INTRODUCTION
In the United States, endometrial cancer is the most common gynecologic malignancy. Risk factors include obesity and advancing age. As these factors are now more prevalent, the incidence of endometrial cancer continues to increase. Fortunately, patients usually seek medical attention early due to vaginal bleeding, and endometrial biopsy leads quickly to diagnosis. The primary treatment is hysterectomy with bilateral salpingo-oophorectomy (BSO) and staging lymphadenectomy for most women. Three quarters will have stage I disease that is curable by surgery alone. Patients with more advanced disease typically require postoperative combination chemotherapy, radiotherapy, or both.
EPIDEMIOLOGY AND RISK FACTORS
In the United States, women have a 3-percent lifetime risk of developing endometrial cancer. Although an estimated 54,870 new cases were diagnosed, only 10,170 deaths are expected in 2015. As noted, most patients are diagnosed early and subsequently cured. As a result, endometrial cancer is the fourth leading cause of cancer, but the seventh leading cause of cancer deaths among women (Siegel, 2015).
Endometrial adenocarcinomas are categorized as type I or type II based on histology. Type I, that is, endometrioid type, makes up 80 to 90 percent of all cases (Felix, 2010). The other 10 to 20 percent are type II cancers, namely, the non-endometrioid histologic types that include serous and clear cell adenocarcinomas. Risk factors for developing endometrial cancer are numerous (Table 33-1). Risks specifically for type I cancers are associated with an excess-estrogen environment.
|
Of these, obesity is the most common cause of endogenous overproduction of estrogen. Excessive adipose tissue increases peripheral aromatization of androstenedione to estrone. In premenopausal women, elevated estrone levels trigger abnormal feedback in the hypothalamic-pituitary-ovarian axis. The clinical result is oligo- or anovulation. In the absence of ovulation, the endometrium is exposed to virtually continuous estrogen stimulation without subsequent progestational effect and without menstrual withdrawal bleeding.
Unopposed estrogen therapy is the next most important potential inciting factor. Fortunately, the malignant potential of continuously administered estrogen was recognized more than three decades ago (Smith, 1975). Currently, it is rare to encounter a woman with a uterus who has taken unopposed estrogen for years. Instead, combined estrogen plus progestin hormonal replacement therapy (combination HRT) is routinely prescribed for postmenopausal women with a uterus to reduce estrogen-related endometrial cancer risk (Strom, 2006). Moreover, in one study, the endometrial cancer risk was lower in women taking continuous combination HRT for greater than 6 months compared with those women who had never taken HRT (Phipps, 2011).
Menstrual and reproductive influences are commonly associated with endometrial cancer. For example, early age at menarche or late age of menopause are both associated with increased risk (Wernli, 2006). Classically, women with polycystic ovarian syndrome (PCOS) are anovulatory and thus also have an increased risk of developing this cancer (Fearnley, 2010; Pillay, 2006).
Environment is implicated in endometrial cancer in several ways. Women in Western and developed societies have a much higher incidence (Parkin, 2005). Obvious confounding variables within these populations, such as obesity and low parity, account for much of this effect. However, a possible role for nutrition—especially a diet with a high animal-fat content—is another explanation (Goodman, 1997). Immigrant populations tend to assume the risks of native populations within one or two generations, highlighting the importance of environmental influences (Liao, 2003).
Older age is linked with endometrial cancer development. The average age at diagnosis is the early 60s, and overall, approximately 80 percent of these cancers are diagnosed in postmenopausal women older than 55 years (Madison, 2004; Schottenfeld, 1995). Approximately 8 percent of endometrial cancers develop in patients younger than 45 years (Howlader, 2014). Of note, Nevadunsky and associates (2014) found that the age at diagnosis of endometrioid cancer decreased linearly with increasing body mass index (BMI).
Family history is another risk for endometrial cancer. Endometrial cancer is the most common extracolonic manifestation in Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) (Hemminki, 2005). This autosomal-dominant syndrome results primarily from mutations in the mismatch repair genes. The mismatch-repair genes associated with Lynch are MLH1, MSH2, MSH6, and PMS2 (Bansal, 2009). Gene mutation prevents repair of base mismatches, which are commonly produced during DNA replication. Inactivity of this DNA repair system leads to mutations that can promote carcinogenesis. Mutation carriers have a risk of developing endometrial cancer that ranges from 40 to 60 percent. Among affected women, the endometrial cancer risk actually exceeds that for colorectal cancer and often develops at a young age (Aarnio, 1999; Delin, 2004). Of endometrial cancer cases, 2 to 5 percent are attributable to Lynch syndrome (Hampel, 2006). In general, most familial cases develop in premenopausal women (Gruber, 1996).
Women who carry mutations in BRCA1 and BRCA2 genes are at increased risk for breast and ovarian cancer. They may also have a slightly elevated risk for endometrial cancer, but only because associated breast cancers are often treated with tamoxifen (Beiner, 2007; Thai, 1998).
Tamoxifen causes a two- to threefold higher risk of developing endometrial cancer by its modest “unopposed” estrogenic effect on the endometrium (Chap. 27). The increased risk of endometrial cancer affects postmenopausal women almost exclusively, and cancer rates increase linearly with the duration and cumulative dose of tamoxifen therapy (Fisher, 1998; van Leeuwen, 1994). Accordingly, women taking tamoxifen are counseled regarding this endometrial risk and should report vaginal spotting, bleeding, or discharge. That said, unless a tamoxifen-treated patient has such symptoms or is identified to be at otherwise high risk for endometrial cancer, routine endometrial surveillance does not increase early detection rates (American College of Obstetricians and Gynecologists, 2014c).
Coexisting medical conditions such as diabetes mellitus, hypertension, and gallbladder disease are more commonly associated with endometrial cancer (Morimoto, 2006; Soliman, 2005). In general, these are frequent sequelae of obesity and an environment of chronic excess estrogen.
In contrast, combination oral contraceptive (COC) use for at least 1 year confers as much as a 30- to 50-percent reduced risk of endometrial cancer, and risk reduction extends for 10 to 20 years (Dossus, 2010; Stanford, 1993). This most likely derives from a chemopreventive effect on the endometrium provided by the progestin component (Maxwell, 2006). Logically, progesterone intrauterine devices (IUDs) also confer long-term endometrial cancer protection (Tao, 2006). Moreover, similar protective effects have been found with inert and copper IUD types (Felix, 2015).
Smokers have a lower risk of developing endometrial cancer. The biologic mechanism is multifactorial but in part involves lower circulating estrogen levels from weight reduction, earlier age at menopause, and altered hormonal metabolism. Both current and past smoking have a long-lasting influence (Viswanathan, 2005).
ENDOMETRIAL HYPERPLASIA
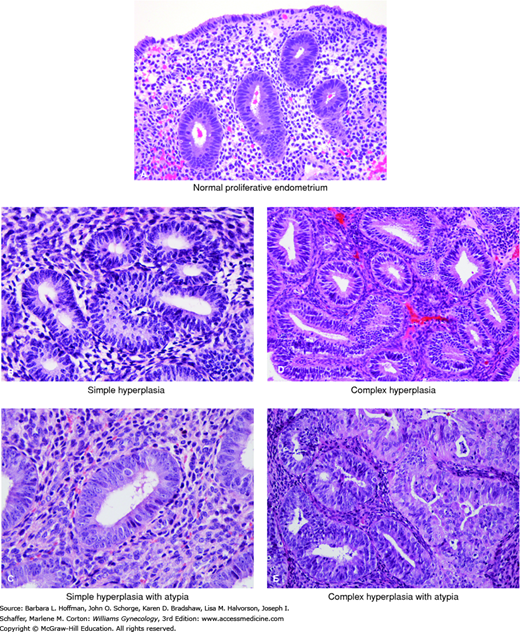
Most endometrial cancers arise following progression of histologically distinguishable hyperplastic lesions. In fact, endometrial hyperplasia is the only known direct precursor of invasive disease. Endometrial hyperplasia is defined as endometrial thickening with proliferation of irregularly sized and shaped glands and an increased gland-to-stroma ratio (Fig. 33-1) (Ellenson, 2011b). In the absence of such thickening, lesions are best designated as disorderly proliferative endometrium or focal glandular crowding.
FIGURE 33-1
A. This high-power view of normal proliferative endometrium shows regularly spaced glands composed of stratified columnar epithelium with bland, slightly elongate nuclei. B. In simple hyperplasia, glands are modestly crowded and typically display normal tubular shape or mild gland-shape abnormalities. Nuclei are bland. C. In this case of simple hyperplasia with atypia, glands are only mildly crowded. Occasional glands show nuclear atypia characterized by nuclear rounding and visible nucleoli. D. In complex hyperplasia, glands are more markedly crowded. Some specimens show architectural abnormalities such as papillary infoldings, although the gland profiles in this case are fairly regular. E. In complex hyperplasia with atypia, glands are markedly crowded and some have papillary infoldings. Nuclei show variable atypia. (Used with permission from Dr. Kelley Carrick.)
Endometrial hyperplasia represents a continuum of histopathologic findings. The classification system used by the World Health Organization (WHO) and International Society of Gynecological Pathologists designates four different types with varying malignant potential (Table 33-2) (Kurman, 1985, 2014). Hyperplasias are classified as simple or complex, based on the absence or presence of architectural abnormalities of the endometrial glands. Abnormalities include gland crowding and complexity (see Fig. 33-1). Most importantly, hyperplasias are additionally labeled as atypical if they demonstrate nuclear atypia of the endometrial gland cells. Atypical endometrial hyperplasias are clearly associated with the subsequent development of adenocarcinoma. Simple atypical hyperplasia is a relatively uncommon diagnosis. In general, most have a complex architecture.
Although endometrial hyperplasias are formally classified in these four different groups, they tend to be morphologically heterogeneous, both within and between individual patients. This histologic diversity explains why only a small number of conserved features are useful as diagnostic criteria. As a result, reproducible scoring of cytologic atypia is often challenging, particularly with a small amount of tissue from a biopsy sample.
Endometrial intraepithelial neoplasia (EIN) is a term introduced to more accurately distinguish the two very different clinical categories of hyperplasia: (1) normal polyclonal endometria diffusely responding to an abnormal hormonal environment and (2) intrinsically proliferative monoclonal lesions that arise focally and confer an elevated risk of adenocarcinoma (Mutter, 2000). This nomenclature emphasizes the malignant potential of endometrial precancers and is in keeping with similar precedents in the cervix (CIN [cervical intraepithelial neoplasia]), vagina (VaIN), and vulva (VIN) (Chap. 29).
Using this system, anovulatory or prolonged estrogen-exposed endometria without atypia are generally designated as endometrial hyperplasias. In contrast, EIN is used to describe all endometria delineated as premalignant by a combination of three morphometric features. The qualities reflect glandular volume, architectural complexity, and cytologic abnormality. The EIN classification system is a more accurate and reproducible way to predict progression to cancer (Baak, 2005; Hecht, 2005). This classification is endorsed by the Society of Gynecologic Oncology and American College of Obstetricians and Gynecologists (2015) but has not been universally implemented.
The risks for developing endometrial hyperplasia generally mirror those for invasive carcinoma (Anastasiadis, 2000; Ricci, 2002). Two thirds of women present with postmenopausal bleeding (Horn, 2004). However, premenopausal women with abnormal uterine bleeding (AUB) are also evaluated as described in Chapter 8.
As hyperplasia is a histologic diagnosis, a Pipelle office endometrial biopsy (EMB) or outpatient dilatation and curettage (D & C) are suitable choices for endometrial sampling. The American College of Obstetricians and Gynecologists (2014a) recommends such sample for women older than 45 years with AUB. EMB is also considered for those younger than 45 with chronic excess estrogen exposure (exogenous or endogenous), failed medical management, and persistent AUB.
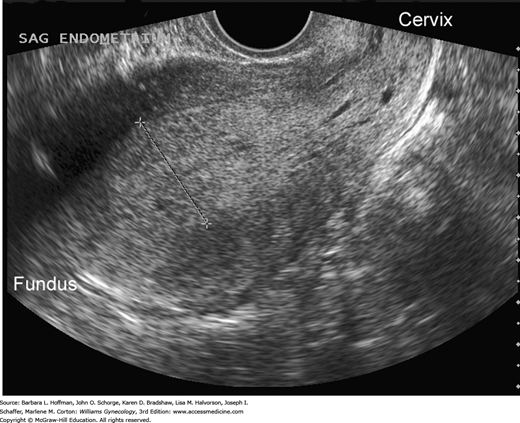
In those with AUB, transvaginal sonography to measure endometrial thickness is also a feasible method for predicting endometrial hyperplasia (Granberg, 1991; Jacobs, 2011). In postmenopausal women, endometrial stripe thickness measurements ≤4 mm are associated with bleeding that is attributed to endometrial atrophy (American College of Obstetricians and Gynecologists, 2013). Postmenopausal women with a thicker endometrium warrant biopsy. Sonography may also identify abnormal echostructural changes in the endometrium. Cystic endometrial changes suggest polyps, homogeneously thickened endometrium may indicate hyperplasia, and a heterogeneous structural pattern is suspicious for malignancy (Figs. 33-2 and 33-3). However, these sonographic findings show great overlap and cannot be used alone.
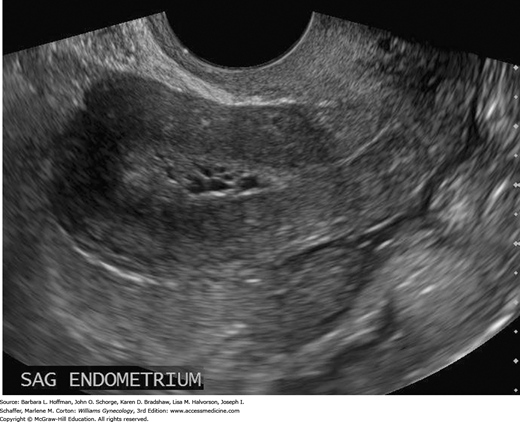
FIGURE 33-3
Transvaginal sagittal image of the endometrium from a 38-year-old woman with chronic oligomenorrhea. The abnormal endometrium is thickened, echogenic, and heterogeneous in echotexture and contains tiny cystic foci. Biopsy revealed grade 1 endometrioid adenocarcinoma, which was confirmed at surgery. (Used with permission from Dr. Elysia Moschos.)
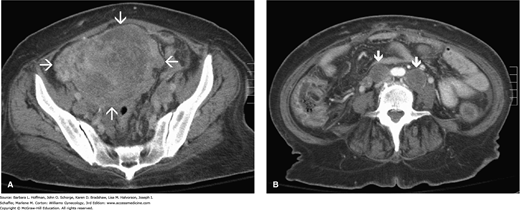
FIGURE 33-4
Computed tomographic (CT) images in the axial plane of a 61-year-old woman with endometrial cancer. A. Massively enlarged and inhomogeneous uterus (arrows) in the upper pelvis. B. At the level of the aortic bifurcation, enlarged lymph nodes are seen bilaterally (arrows), consistent with lymph node involvement. (Used with permission from Dr. Diane Twickler.)
For premenopausal women, transvaginal sonography is often performed to exclude structural sources of abnormal bleeding. Similarly, researchers have attempted to create endometrial thickness guidelines. However, endometrial thicknesses can vary considerably among premenopausal women during normal menstrual cycling. From studies, suggested evidence-based abnormal thresholds range from >4 mm to >16 mm (Breitkopf, 2004; Goldstein, 1997; Shi, 2008). Thus, consensus for an endometrial thickness threshold has not been established for this group. That said, in reproductive-aged women with a thick endometrium combined with other hyperplasia risk factors, EMB may be prudent.
Of other tools, hysteroscopy is more sensitive for focal endometrial lesions. Hyperplastic endometrium is grossly indistinct, and thus hysteroscopy has poor sensitivity for this diagnosis (Ben Yehuda, 1998; Garuti, 2006).
Occasionally, an adnexal mass may be palpable during examination and in most cases is a benign ovarian cyst. However, any solid features noted during transvaginal sonography raises the possibility of a coexisting ovarian granulosa cell tumor. These tumors produce excess estrogen that results in up to a 30-percent risk of endometrial hyperplasia or less commonly, endometrial carcinoma (Chap. 36) (Ayhan, 1994).
Management of women with endometrial hyperplasia mainly depends on a patient’s age, comorbid risks for surgery, desire for fertility, and specific histologic features such as cytologic atypia. Traditional treatment has been surgery. Hormonal therapy is another option and includes oral or injectable progestins or the progestin (levonorgestrel-releasing) IUD.
There is some inconsistency of diagnosis and uncertainty in predicting the stability of individual lesions. Specifically, several studies have documented low reproducibility for WHO classifications of endometrial hyperplasia (Allison, 2008; Sherman, 2008; Zaino, 2006). In addition, there is no way to anticipate which types will involute with progestin therapy. However, as long as an endometrial sample is representative and a provider has no reason to suspect a coexisting invasive carcinoma, the decision to treat endometrial hyperplasia through hormonal or surgical means relies on clinical judgment.
Nonatypical lesions may spontaneous regress without therapy. However, progestins are generally used to address the underlying etiology, that is, chronic anovulation and excess estrogen (Terakawa, 1997). Premenopausal women with nonatypical endometrial hyperplasia typically require a 3- to 6-month course of low-dose progestin therapy. Cyclic medroxyprogesterone acetate (MPA) (Provera) given orally for 12 to 14 days each month at a dose of 10 to 20 mg daily is commonly used. Continuous daily dosing with MPA 10 mg is suitable and may be more effective than cyclic administration in reversing hyperplastic changes. Another frequently used option is COC pills for those without contraindications. The levonorgestrel-releasing IUD is also effective (Gallos, 2010; Ørbo, 2014; Scarselli, 2011).
In general, follow-up endometrial biopsy is performed to document regression. In those with an IUD, endometrial biopsy can be performed without device removal. After regression, a key point is to continue endometrial protection. Thus, once hyperplastic changes resolve, patients are continued on progestins and observed until menopause. Additional endometrial sampling is required for new bleeding.
Postmenopausal women with nonatypical endometrial hyperplasia may also be treated with low-dose oral cyclic MPA or a continuous 10-mg daily regimen. However, it is particularly important in older women to be confident that the sample obtained is adequate for excluding cytologic atypia. D & C may be indicated in some circumstances, especially if the tissue from Pipelle sampling is scant or if recurrent bleeding is noted.
In most cases, a form of progestin therapy is used to treat endometrial hyperplasia without atypia. But, affected postmenopausal patients who have a contraindication to progestin therapy or who cannot tolerate the therapy can be expectantly managed. Complex hyperplasia without atypia is usually treated chronically with progestins. With either complex or simple hyperplasia without atypia, office endometrial biopsy is recommended every 3 to 6 months until lesion resolution is achieved.
In cases of endometrial hyperplasia without atypia, the risk of progression to endometrial cancer is low (1 to 3 percent). The overall clinical and pathologic regression rates to progestin therapy range from 70 to 80 percent for nonatypical endometrial hyperplasia (Rattanachaiyanont, 2005; Reed, 2009). Patients with persistent disease on repeated biopsy may be switched to a higher-dose regimen such as MPA, 40 to 100 mg orally daily. Also, megestrol acetate (Megace), 160 mg daily or 80 mg twice daily, is suitable. It can be increased even up to 160 mg twice daily if no regression is initially achieved. Again, a clinician must confirm that hormonal ablation has occurred by resampling the endometrium after a suitable therapeutic interval, usually 3 to 6 months. Hysterectomy may also be considered for lesions that are refractory to medical management.
If surgery is selected, a minimally invasive surgery (MIS) approach is considered, and options are laparoscopic, robotic, or vaginal hysterectomy. In cases in which hyperplasia has been proven or is suspected, the uterus is removed in toto and without morcellation, which might disseminate the lesion. Because the lesion may extend into the lower uterine segment or upper endocervix, supracervical hysterectomy is not appropriate for women undergoing hysterectomy for treatment of endometrial hyperplasia.
Hysterectomy is the preferred treatment for women with atypical endometrial hyperplasia because the risk of progression to cancer over time approximates 29 percent. There is also a high rate of finding concurrent invasive malignancy coexistent with the atypical hyperplasia (Horn, 2004; Trimble, 2006). In postmenopausal women, a hysterectomy with removal of both tubes and ovaries is recommended.
In premenopausal women who have completed childbearing, hysterectomy is performed for atypical hyperplasia. Risk-reducing salpingectomy is encouraged to potentially lower cancer risk that arises from the fallopian tubes (American College of Obstetricians and Gynecologists, 2015d). For premenopausal women, removal of the ovaries is optional. The deciding factors mirror those for women contemplating BSO for other benign indications and are outlined fully in Section 43-12.
Premenopausal women who strongly wish to preserve fertility can be treated with progestins (Trimble, 2012). High-dose progestin therapy, megestrol acetate 80 mg orally twice daily, is an option for motivated patients who will be compliant with surveillance (Randall, 1997). The IUD that releases 20 μg of intrauterine levonorgestrel daily (Mirena) is also suitable (Ørbo, 2014). Poor surgical candidates may also warrant an attempt at hormonal ablation with progestins. Resolution of the hyperplasia must be confirmed by serial endometrial biopsies every 3 months until response is documented. Otherwise, hysterectomy is recommended. Following hyperplasia resolution, surveillance and progestins continue long-term due to the potential for eventual progression to carcinoma (Rubatt, 2005). Once fertility is complete, hysterectomy is again recommended.
The Gynecologic Oncology Group (GOG) performed a prospective cohort study of 289 patients who had a diagnosis of atypical endometrial hyperplasia. Participants underwent hysterectomy within 3 months of their biopsy, and 43 percent were found to have a concurrent endometrial carcinoma (Trimble, 2006). Suh-Burgmann and associates (2009) found a similarly high number of 48 percent. Results demonstrate the difficulty in attaining an accurate diagnosis before hysterectomy and the potential risks of conservative hormonal treatment.
Generalists in obstetrics and gynecology who perform hysterectomy for atypical endometrial hyperplasia should recognize the possibility of a coexisting invasive malignancy and the possible need for surgical staging. At a minimum, peritoneal washings are obtained prior to performing a hysterectomy. In addition, the uterus should be opened and examined in the operating room. From this, a frozen section analysis can be performed to search for concurrent cancer and determine grade and depth of invasion if found. Any suspicion for myometrial invasion is an appropriate indication for intraoperative consultation with a gynecologic oncologist.
ENDOMETRIAL CANCER
Endometrial cancer is a biologically and histologically diverse group of neoplasms characterized by a dualistic model of pathogenesis. As noted, type I endometrioid adenocarcinomas comprise most cases. They are estrogen-dependent, low grade, and derived from atypical endometrial hyperplasia. In contrast, type II cancers are serous or clear cell histology, have no precursor lesion, and portend a more aggressive clinical course (Table 33-3). The morphologic and clinical differences are paralleled by genetic distinctions. Namely, type I and II tumors carry mutations of independent sets of genes (Bansal, 2009; Hecht, 2006). The two pathways of endometrial cancer pathogenesis have significant overlap and thus result in a spectrum of histologic features.
| Feature | Type I | Type II |
| Chronic estrogen Menopause status Hyperplasia Race Grade Invasiona Behavior Subtypes | Present Pre-/peri- Present White Low Minimal Stable Endometrioid | Absent Post- Absent Black High Deep Aggressive Serous Clear cell |
Education can be effective prevention, as many endometrial cancer risks are alterable. Women with PCOS may benefit from weight loss and chronic progestin supplementation (Chap. 17). Assessing and managing obesity as described in Chapter 1 may also lower risks.
For women at average risk or increased risk, routine screening of hyperplasia or endometrial cancer is not advocated. Instead, at the onset of menopause, women are counseled on the risks and symptoms of endometrial cancer and strongly encouraged to report unexpected bleeding or spotting to their provider. One screening exception is the woman with Lynch syndrome. For these individuals, EMB is recommended every 1 to 2 years beginning at age 30 to 35 years (American College of Obstetricians and Gynecologists, 2014b; Smith, 2015).
Genetic testing criteria have been published to identify the individual with Lynch syndrome (Table 33-4) (Lancaster, 2015). Lynch syndrome cancers include colon, endometrium, small bowel, renal pelvis and ureter, and ovary, among others (Vasen, 1999). Referral for genetic counseling can further clarify which patients may benefit from specific germline testing (Balmana, 2006; Chen, 2006). Endometrial cancer is the most common “sentinel cancer,” thus, obstetrician-gynecologists play a pivotal role in the identification of women with Lynch syndrome (Lu, 2005).
Patients with endometrial or colorectal cancer and tumor evidence of:
|
First-degree relative with endometrial or colorectal cancer who was diagnosed:
|
| First- or second-degree relative with a known DNA mismatch repair gene mutation |
Since women with Lynch syndrome have such a high lifetime risk of developing endometrial cancer (40 to 60 percent), prophylactic hysterectomy is recommended once affected women reach the early to mid 40s. In a cohort of 315 HNPCC-mutation carriers, Schmeler and coworkers (2006) confirmed the benefit of this approach by reporting a 100-percent endometrial cancer risk reduction. In general, BSO is also performed due to the 9- to 12-percent lifetime risk of ovarian cancer. Prior to hysterectomy, colon cancer screening with colonoscopy should be up to date (American College of Obstetrician and Gynecologists, 2014b).
Early diagnosis of endometrial cancer is almost entirely dependent on the prompt recognition and evaluation of irregular vaginal bleeding. In premenopausal women, a clinician must maintain a high index of suspicion for a history of prolonged, heavy menstruation or intermenstrual spotting, because many other benign disorders give rise to similar symptoms (Table 8-1). Postmenopausal bleeding is particularly worrisome, leading to a 5- to 10-percent likelihood of diagnosing endometrial carcinoma (Gredmark, 1995; Iatrakis, 1997). Abnormal vaginal discharge may be another symptom in older women.
Unfortunately, some patients do not seek medical attention despite months or years of heavy, irregular bleeding. In more advanced disease, pelvic pressure and pain may reflect uterine enlargement or extrauterine tumor spread. Patients with serous or clear cell tumors often present with signs and symptoms suggestive of advanced epithelial ovarian cancer that include pelvic pain or pressure, bloating, early satiety, and increasing abdominal girth (Chap. 35).
Pap testing is not an indicated test to diagnose endometrial cancer, and 50 percent of women with endometrial cancer will have normal findings (Gu, 2001). Liquid-based cytology appears to increase the detection of glandular abnormalities, but not enough to cause a shift in clinical practice (Guidos, 2000; Schorge, 2002). However, some findings from Pap testing should prompt further investigation. Benign endometrial cells are occasionally recorded on a routine Pap test report in women 45 years or older. In premenopausal women, this is often a finding of limited importance, especially if a test is obtained following menses. However, postmenopausal women with such findings have a 3- to 5-percent risk of endometrial cancer (Simsir, 2005). In those using HRT, the prevalence of benign endometrial cells on smears is increased, and the associated risk of malignancy is less (1 to 2 percent) (Mount, 2002). Although endometrial biopsy is considered in asymptomatic postmenopausal women if this finding is reported, most patients ultimately diagnosed with hyperplasia or cancers also have concomitant abnormal bleeding (Ashfaq, 2001).
In contrast, atypical glandular cells found during Pap testing carry higher risks for underlying cervical or endometrial neoplasia. Accordingly, evaluation of a glandular abnormality includes colposcopy and endocervical curettage (ECC). It may also include endometrial sampling in nonpregnant patients older than 35 years or in those younger if there is a history of abnormal bleeding, if risk factors for endometrial disease are noted, or if the cytology specifies that the atypical glandular cells are of endometrial origin.
Office Pipelle biopsy is preferred for the initial evaluation of women with bleeding suspicious for malignancy (Feldman, 1993). However, if sampling techniques fail to provide sufficient diagnostic information or if abnormal bleeding persists, D & C may be required to clarify the diagnosis.
The American College of Obstetricians and Gynecologists (2015b) considers hysteroscopy acceptable for AUB evaluation in those without suspected advanced-stage uterine or cervical cancer. However, hysteroscopy is more sensitive for focal endometrial lesions and thus has proved less helpful in diagnosing early endometrial cancer. In those cases in which hysteroscopy is used to evaluate abnormal bleeding and in which cancer is ultimately diagnosed, an increased incidence of positive peritoneal cytology has been noted during subsequent staging surgery (Obermair, 2000; Polyzos, 2010; Zerbe, 2000). Although the risk of peritoneal contamination by cancer cells may be increased by retrograde efflux of hysteroscopic media, patient prognosis overall does not appear to be worsened (Cicinelli, 2010; Revel, 2004).
The only clinically useful tumor marker in the management of endometrial cancer is a serum CA125 level. Preoperatively, an elevated level indicates the possibility of more advanced disease (Powell, 2005). In practice, it is most useful in patients with advanced disease or serous subtypes to assist in monitoring response to therapy or during posttreatment surveillance. However, even in this setting, its utility in the absence of other clinical findings is limited (Price, 1998).
In general, for women with a well-differentiated type I endometrioid tumor, chest radiography is the only required preoperative imaging study. All other preoperative testing is directed toward general surgical preparation (Chap. 39).
Computed tomography (CT) or magnetic resonance (MR) imaging is usually not necessary (American College of Obstetricians and Gynecologists, 2015c). However, CT scanning can be obtained preoperatively in cases with higher-grade lesions to assess for lymph node involvement or metastatic disease. MR imaging can occasionally help distinguish an endometrial cancer with cervical extension from a primary endocervical adenocarcinoma (Nagar, 2006). Moreover, women with serous features or other high-risk histology on preoperative biopsy and those with physical examination findings suggesting advanced disease are most appropriate for abdominopelvic CT scanning (Fig. 33-4). In these cases, advance knowledge of intraabdominal disease may help guide surgery and treatment. MR imaging is also recommended for women who are considering fertility-sparing management with hormonal therapy, since it may not be an option if deep invasion is found.
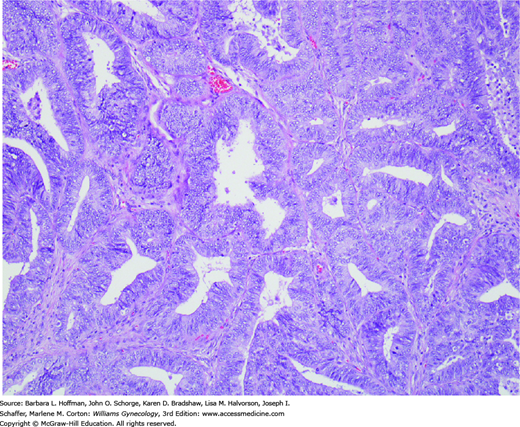
FIGURE 33-5
Endometrioid adenocarcinomas are composed of neoplastic glands resembling those of the normal endometrium. Cells are typically tall columnar with mild to moderate nuclear atypia. They form glands that are abnormally crowded or “back-to-back.” Gland cribriforming, confluence, and villous structures are also common. It is these architectural forms, with the associated disappearance of intervening stroma, that distinguish well-differentiated endometrioid adenocarcinoma from complex hyperplasia. (Used with permission from Dr. Kelley Carrick.)
Although most endometrial cancers are cured by hysterectomy and BSO, primary management by gynecologic oncologists has advantages. It is an efficient use of health care resources, minimizes potential morbidity, is more likely to lead to staging, and improves the survival of patients with high-risk disease (Chan, 2011; Roland, 2004). Therefore, preoperative consultation is generally advisable for any patient with endometrial cancer who is being prepared for surgery by a generalist in obstetrics and gynecology. Postoperatively, a gynecologic oncologist should be consulted if cervical extension, extrauterine disease, or positive peritoneal washing cytology was found during surgery.
If treated by an oncologist, early-stage patients treated by surgery alone will return in many cases to their primary obstetrician-gynecologist for surveillance. Consultation is again recommended if recurrent disease is later suspected or identified.
When an endometrial cancer is unexpectedly diagnosed after hysterectomy performed by a generalist for other indications, consultation is also recommended. Possible therapeutic options include no further therapy and surveillance only, reoperation to complete surgical staging, or radiotherapy to prevent local recurrence. In general, the survival advantages of staging must be weighed against the complications from another surgical procedure (American College of Obstetricians and Gynecologists, 2015c). Fortunately, the advent of laparoscopic and robotic delayed staging offers the potential for less morbidity (Spirtos, 2005).
The spectrum of aggressiveness within the histopathologic types of endometrial cancer is broad (Table 33-5). Most patients have endometrioid adenocarcinomas that behave indolently. However, some will have an unfavorable histology that portends a much more aggressive tumor. In addition, the degree of tumor differentiation is an important predictor of disease spread.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree