INTRODUCTION
Cervical cancer is the most common gynecologic cancer in women worldwide. Most of these cancers stem from infection with the human papillomavirus (HPV), although other host factors affect neoplastic progression following initial infection. Compared with other gynecologic malignancies, cervical cancer develops in a younger population. Thus, screening for this neoplasia typically begins in young adulthood.
Most early cancers are asymptomatic. Thus, diagnosis usually follows histologic evaluation of biopsies taken during colposcopic examination or from a grossly abnormal cervix. This cancer is staged clinically, and this in turn directs treatment. In general, early-stage disease is effectively eradicated surgically. For advanced disease, chemoradiation is primarily selected. As expected, disease prognosis differs with tumor stage, and stage is the most important indicator of long-term survival.
Prevention lies mainly in identifying and treating women with high-grade dysplasia, and in HPV vaccination. Accordingly, as detailed in Chapter 29, regular screening is recommended and HPV vaccination is encouraged to lower rates of cervical cancer in the future.
INCIDENCE
Worldwide, cervical cancer is common, and it ranks fourth among all malignancies for women (World Health Organization, 2012). In general, higher incidences are found in developing countries, and these countries contribute 85 percent of reported cases annually. Mortality rates are similarly higher in these populations (Torre, 2015). This incidence and survival disparity highlights successes achieved by long-term cervical cancer screening programs.
In the United States, cervical cancer is the third most common gynecologic cancer and the 11th most common solid malignant neoplasm among women. Women have a 1 in 132 lifetime risk of developing this cancer. In 2015, the American Cancer Society estimated 12,900 new cases and 4100 deaths from this malignancy (Siegel, 2015). Of U.S. women, black women and those in lower socioeconomic groups have the highest age-adjusted cervical cancer death rates, and Hispanic women have the highest incidence rates (Table 30-1). This trend is thought to stem mainly from financial and cultural characteristics affecting access to screening and treatment. The age at which cervical cancer develops is in general earlier than that of other gynecologic malignancies, and the median age at diagnosis is 49 years (Howlader, 2014).
RISKS
In addition to demographic differences, other risks may alter HPV acquisition or action. Notably, the greatest risk is the lack of regular cervical cancer screening (Abed, 2006; Leyden, 2005). Most communities adopting such screening have documented decreased incidences of this cancer (Jemal, 2006).
HPV is the primary etiologic infectious agent associated with cervical cancer (Ley, 1991; Schiffman, 1993). Although other sexually transmitted factors, including herpes simplex virus 2, may play a concurrent causative role, 99.7 percent of cervical cancers are associated with an oncogenic HPV subtype (Walboomers, 1999). In one study, 57 percent of invasive cervical cancer cases were attributable to HPV serotype 16. Serotype 18 was associated with 16 percent of invasive disease cases (Li, 2010). Each of these serotypes can lead to either squamous cell carcinoma or adenocarcinoma of the cervix. However, HPV 16 is more commonly associated with squamous cell carcinoma of the cervix, whereas HPV 18 is a risk factor for cervical adenocarcinoma (Bulk, 2006).
Of other risks, lower educational attainment, older age, obesity, smoking, and neighborhood poverty are independently related to lower rates of cervical cancer screening. Specifically, those living in impoverished neighborhoods have limited access to testing and may benefit from screening outreach programs (Datta, 2006).
Cigarette smoking, both active and passive, increases cervical cancer risk. Among HPV-infected women, current and former smokers have a two- to threefold increased incidence of high-grade squamous intraepithelial lesion (HSIL) or invasive cancer. Passive smoking is also associated with increased risk, but to a lesser extent (Trimble, 2005). The mechanism underlying the association between smoking and this cancer is unclear, but smoking may alter HPV infection in those who smoke. For example, “ever smoking” has been associated with reduced clearance of high-risk HPV (Koshiol, 2006; Plummer, 2003). Tobacco smoke may also alter viral oncoprotein expression in cells in which the HPV is not integrated into the host genome (Wei, 2014).
Parity has a significant association with cervical cancer. Specifically, women with seven prior full-term pregnancies have an approximately fourfold risk, and those with one or two have a twofold risk compared with nulliparas (Muñoz, 2002).
Long-term combination oral contraceptive (COC) use is another risk. In women who are positive for cervical HPV DNA and who use COCs, cervical carcinoma rates increase by up to fourfold compared with women who are HPV-positive and never users (Moreno, 2002). Additionally, current COC users and women who are within 9 years of use have a significantly higher risk of developing both squamous cell and adenocarcinoma of the cervix (International Collaboration of Epidemiological Studies of Cervical Cancer, 2006). Encouragingly, the relative risk to COC users appears to decline after cessation. Data from 24 epidemiologic studies showed that by 10 or more years following COC cessation, cervical cancer risk returns to that of never users (International Collaboration of Epidemiological Studies of Cervical Cancer, 2007).
Sexual activity logically has an association as HPV is sexually transmitted. Having more than six lifetime sexual partners elevates the relative risk of cervical cancer. Similarly, an early age of first intercourse before age 20 confers an increased risk of developing this malignancy. Intercourse after age 21 only shows a trend toward an elevated risk. Moreover, abstinence from sexual activity and barrier protection during sexual intercourse decrease cervical cancer incidence (International Collaboration of Epidemiological Studies of Cervical Cancer, 2006).
Immunosuppressed women have an increased risk of developing cervical cancer. Cervical cancer is an acquired immune deficiency syndrome (AIDS)-defining illness. The standardized incidence ratio (SIR) of developing this cancer in HIV-infected women is 5.82. For transplant recipients the SIR is 2.013 for this malignancy (Grulich, 2007). Women with autoimmune diseases who use immunosuppressants do not appear to have an increased cervical cancer risk, except for azathioprine users (Dugue, 2015).
PATHOPHYSIOLOGY
Most women readily clear HPV, but those with persistent infection may develop preinvasive dysplastic cervical lesions. From such lesions, squamous cell carcinoma of the cervix typically arises at the squamocolumnar junction (Bosch, 2002). In general, progression from dysplasia to invasive cancer requires several years, although times can vary widely. The molecular alterations involved with cervical carcinogenesis are complex and not fully understood. Carcinogenesis currently is suspected to result from the interactive effects among environmental insults, host immunity, and somatic-cell genomic variations (Helt, 2002; Jones, 1997, 2006; Wentzensen, 2004).
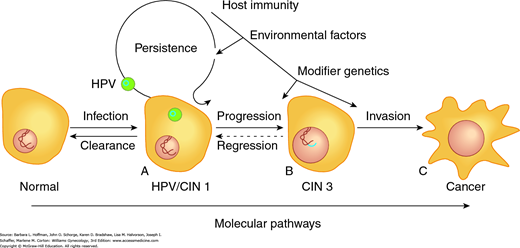
Increasing evidence suggests that HPV oncoproteins may be a critical component of continued cancer cell proliferation (Mantovani, 1999; Munger, 2001). Unlike low-risk serotypes, oncogenic HPV serotypes can integrate into human DNA (Fig. 30-1). As a result, with infection, oncogenic HPV’s early replication proteins E1 and E2 enable the virus to replicate within cervical cells. These proteins are expressed at high levels early in HPV infection. They can lead to cytologic changes detected as low-grade squamous intraepithelial (LSIL) cytologic findings on Pap smears.
FIGURE 30-1
Critical end points lie on the spectrum of cervical dysplasia. A. This initial point is the cell at risk due to active HPV infection. The HPV genome (blue ring) exists as a plasmid, separate from the host DNA. B. The clinically relevant preinvasive lesion, cervical intraepithelial neoplasia 3 (CIN 3) or carcinoma in situ (CIS), is an intermediate stage in cervical cancer development. The HPV genome has become integrated into the host DNA, resulting in increased proliferative ability. C. Interactive effects between environmental insults, host immunity, and somatic cell genomic variations lead to invasive cervical cancer.
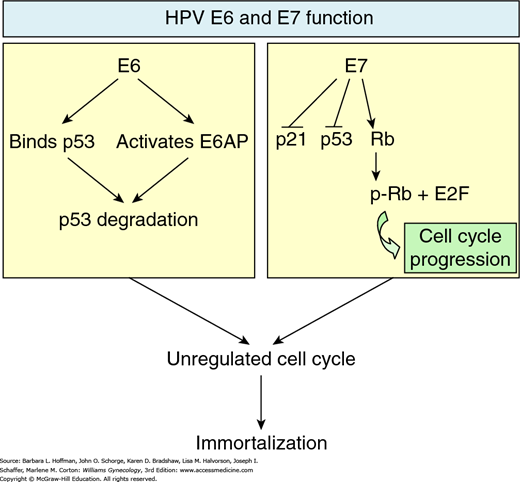
Amplification of viral replication and subsequent transformation of normal cells into tumor cells may follow (Mantovani, 1999). Specifically, the viral gene products E6 and E7 oncoproteins are implicated in this transformation (Fig. 30-2). E7 protein binds to the retinoblastoma (Rb) tumor suppressor protein, whereas E6 binds to the p53 tumor suppressor protein. In both instances, binding leads to degradation of these suppressor proteins. The E6 effect of p53 degradation is well studied and linked with the proliferation and immortalization of cervical cells (Jones, 1997, 2006; Mantovani, 1999; Munger, 2001).
FIGURE 30-2
Effects of E6 and E7 oncoproteins. On the left, viral oncoprotein E6 directly binds p53 and also activates E6AP to degrade p53 tumor suppressor protein. On the right, E7 oncoprotein phosphorylates retinoblastoma tumor suppressor protein, resulting in release of E2F transcription factors, which are involved in cell cycle progression. E7 also downregulates p21 tumor suppressor protein production and subverts p53 function. The cumulative effect of E6 and E7 oncoproteins eventually results in cell cycle alteration, promoting uncontrolled cell proliferation.
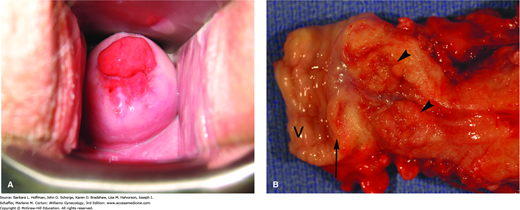
Following tumorigenesis, the pattern of local growth may be exophytic if a cancer arises from the ectocervix, or may be endophytic if it arises from the endocervical canal (Fig. 30-3). Lesions lower in the canal and on the ectocervix are more likely to be clinically visible during physical examination. Alternatively, growth may be infiltrative, and in these cases, ulcerated lesions are common if necrosis accompanies this growth. As primary lesions enlarge and lymphatic involvement progresses, local invasion increases and will eventually become extensive.
FIGURE 30-3
Cervical adenocarcinoma. A. Invasive cancer originating from the endocervix. (Photograph contributed by Dr. David Miller.) B. Exophytic growth of cervical adenocarcinoma into the endocervical canal (arrowheads). In this radical hysterectomy specimen, proximal vagina (V) is excised with the cervix, and an arrow marks the ectocervix.
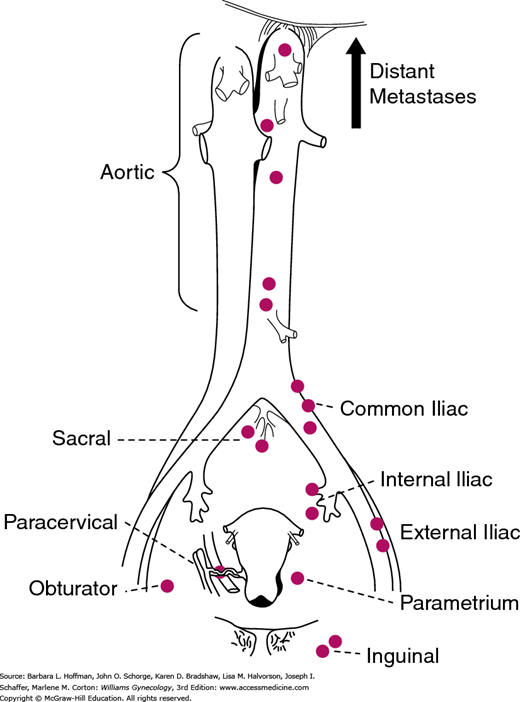
The pattern of tumor spread typically follows cervical lymphatic drainage. Thus, familiarity with this drainage aids understanding the surgical steps of radical hysterectomy performed for this cancer (Section 46-1). The cervix has a rich network of lymphatics, which follow the course of the uterine artery (Fig. 30-4). These channels drain principally into the paracervical and parametrial lymph nodes. These lymph nodes are clinically important and thus are removed as part of parametrial resection during radical hysterectomy. From the parametrial and paracervical nodes, lymph subsequently flows into the obturator lymph nodes and into the internal, external, common iliac lymph nodes, and ultimately the paraaortic lymph nodes. Accordingly, pelvic and common iliac lymph nodes are also traditionally removed concurrently with radical hysterectomy. In contrast, lymphatic channels from the posterior cervix course through the rectal pillars and the uterosacral ligaments to the rectal lymph nodes. These nodes are also encountered and are removed during the extended resection of the uterosacral ligaments that is characteristic of radical hysterectomy.
FIGURE 30-4
Lymphatic drainage of the cervix. The parametrial lymph nodes are removed as part of the radical hysterectomy. Lymph node dissection for cervical cancer includes removal of pelvic lymph nodes (from the external iliac artery and vein, internal iliac artery, and the common iliac artery) with or without a paraaortic lymph node dissection to the level of the inferior mesenteric artery. (Reproduced with permission from Henriksen E: The lymphatic spread of carcinoma of the cervix and of the body of the uterus; a study of 420 necropsies, Am J Obstet Gynecol 1949 Nov;58(5):924–942.)
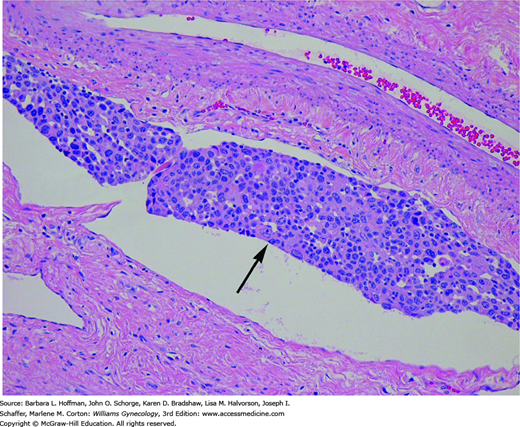
As tumor invades deeper into the stroma, it enters blood capillaries and lymphatic channels (Fig. 30-5). Termed lymphovascular space involvement (LVSI), this type of invasive growth is not included in the clinical staging of cervical cancer. However, its presence is regarded as a poor prognostic indicator, especially in early-stage cervical cancers. Thus, the presence of LVSI often requires tailoring the planned surgical procedure and adjuvant radiation treatment.
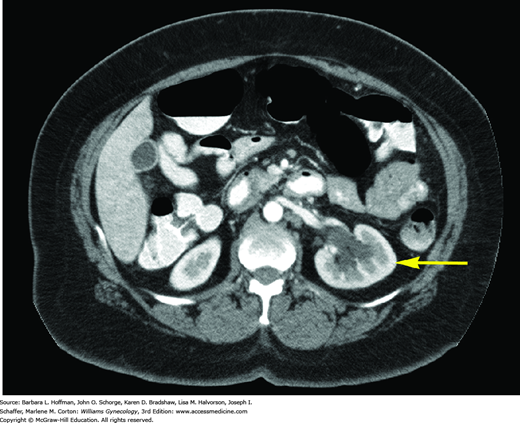
With extension through the parametria to the pelvic sidewall, ureteral blockage frequently develops, resulting in hydronephrosis (Fig. 30-6). Additionally, the bladder may be invaded by direct tumor extension through the vesicouterine ligaments (bladder pillars). The rectum is invaded less often because it is anatomically separated from cervix by the posterior cul-de-sac. Distant metastasis results from hematogenous dissemination, and the lungs, ovaries, liver, and bone are the most frequently affected.
HISTOLOGIC TYPES
The two most common histologic subtypes of cervical cancer are squamous cell and adenocarcinoma (Table 30-2). Of these, squamous cell tumors predominate, comprise about 70 percent of all cervical cancers, and arise from the ectocervix. Over the past 30 years, the incidence of squamous cell cancers has declined, whereas that of cervical adenocarcinoma has risen. These changes may be attributed to an improved method of screening for early squamous lesions of the cervix and an increase in HPV prevalence (Vizcaino, 2000). Squamous cell carcinomas can be subdivided into keratinizing and nonkeratinizing carcinomas (Fig. 30-7).
| Squamous |
| Keratinizing |
| Nonkeratinizing |
| Papillary |
| Adenocarcinoma |
| Mucinous |
| Endocervical |
| Intestinal |
| Minimal deviation |
| Villoglandular |
| Endometrioid |
| Serous |
| Clear cell |
| Mesonephric |
| Mixed cervical carcinoma |
| Adenosquamous |
| Glassy cell |
| Neuroendocrine cervical tumor |
| Large cell neuroendocrine |
| Small cell neuroendocrine |
| Others |
| Sarcoma |
| Lymphoma |
| Melanoma |
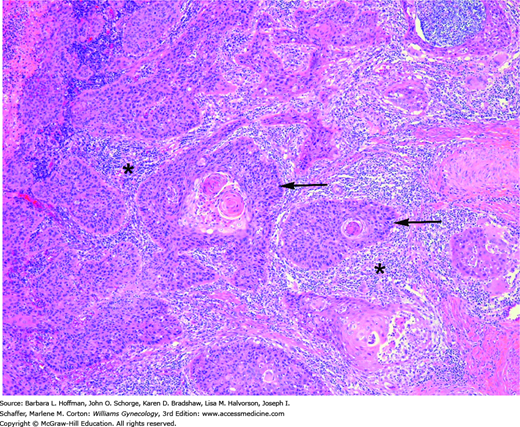
FIGURE 30-7
Squamous cell cervical cancer. Irregular nests of malignant cells (arrows) show eosinophilic keratin pearls at their center, which is a classic diagnostic feature. These nests invade the stroma accompanied by a brisk lymphocytic response (asterisks). (Used with permission from Dr. Raheela Ashfaq.)
Adenocarcinomas are a group of cervical cancers composed of the subtypes listed in Table 30-2. In contrast to squamous cell cervical carcinoma, adenocarcinomas make up 25 percent of cervical cancers and arise from the endocervical mucus-producing columnar cells. Because of this origin within the endocervix, adenocarcinomas are often occult and may be advanced before becoming clinically evident. They often give the cervix a palpable barrel shape during pelvic examination.
Adenocarcinomas exhibit various histologic patterns composed of diverse cell types. Of these, mucinous adenocarcinomas are the most common and are subdivided as shown in Table 30-2. The mucinous endocervical type retains resemblance to normal endocervical tissue (Fig. 30-8). The intestinal type resembles intestinal cells and may include goblet cells. Minimal deviation adenocarcinoma, also known as adenoma malignum, is characterized by cytologically bland glands that are abnormal in size and shape. These tumors contain an increased number of glands positioned at a deeper level than normal endocervical glands. Women with Peutz-Jeghers syndrome are at increased risk of developing adenoma malignum. Villoglandular adenocarcinomas are made up of surface papillae. Endometrioid adenocarcinomas are the second most frequently identified and display glands resembling those of the endometrium. Serous carcinoma is identical to serous carcinomas of the ovaries or uterus and is rare. Clear cell adenocarcinoma accounts for less than 5 percent of cervical adenocarcinomas and is named for its clear cytoplasm (Jaworski, 2009). Rarely, adenocarcinomas arise in mesonephric remnants in the cervix and are termed mesonephric adenocarcinomas.
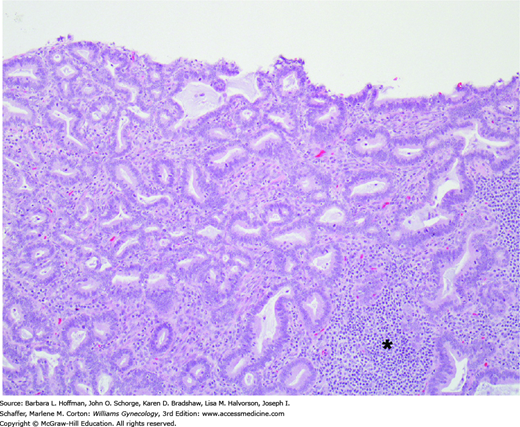
FIGURE 30-8
This invasive cervical adenocarcinoma is characterized by columnar cells with moderate nuclear atypia and mitotic activity. Tumor cells form glands that resemble native endocervical glands but invade the stroma haphazardly. A chronic inflammatory infiltrate is present on the right (asterisk).
Evidence describing the prognosis of squamous cell carcinoma compared with that of adenocarcinoma is contradictory. For stage IB and IIA cervical cancer, one study showed a statistically significant lower overall survival rate in those with adenocarcinoma compared with women with squamous cell carcinoma (Landoni, 1997). However, the Gynecologic Oncology Group (GOG) found in a subsequent study that overall survival rates in women with stage IB squamous and adenocarcinomas of the cervix are similar (Look, 1996). For advanced-stage (stage IIB to IVA) cancers, evidence suggests that cervical adenocarcinomas may portend a poorer overall survival rate compared with similarly staged squamous cell carcinomas (Eifel, 1990; Lea, 2002). The 2006 International Federation of Obstetricians and Gynecologists (FIGO) annual report, which reported on more than 11,000 squamous carcinomas and 1613 adenocarcinomas, demonstrated that women with adenocarcinomas have worse overall survival rates at every stage compared with those with squamous cell carcinoma (Quinn, 2006). In sum, evidence suggests that adenocarcinoma of the cervix is a high-risk cell type.
Mixed cervical carcinomas are rare. Of these, adenosquamous carcinomas do not differ grossly from adenocarcinomas of the cervix. The squamous component is poorly differentiated and shows little keratinization. Glassy cell carcinoma describes a form of poorly differentiated adenosquamous carcinoma in which cells display cytoplasm with a ground-glass appearance.
Neuroendocrine tumors of the cervix include large cell and small cell tumors. These rare malignancies are highly aggressive, and even early-stage cancers have a relatively low disease-free survival rate despite treatment with radical hysterectomy and adjuvant chemotherapy (Albores-Saavedra, 1997; Viswanathan, 2004). Often, neuroendocrine markers, including chromogranin, synaptophysin, and CD56, are used to confirm the diagnosis. Uncommonly, endocrine and paraendocrine tumors are associated with these neuroendocrine tumors.
Of other rare types, the cervix may be the site of sarcomas, malignant lymphomas, and melanomas. Cervical leiomyosarcomas and cervical stromal sarcomas have a poor prognosis, similar to that for uterine sarcomas. Melanomas often present as ulcerated blue or black nodules and also have a poor prognosis.
DIAGNOSIS
Some women diagnosed with this cancer are asymptomatic. In others, early-stage cervical cancer may create a watery, blood-tinged vaginal discharge. Intermittent vaginal bleeding that follows coitus or douching can also be noted. As a malignancy enlarges, bleeding typically intensifies, and occasionally, a woman presents with uncontrolled hemorrhage from a tumor bed. In such cases, bleeding can often be controlled with a combination of Monsel paste (ferric subsulfate) and vaginal packing. Topical acetone can also be used to obtain hemostasis, especially in cases refractory to Monsel paste (Patsner, 1993). Burning from acetone makes it a less desirable choice than Monsel paste. With vaginal packing, the patient ideally remains at bed rest, and a Foley catheter is concurrently inserted to drain the bladder. The pack may interfere with normal voiding, and the catheter also allows for accurate monitoring of urine output during volume repletion. If bleeding continues, emergent radiation can be delivered. Alternatively, internal iliac artery embolization or ligation can be performed in cases of refractory hemorrhage. However, caution is used for these latter two options, as tumor oxygenation is decreased if these blood supplies are occluded. Radiation therapy is a primary component of advanced-stage cancer, and as noted in Chapter 28, radiation’s effects are diminished in low-oxygen environments and can translate into worsening disease-specific survival rates (Kapp, 2005). In those with significant bleeding, hemodynamic support of the patient follows that described in Chapter 40.
Most women with this cancer have normal general physical examination findings. In those with suspected cervical cancer, a thorough external genital and vaginal examination is performed. Because HPV is a shared risk factor for cervical, vaginal, vulvar, and anal cancers, concomitant lesions are sought. With speculum examination, the cervix may appear grossly normal if cancer is microinvasive. Visible disease displays varied appearances. Lesions may be an exophytic or endophytic growth; a polypoid mass, papillary tissue, or barrel-shaped cervix; a cervical ulceration or granular mass; or necrotic tissue. A watery, purulent, or bloody discharge can also be seen. For this reason, cervical cancer may mirror the appearance of a cervical leiomyoma, cervical polyp, vaginitis, cervical eversion, cervicitis, threatened abortion, placenta previa, cervical pregnancy, condyloma acuminata, herpetic ulcer, chancre, or a prolapsing uterine leiomyoma, polyp, or sarcoma.
During bimanual examination, a clinician may palpate an enlarged uterus resulting from tumor invasion and growth. Alternatively, hematometra or pyometra may expand the endometrial cavity following obstruction of fluid egress by a primary cervical cancer. In this case, the uterus may feel enlarged and boggy. Advanced cervical cancer cases can extend into the vagina, and disease extent can be appreciated during anterior vaginal wall palpation or during rectovaginal examination. With posterior spread, palpation of the rectovaginal septum between the index and middle finger of an examiner’s hand reveals a thick, hard, irregular septum. The proximal posterior vaginal wall is most commonly invaded. In addition, during digital rectal examination, parametrial, uterosacral, and pelvic sidewall involvement can be appreciated. Either one or both parametria may be invaded, and involved tissues feel thick, irregular, firm, and less mobile. A fixed mass indicates that tumor has probably extended to the pelvic sidewalls. However, a central lesion can become as large as 8 to 10 cm in diameter before reaching the sidewall.
With advancing disease, enlarged supraclavicular or inguinal lymphadenopathy suggest lymphatic tumor spread. Lower extremity edema and low back pain, often radiating down the posterior leg, may reflect compression of the sciatic nerve root, lymphatics, veins, or ureter by an expanding tumor. With ureteral obstruction, hydronephrosis and uremia can follow and may occasionally be the initial presenting finding. In these cases, ureteral stenting or percutaneous nephrostomy tube insertion are usually required. Kidney function is ideally preserved for chemotherapy. Additionally, with tumor invasion into the bladder or rectum, hematuria and/or vesicovaginal or rectovaginal fistula may be found.
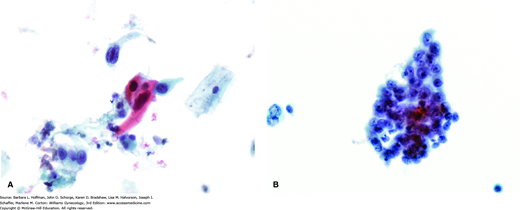
Histologic evaluation of cervical biopsy is the primary tool to diagnose cervical cancer. Although Papanicolaou (Pap) tests are performed extensively to screen for this cancer, this test does not always detect cervical cancer. Specifically, Pap testing has only a 53- to 80-percent sensitivity for detecting high-grade lesions on any given single test (Agorastos, 2015; Benoit, 1984; Soost, 1991). Thus, the preventive power of Pap testing lies in regular serial screening (Fig. 30-9). Moreover, in women who have stage I cervical cancer, only 30 to 50 percent of single cytologic smears obtained are read as positive for cancer (Benoit, 1984). Hence, Pap testing alone for evaluation of a suspicious lesion is discouraged. Instead, these lesions are directly biopsied with Tischler biopsy forceps or a Kevorkian curette (Fig. 29-16). When possible, biopsies are taken from the tumor periphery, as central portions often contain only necrotic tissue, which will fail to yield a diagnosis. Moreover, biopsies ideally include underlying stroma, so that invasion, if present, can be assessed.
FIGURE 30-9
A. Pap smear, squamous cell carcinoma. Some show spindled tumor cells and/or cytoplasmic keratinization, as evidenced by dense orangeophilic cytoplasm. B. Pap smear, endocervical adenocarcinoma. This shows malignant cytologic features including nuclear pleomorphism, nuclear membrane abnormalities, and nucleolar prominence. Cytoplasm tends to be more delicate than in squamous carcinoma and may contain mucin. (Photographs contributed by Ann Marie West, MBA, CT[ASCP].)
If abnormal Pap test findings are noted, colposcopy is often performed, and adequate cervical and endocervical biopsies are obtained. In some cases, cold knife conization is needed for this. Indications for colposcopy and conization are outlined in Chapter 29, and cervical punch biopsies or conization specimens are the most accurate for allowing assessment of cervical cancer invasion. Both sample types typically contain underlying stroma and enable differentiation between invasive and in situ carcinomas. For this determination, conization specimens provide a larger tissue sample and are most helpful.
STAGING
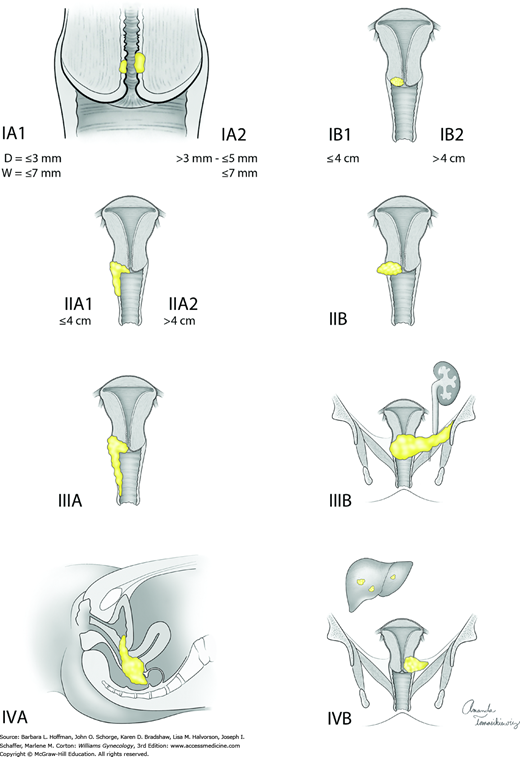
Cervical cancers are staged clinically. Allowable components of staging include cold-knife conization, pelvic examination under anesthesia, cystoscopy, proctoscopy, chest radiograph, and intravenous pyelogram (or this portion of the computed tomography [CT] scan can be used). Table 30-3 lists these and also contains radiologic and laboratory tools that are not included in formal staging but may contribute additional information. Bullous edema is not sufficient for the diagnosis of bladder involvement, and this involvement must be biopsy proven. Lymph node metastasis does not change the clinical stage. The staging system widely used for cervical cancer is that developed by the FIGO in collaboration with the World Health Organization (WHO) and the International Union Against Cancer (UICC). This staging was updated in 2009 and is detailed in Table 30-4 and Figure 30-10. In this chapter, early-stage disease refers to FIGO stages I through IIA. The term advanced-stage disease describes stages IIB and higher.
| Testing | To Identify: |
| Laboratory | |
|
|
| Radiologic | |
|
|
| Procedural | |
|
|
| Stage | Characteristics |
| 0 | Carcinoma in situ, cervical intraepithelial lesion (CIN) 3 |
| I | Carcinoma is strictly confined to cervix (extension to corpus should be disregarded) |
| IA | Microscopic lesion, invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm |
| IA1 | Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm |
| IA2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm in depth and no wider than 7 mm |
| IB | Clinical lesions confined to the cervix or preclinical lesions greater than IA |
| IB1 | Clinical lesions no greater than 4 cm in size |
| IB2 | Clinical lesions greater than 4 cm in size |
| II | Carcinoma extends beyond cervix but has not extended to pelvic wall; it involves vagina, but not as far as the lower third |
| IIA | No obvious parametrial invasion |
| IIA1 | Clinical lesions no greater than 4 cm in size |
| IIA2 | Clinical lesions greater than 4 cm in size |
| IIB | Obvious parametrial involvement |
| III | Carcinoma has extended to the pelvic wall; on rectal examination there is no cancer-free space between tumor and pelvic wall; tumor involves lower third of vagina; all cases with hydronephrosis or nonfunctioning kidney should be included, unless they are known to be due to another cause |
| IIIA | No extension to pelvic wall, but involvement of lower third of vagina |
| IIIB | Extension to pelvic wall, or hydronephrosis or nonfunctioning kidney due to tumor |
| IV | Carcinoma has extended beyond true pelvis or has clinically involved mucosa of bladder or rectum |
| IVA | Spread of growth to adjacent pelvic organs |
| IVB | Spread to distant organs |
Within each oncology chapter of this text, staging of each cancer type (cervix, vulva, vagina, uterus, ovary) will be presented. FIGO classification is used for gynecologic cancers. In contrast, the American Joint Committee on Cancer (AJCC) developed the TNM Staging System, which is based on the extent of the tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M). Breast cancer is staged with this latter system, as shown in Table 12-5.
RADIOLOGIC IMAGING
As discussed, cervical cancer is staged clinically, and accurate evaluation is critical to appropriate treatment planning. For example, early-stage tumors may be treated surgically, whereas more advanced tumors require radiation and/or chemotherapy. Although imaging does not affect assignment of stage (except lung metastases seen on chest radiograph and hydronephrosis seen on CT scan), imaging results can tailor treatment for an individual. In addition, lymph node metastases, although not included in the FIGO system, worsen patient prognosis and may be identified with imaging. Thus, radiologic tools such as CT scanning, magnetic resonance (MR) imaging, or positron emission tomography (PET) scanning are commonly used as adjuncts in initial cervical cancer evaluation. However, no uniform approach to the use of these has been developed.
For defining anatomy, this high-resolution imaging tool offers superior contrast resolution at soft-tissue interfaces. Thus, MR imaging effectively measures tumor size, delineates cervical tumor boundaries, and identifies surrounding bladder, rectal, or parametrial invasion. Unfortunately, MR imaging is less accurate for diagnosing microscopic or deep cervical stromal invasion or identifying minimal parametrial extension (Mitchell, 2006). These particular distinctions are important as both stromal and parametrial invasion may affect suitability for a given planned treatment. In addition, false-negative findings occur with small volumes of disease and with tissue foci in which cancer cannot be differentiated from scar or necrosis. In these cases, PET scanning identifies metabolic rather than anatomic changes and can be a complementary tool.
For primary cervical cancer, MR imaging is superior to CT for determining carcinoma size, local tumor extension, and lymph node involvement (Bipat, 2003; Mitchell, 2006; Subak, 1995). MR imaging is often preferred for patients being considered for fertility-sparing radical trachelectomy (Abu-Rustum, 2008; Olawaiye, 2009). Overall, however, both MR imaging and CT perform similarly in cervical cancer (Hricak, 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree