INTRODUCTION
Cancer found in the vagina is most likely metastatic disease. Primary vaginal carcinoma is rare and makes up only 3 percent of all gynecologic malignancies (Siegel, 2015). This low incidence reflects the infrequency with which primary carcinoma arises in the vagina and the strict criteria for its diagnosis. According to International Federation of Gynecology and Obstetrics (FIGO) staging criteria, a vaginal lesion that involves adjacent organs such as the cervix or vulva, by convention, is deemed primary cervical or vulvar, respectively (Pecorelli, 1999). The most common histologic type of primary vaginal cancer is squamous cell carcinoma, followed by adenocarcinoma (Platz, 1995).
RELEVANT ANATOMY
During embryogenesis, the müllerian ducts fuse caudally to form the uterovaginal canal (Chap. 18). The canal’s distal portion forms the proximal vagina, whereas the distal vagina arises from the urogenital sinus. The uterovaginal canal is lined by columnar epithelium, which is subsequently replaced by squamous cells migrating cephalad from the urogenital sinus. These squamous cells stratify, and the vaginal epithelium matures and thickens. Underlying this epithelium, muscularis and adventitial layers surround the vaginal tube.
With vaginal cancer, local extension and lymphatic invasion are common patterns of spread. The lymphatic channels that drain the vagina form extensive, complex, and variable anastomoses. As a result, any node in the pelvis, groin, or anorectal area may drain any part of the vagina. Of these, the external, internal, and common iliac lymph nodes are the primary sites of vaginal lymphatic drainage. Thus, pelvic lymphadenectomy, which samples these nodal groups, is commonly performed during primary surgical excision of proximally located vaginal cancers. Alternatively, lymphatic vessels of the posterior vagina may empty into the inferior gluteal, presacral, or perirectal nodes, and those of the vagina’s distal third may drain to the superficial and deep inguinal lymph nodes (Frank, 2005).
Hematogenous spread of vaginal cancer is less frequent, and venous drainage consists of the uterine, pudendal, and rectal veins, which drain into the internal iliac vein. Arterial blood supply to the vagina comes primarily from internal iliac artery branches, which include the uterine, vaginal, middle rectal, and internal pudendal arteries (Fig. 38-12).
INCIDENCE
According to estimates for 2015, 4070 new cases of vaginal cancer will be diagnosed in the United States, and there will be 910 deaths (Siegel, 2015). The overall incidence is 0.45 cases per 100,000 women but is notably lower in whites (0.42 cases) compared with black and Hispanic women (0.73 and 0.56 cases, respectively). Vaginal cancer rates increase with age and peak among women ≥80 years. The median age at diagnosis is 58 (Watson, 2009). Of histologic forms, squamous cell carcinoma accounts for 70 to 80 percent of all primary vaginal cancer cases (Beller, 2003; Platz, 1995).
SQUAMOUS CELL CARCINOMA
Squamous cell cancer of the vagina arises within its stratified nonkeratinized epithelium (Fig. 32-1). As with other cancers of the lower reproductive tract, human papillomavirus (HPV) has been closely linked with squamous cell vaginal cancer (Chap. 30). A systematic review found an HPV prevalence of 65 percent in samples from invasive vaginal cancer, and a 93-percent prevalence in high-grade vaginal dysplasia lesions. HPV 16 was the most common type and was present in 55 percent of vaginal cancer samplings (Smith, 2009). A retrospective cross-sectional study involving 31 countries found similar results (Alemany, 2014).
FIGURE 32-1
Sections showing invasive squamous cell carcinoma of the vagina. A. Invasive, well-differentiated squamous cell carcinoma of the vagina (bracket) invading the subepithelial stroma (×4). B. Invasive, well-differentiated squamous cell carcinoma of the vagina (×10). Invasive tumor is composed of irregular nests of malignant squamous cells with keratin pearls (arrow) and intercellular bridges. (Used with permission from Dr. Kelley Carrick.)
Because of this association with HPV infection, vaginal in situ and invasive squamous cell carcinomas share risk factors similar to those for cervical cancer. Some of these include multiple lifetime sexual partners, early age at first intercourse, and current cigarette smoking. Women with a vulvar or cervical cancer history are also at increased risk. This last association may stem from the field effect of HPV affecting multiple lower genital tract epithelia or may result from direct tumor spread.
Vaginal intraepithelial neoplasia (VaIN) is a precursor to invasive vaginal cancer, and approximately 2 to 3 percent of patients with VaIN will progress to invasive cancer (Dodge, 2001; Ratnavelu, 2013). The quadrivalent HPV vaccine is effective in preventing VaIN 2 and 3 associated with HPV 16 or 18 (Joura, 2007). It is possible that use of this vaccine will decrease invasive vaginal cancer rates in the future.
Vaginal bleeding is the most common complaint associated with vaginal cancer, although pelvic pain and vaginal discharge also may be noted. Less frequently, lesions involving the anterior vaginal wall may lead to dysuria, hematuria, or urgency. Alternatively, constipation may result from posterior wall masses. Most vaginal cancers develop in the upper third of the vagina. Moreover, of those with cancers, women who have had a prior hysterectomy are significantly more likely to have lesions in the upper vagina (70 percent) than those without prior hysterectomy (36 percent) (Chyle, 1996).
During pelvic evaluation in all women, the vagina is inspected as the speculum is inserted or removed. If a gross lesion is found, vaginal cancer usually can be diagnosed by punch biopsy in the office. Biopsy may be obtained with Tischler biopsy forceps (Fig. 29-16). An Emmett hook, one type of skin hook, may be useful to elevate and stabilize vaginal tissue during biopsy. Monsel paste is applied as needed for hemostasis. If a gross lesion is not detectable, vaginoscopy can guide directed biopsy. Bimanual examination assists in determining the tumor size, and rectovaginal examination is especially important for posterior wall lesions.
Once cancer is diagnosed, no specific laboratory testing other than that used generally for preoperative preparation such as complete blood count and serum chemistry panel is required.
Vaginal cancer staging is similar to that for cervical cancer and is completed clinically by physical examination and with the assistance of cystourethroscopy and/or proctosigmoidoscopy depending on tumor location. Chest radiography aids the search for metastatic disease (Table 32-1 and Table 32-2). If needed, general anesthesia can permit a more detailed pelvic examination for accurate staging. Proctosigmoidoscopy to a depth of at least 15 cm can detect local bowel invasion, whereas cystourethroscopy assists identification of bladder or urethral involvement.
| Vaginal biopsy |
| Physical examination |
| Endocervical curettage |
| Endometrial biopsy |
| Cystourethroscopy |
| Proctosigmoidoscopy |
| Chest radiograph |
| Abdominopelvic CT scan, MR imaging, or PET CT a |
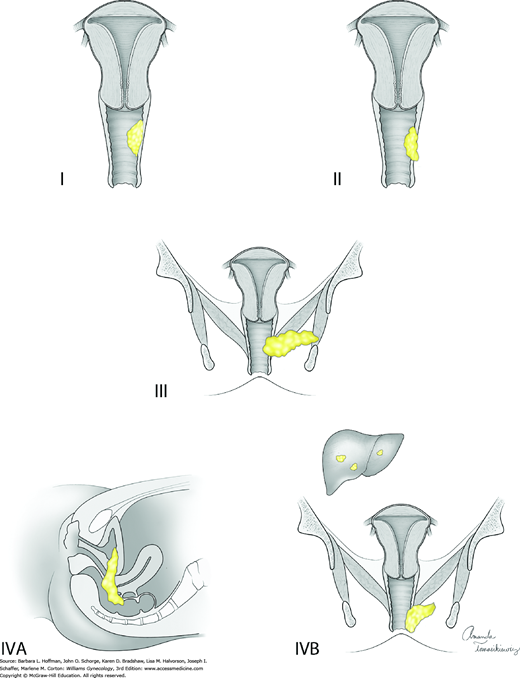
| Stage | Characteristics |
| I | The carcinoma is limited to the vaginal wall |
| II | The carcinoma has involved the subvaginal tissue but has not extended to the pelvic wall |
| III | The carcinoma has extended to the pelvic wall |
| IV | The carcinoma has extended beyond the true pelvis or has involved the mucosa of the bladder or rectum; bullous edema as such does not permit a case to be allotted to stage IV |
| IVA | Tumor invades bladder and/or rectal mucosa and/or direct extension beyond the true pelvis |
| IVB | Spread to distant organs |
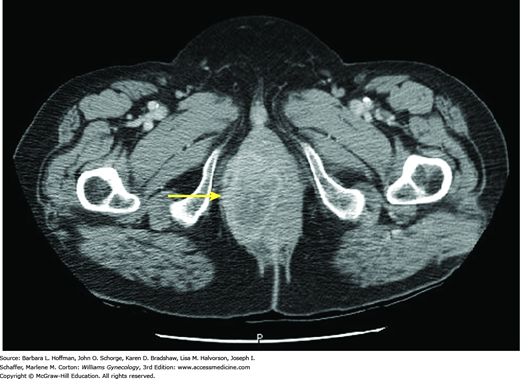
Computed tomography (CT) scanning, magnetic resonance (MR) imaging, and fluorodeoxyglucose-positron emission tomography (FDG-PET) may also be useful in treatment planning but are not classically used to determine disease stage. CT scanning can delineate the size and extent of many tumors (Fig. 32-2). However, if the extent of cancer expansion is unclear, MR imaging is the most useful radiologic tool available to visualize the vagina due to its superior soft tissue resolution. FDG-PET can also be selected to evaluate lymph node involvement and distant metastases. In one study, FDG-PET was more sensitive than CT for detection of abnormal lymph nodes (Lamoreaux, 2005).
The prognosis of vaginal squamous cell carcinoma has improved since the 1950s, when the 5-year survival rate was only 18 percent. Advances in radiation technology and earlier diagnosis are largely responsible for the improved 5-year survival rate, which now ranges from 45 to 68 percent for all stages (Ghia, 2011; Hellman, 2006).
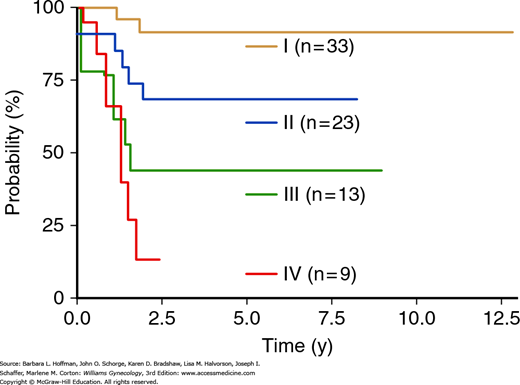
The prognosis of vaginal squamous cell carcinoma depends primarily on FIGO stage (Fig. 32-3 and Table 32-2) (Frank, 2005; Peters, 1985). Other factors associated with poor prognosis include larger tumor size, adenocarcinoma cell type, and older age (Hellman, 2006; Tjalma, 2001; Tran, 2007). The 5-year disease-specific survival rate is 85 to 92 percent for women with stage I disease, 68 to 78 percent for those with stage II, and 13 to 58 percent for those with stage III or IV (Fig. 32-4) (Frank, 2005; Tran, 2007).
FIGURE 32-4
Disease-specific survival stratified by International Federation of Gynecology and Obstetrics (FIGO) stage. (Reproduced with permission from Tran PT, Su Z, Lee P, et al: Prognostic factors for outcomes and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol 2007 Jun;105(3):641–649.)
Because of vaginal cancer’s rarity, data are limited to guide evidence-based treatment. Therefore, therapy is individualized and based on factors such as tumor type, stage, location, and size.
For stage I disease, both surgery and radiotherapy are options. However, surgery is preferred for most if negative surgical margins can be achieved. Surgery includes radical vaginectomy, radical hysterectomy (for women with an intact uterus), and pelvic lymphadenectomy for most tumors located in the upper third of the vaginal vault. A review of the National Cancer Data Base showed that women with stage I disease treated with surgery alone had a significantly improved 5-year survival rate compared with those treated with radiation (90 percent versus 63 percent) (Creasman, 1998). However, other reports have found no significant difference in disease-free survival rates in women with stage I disease treated with surgery compared with radiation alone (Stock, 1995). Radiotherapy may be delivered by external beam, with or without brachytherapy. Further, brachytherapy alone has been used successfully to treat selected small stage I lesions (Nori, 1983; Perez, 1999; Reddy, 1991).
Depending on circumstances and the treating clinician, primary surgery or radiation may be selected for stage II cancers. Stock and colleagues (1995)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree