diagnoses and have the worst prognosis (Trimble, 2002). For these advanced-stage tumors, the most reliable prognostic indicators are the presence of invasive peritoneal implants or residual disease after surgery (Morice, 2014; Seidman, 2000).
Due to the indolent nature of these tumors, symptomatic recurrence often takes place years or even decades after diagnosis (Silva, 2006). Approximately 70 percent of relapses have only LMP histology. Malignant transformation into an invasive ovarian cancer develops in the other 30 percent. Most of these are low-grade carcinomas, but approximately one third will have high-grade features, which adversely affects prognosis (du Bois, 2013; Harter, 2014). As in primary ovarian LMP tumors, complete surgical excision is the most effective therapy for recurrent disease (Crane, 2015). Chemotherapy is reserved for patients with invasive features, but low-grade tumors tend to be particularly resistant to standard agents, such as carboplatin and paclitaxel. Typically, multiple different regimens are used, including hormonal therapy (Gourley, 2014).
EPITHELIAL OVARIAN CANCER
There are at least three distinct tumorigenic pathways to account for the heterogeneity of epithelial ovarian cancer. First, relatively few cases seem to arise from an accumulation of genetic alterations that leads to malignant transformation of benign cysts to LMP tumors and ultimate progression to invasive ovarian carcinoma (Makarla, 2005). Typically, these invasive tumors are low-grade and clinically indolent, and K-ras oncogenic mutations occur early. The ras family of oncogenes includes K-ras, H-ras, and N-ras. Their protein products participate in cell cycle regulation and cell proliferation control. As such, ras mutations are implicated in carcinogenesis by their inhibition of cellular apoptosis and promotion of cellular proliferation (Mammas, 2005).
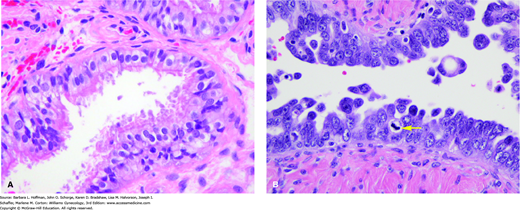
Second, at least 10 percent of epithelial ovarian carcinomas, invariably high-grade serous tumors, result from an inherited predisposition. Women born with a BRCA gene mutation require only one “hit” to the other normal copy (allele) to “knock out” the BRCA tumor-suppressor gene product. As a result, BRCA-related cancers develop approximately 15 years before sporadic cases. Current data suggests that serous tubal intraepithelial carcinoma (STIC) is a precursor condition for a significant percentage of serous carcinomas, which were formerly thought to arise spontaneously on the ovarian or peritoneal surface (Fig. 35-4) (Levanon, 2008; Medeiros, 2006; Perets, 2013). Thereafter, BRCA-related serous cancers appear to have a unique molecular pathogenesis, requiring p53 inactivation to progress (Buller, 2001; Landen, 2008; Schorge, 2000). p53 is a tumor suppressor gene. Its protein product prohibits cells from entering subsequent stages of cell division and thereby halts uncontrolled tumor cell replication. Mutations in p53 are linked with various cancers. In fact, loss of BRCA and p53 protein function has been detected prior to invasion, further supporting its importance as an early triggering event (Werness, 2000).
FIGURE 35-4
A. Normal fallopian tube epithelium is composed of three cell types—ciliated cells, secretory cells, and intercalary cells. B. Serous carcinoma in situ of the fallopian tube. The cells of serous carcinoma lining this tube are markedly atypical, with nuclear pleomorphism, chromatin coarseness, loss of nuclear polarity, mitotic activity (arrow), and epithelial proliferation/tufting. (Used with permission from Dr. Kelley Carrick.)
Third, most carcinomas appear to originate de novo from ovarian surface epithelial cells that are sequestered in cortical inclusion cysts (CICs) within the ovarian stroma. Numerous inciting events and subsequent pathways have been proposed. For example, cyclic repair of the ovarian surface during long periods of repetitive ovulation requires abundant cellular proliferation. In these women, spontaneous p53 mutations arising during the DNA synthesis that accompanies this proliferation appear to play a primary carcinogenetic role (Schildkraut, 1997). Ultimately, the replicative stress and DNA damage transforms the entrapped surface epithelial cells within CICs into any of the histologic ovarian cancer variants (Levanon, 2008).
Ovarian cancer is typically portrayed as a “silent” killer that lacks appreciable early signs or symptoms. This is a misconception. Actually, patients are often symptomatic for several months before the diagnosis, even with early-stage disease (Goff, 2000). The difficulty is distinguishing these symptoms from those that normally occur in women.
In general, persistent symptoms that are more severe or frequent than expected and have a recent onset warrant further diagnostic investigation. Commonly, increased abdominal size, bloating, urinary urgency, and pelvic pain are reported. Additionally, fatigue, indigestion, inability to eat normally, constipation, and back pain may be noted (Goff, 2004). Abnormal vaginal bleeding occurs rarely. Occasionally, patients may present with nausea, vomiting, and a partial bowel obstruction if carcinomatosis is particularly widespread. Unfortunately, many women and clinicians are quick to attribute most symptoms to menopause, aging, dietary changes, stress, depression, or functional bowel problems, and diagnosis is often delayed.
A pelvic or pelvic-abdominal mass is palpable in most patients with ovarian cancer during bimanual evaluation. Malignant tumors tend to be solid, nodular, and fixed, but there are no classic findings that distinguish these growths from benign tumors. Paradoxically, a huge mass filling the pelvis and abdomen more often represents a benign or borderline tumor. To aid surgical planning, a rectovaginal examination is also performed. For example, a woman with cancer involving the rectovaginal septum may need to be positioned in dorsal lithotomy to perform a low anterior colon resection as a part of tumor excision.
The presence of a fluid wave, or less commonly, flank bulging, suggests the presence of significant ascites. In a woman with a pelvic mass and ascites, the diagnosis is ovarian cancer until proven otherwise. However, ascites without an identifiable pelvic mass suggests the possibility of cirrhosis or other primary malignancies such as gastric or pancreatic cancers. In advanced disease, examination of the upper abdomen usually reveals a central mass signifying omental caking.
Auscultation of the chest is also important, since patients with malignant pleural effusions may not be overtly symptomatic. The remainder of the examination includes palpation of the peripheral nodes in addition to a general physical assessment.
A routine complete blood count and metabolic panel often demonstrates a few characteristic features. Of affected women, 20 to 25 percent will present with thrombocytosis (platelet count >400 × 109/L) (Li, 2004). Malignant ovarian cells releasing cytokines are believed to increase platelet production rates. Hyponatremia, typically ranging between 125 and 130 mEq/L, is another common finding. In these patients, tumor secretion of a vasopressin-like substance can cause a clinical picture suggesting a syndrome of inappropriate antidiuretic hormone (SIADH).
The serum CA125 level is integral to epithelial ovarian cancer management. In 90 percent of women presenting with malignant nonmucinous tumors, CA125 levels are elevated. However, there are caveats during adnexal mass evaluation. Half of stage I ovarian cancers will have a normal CA125 measurement (false-negative). Also, an elevated value (false-positive) may be associated with various common benign indications such as pelvic inflammatory disease, endometriosis, leiomyomas, pregnancy, and even menstruation. Thus, in postmenopausal women with a pelvic mass, a CA125 measurement may better predict a higher likelihood of malignancy (Im, 2005).
Another marker, the human epididymal protein 4 (HE4) tumor marker, is approved by the U.S. Food and Drug Administration (FDA), along with CA125, when used in the Risk of Ovarian Malignancy Algorithm (ROMA) to determine the likelihood of finding malignancy at surgery in women with an adnexal mass. The ROMA score is derived from the results of both blood tests, plus menopausal status (Moore, 2009, 2010).
OVA1 is another biomarker blood test panel that may be used for the preoperative triage of women with an identified ovarian mass when surgery is planned (Ueland, 2011; Ware Miller, 2011). Scores ≥5.0 in premenopausal and scores ≥4.4 in postmenopausal women suggest a need for gynecologic oncologist consultation. Importantly, this test is not a screening tool and is reserved for those with a known surgical mass to aid preoperative triage (Vermillion Inc, 2012; Zhang, 2010). Validation studies evaluating ROMA and OVA1 are limited, and their role in preoperative triage is yet to be clearly defined. As a result, they are not necessarily recommended for determining the status of an undiagnosed pelvic mass (Morgan, 2014). Last, when a mucinous ovarian tumor is identified, serum tumor markers that may be better indicators of disease are cancer antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA).
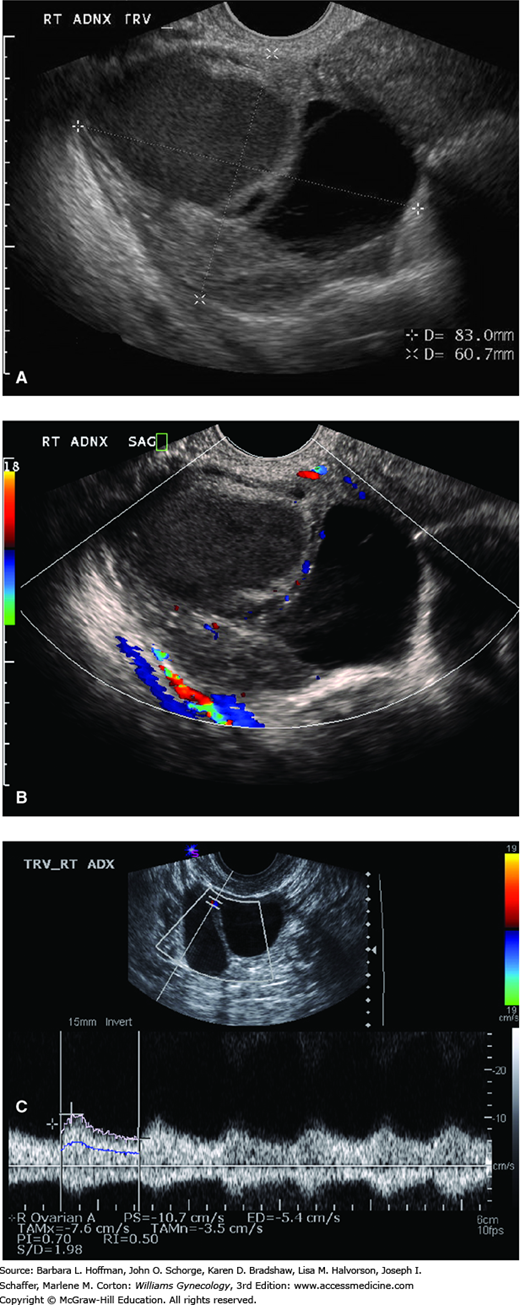
Transvaginal sonography is typically the most useful imaging test to differentiate benign tumors and early-stage ovarian cancers (Chap. 2). In general, malignant tumors are multiloculated, solid or echogenic, and large (>5 cm), and they have thick septa with areas of nodularity (Fig. 35-5A). Other features may include papillary projections or neovascularization—demonstrated by adding color Doppler (Figs. 35-5B and 35-5C). Although several presumptive models have been described in an attempt to distinguish benign masses from ovarian cancers preoperatively, none have been universally implemented (Timmerman, 2005; Twickler, 1999).
FIGURE 35-5
Sonograms of an ovarian cyst. A. Transvaginal sonogram depicts a complex ovarian mass (calipers). Cystic and solid components and a thick intracystic septum are seen. These findings increase clinical concern for malignancy. B. Color Doppler transvaginal sonogram shows neovascularization within this ovarian tumor. C. Transvaginal Doppler study of ovarian mass vessels reveals decreased impedance. (Used with permission from Dr. Diane Twickler.)
In patients with advanced disease, sonography is less helpful. The pelvic sonogram may be particularly difficult to interpret if a large mass encompasses the uterus, adnexa, and surrounding structures. Ascites, if present, is easily detected, but in general, abdominal sonography has limited use.
Of radiographic tests, patients with suspected ovarian cancer should have a chest radiograph to detect pulmonary effusions or infrequently, pulmonary metastases. Rarely, a barium enema is clinically helpful in excluding diverticular disease or colon cancer or in identifying ovarian cancer involvement of the rectosigmoid.
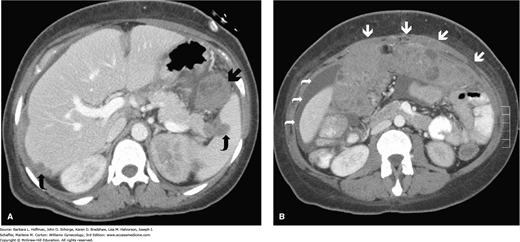
Computed tomography (CT) scanning has a primary role in treatment planning for women with advanced ovarian cancer. Preoperatively, implants in the liver, retroperitoneum, omentum, or other intraabdominal site are detected to thereby guide surgical cytoreduction or demonstrate obviously unresectable disease (Fig. 35-6) (Suidan, 2014). However, CT is not particularly reliable in detecting intraperitoneal disease smaller than 1 to 2 cm in diameter. Moreover, CT scanning accuracy is poor for differentiating a benign ovarian mass from a malignant tumor when disease is limited to the pelvis. In these cases, transvaginal sonography is superior. Other radiologic studies such as magnetic resonance (MR) imaging, bone scans, and positron emission tomography (PET) in general provide limited additional information preoperatively.
FIGURE 35-6
Computed tomographic scans in a woman with ovarian cancer. A. Axial CT scan at the level of the liver and spleen reveals metastatic lesions in the spleen and liver (curved arrows) and a bulky lesion at the splenorenal ligament (arrow). B. More caudal axial CT reveals ascites (curved arrows) and marked omental caking (arrows). (Used with permission from Dr. Diane Twickler.)
A woman with a pelvic mass and ascites can usually be assumed to have ovarian cancer until surgically proven otherwise. Thus, few patients require diagnostic paracentesis. Moreover, this procedure is typically avoided diagnostically as cytologic results are usually nonspecific and abdominal wall metastases may form at the needle entry site (Kruitwagen, 1996). However, paracentesis may be indicated for those with ascites in the absence of a pelvic mass.
Aside from diagnosis, paracentesis may also relieve volume-related symptoms in those with large accumulations. This may be done at the bedside, using connector tubing and vacuum bottles, or completed by an interventional radiologist. Relative dehydration is common afterward and manifest by thirst, oliguria, and short-term creatinine level rise, which all correct with normal oral intake.
Using the currently available diagnostic modalities, clinicians often face tremendous difficulty in distinguishing benign from malignant. However, ascites or evidence of metastases should prompt consultation with an oncologist (American College of Obstetricians and Gynecologists, 2011). Additionally, premenopausal women with elevated CA125 levels (i.e., >200 U/mL) or an OVA1 score ≥5.0 and postmenopausal women with any CA125 level elevation or an OVA1 score ≥4.4 are at higher risk.
Ideally, for patients with suspicious adnexal masses, surgery is performed in a hospital with a pathologist able to reliably interpret an intraoperative frozen section. At minimum, samples for peritoneal cytology are obtained when the abdomen is entered. The mass is then removed intact through an incision that permits thorough staging and resection of possible metastatic sites (American College of Obstetricians and Gynecologists, 2011).
If malignancy is diagnosed, then surgical staging is completed. However, in a study of more than 10,000 women with ovarian cancer, almost half of those with early-stage disease did not undergo the recommended surgical procedures (Goff, 2006
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree