INTRODUCTION
Office gynecology frequently involves the diagnosis and management of preinvasive lower genital tract disease, most often involving the uterine cervix. Since widespread introduction of the Papanicolaou (Pap) test in the 1950s, cervical cancer screening has reduced the incidence of and mortality rate from invasive cervical cancer by more than 70 percent (Howlader, 2014). This is true despite a continued rise in the incidence of preinvasive lesions (Kurdgelashvili, 2013). Approximately 7 percent of U.S. women who undergo Pap testing will have an abnormal result (Wright, 2012). An abnormal screening test prompts further patient evaluation, usually with colposcopy and biopsy. Histologic results are more definitive and inform appropriate management.
LOWER GENITAL TRACT NEOPLASIA
In the lower genital tract (LGT), the term intraepithelial neoplasia refers to squamous epithelial lesions that are potential precursors of invasive cancer. These lesions demonstrate a range of histologic abnormality from mild to severe based on cytoplasmic, nuclear, and histologic changes. The severity of a squamous intraepithelial lesion is graded by the proportion of epithelium with abnormal cells from the basement membrane upward toward the surface. In the case of cervical intraepithelial neoplasia (CIN), abnormal cells confined to the lower third of the squamous epithelium are referred to as mild dysplasia or CIN 1, extending into the middle third as moderate dysplasia or CIN 2, into the upper third as severe dysplasia or CIN 3, and full-thickness involvement as carcinoma in situ (CIS) (Fig. 29-1). Squamous neoplasia of the vagina, vulva, perianal, and anal squamous epithelia (VaIN, VIN, PAIN, and AIN, respectively) are graded similarly with the caveat that VIN 1 is no longer recognized. The natural history of these extracervical lesions is less understood than for CIN. In contrast, the cervical columnar epithelium does not demonstrate an analogous neoplastic disease spectrum because it is only one cell-layer thick. Histologic abnormalities are therefore limited to either adenocarcinoma in situ (AIS) or adenocarcinoma.
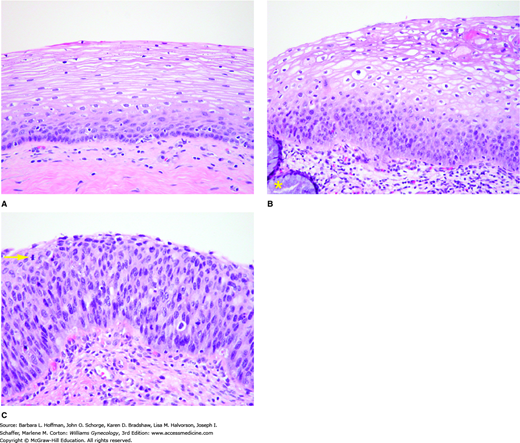
FIGURE 29-1
A. Normal ectocervical epithelium is a nonkeratinizing, stratified squamous epithelium. Mitoses are normally confined to the lower layers, namely, the basal and parabasal epithelial layers. B. Low-grade squamous intraepithelial lesion (LSIL). This biopsy’s location at the transformation zone is indicated by the presence of both columnar epithelium (asterisk) and squamous epithelium. Low-grade SIL has a disordered proliferation of squamous cells and increased mitotic activity confined to the basal one third of the epithelium. Koilocytotic atypia, which is indicative of proliferative HPV infection, involves the more superficial epithelium. Koilocytosis is typified by nuclear enlargement, coarse chromatin, nuclear “wrinkling,” and perinuclear halos. C. This high-grade SIL shows disordered, highly atypical squamous cells and increased mitotic activity involving the full thickness of the epithelium. Note the mitotic figure located close to epithelial surface (yellow arrow). (Used with permission from Dr. Kelley Carrick.)
The concept of cervical neoplasia as a spectrum has come under question with increasing insight into human papillomavirus (HPV) infection. Mild squamous dysplasia is now recognized as evidence of HPV infection, most of which is transient and unlikely to progress. Moderate to severe dysplastic squamous lesions are considered to be true cancer precursors. Current cytology reporting reflects this two-tier concept (Solomon, 2002). In 1989, the Bethesda System nomenclature replaced CIN with squamous intraepithelial lesion (SIL). Because cytologic and histologic changes of HPV infection and CIN 1 cannot be distinguished reliably and because of their like natural histories, they are categorized together as low-grade squamous intraepithelial lesions (LSIL). Similarly, CIN 2, CIN 3, and CIS are difficult to distinguish, are truer cancer precursors, and are all designated as high-grade squamous intraepithelial lesions (HSIL). The diagnostic distinction between LSIL and HSIL is more reliable, biologically plausible, and clinically meaningful than diagnoses using the CIN system. This two-tiered nomenclature is now recommended, and guidelines for the management of these lesions are grouped accordingly (Darragh, 2012).
ANATOMIC CONSIDERATIONS
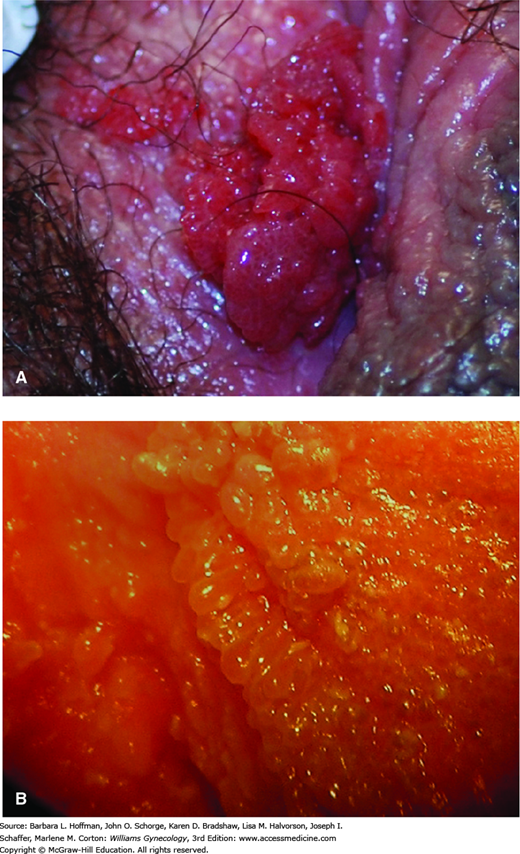
Precancerous lesions of the female LGT are often multifocal, can involve any of its structures, and may appear similar to benign processes. For example, micropapillomatosis labialis is a benign anatomic variant characterized by minute epithelial projections on the inner labia minora (Fig. 29-2). This condition can be easily mistaken for HPV-related lesions, but true HPV lesions tend to be multifocal and asymmetric, and to have multiple papillations arising from a single base (Ferris, 2004). Micropapillomatosis often shows spontaneous regression, and treatment is not indicated (Bergeron, 1990).
FIGURE 29-2
Benign lower genital tract lesions. A. Condylomata tend to be multifocal, asymmetric, and have multiple papillations arising from a single base. B. Micropapillomatosis labialis is a normal variant of vulvar anatomy encountered along the inner aspects of the labia minora and lower vagina. In contrast to condylomata, projections are uniform in size and shape and arise singly from their base attachments.
The vagina is lined by nonkeratinized squamous epithelium, and glands are absent. However, areas of columnar epithelium are occasionally found within the vaginal squamous mucosa, a condition termed adenosis. It is often attributable to in utero exposure to exogenous estrogen, particularly diethylstilbestrol (DES) (Trimble, 2001). These areas are red patches within the squamous epithelium and can be mistaken for ulcers or other lesions. With DES-related adenosis, careful palpation of the vagina is warranted in addition to visual inspection, as clear cell adenocarcinoma may be palpable before becoming visible.
During embryogenesis, upward migration of stratified squamous epithelium from the urogenital sinus and vaginal plate is thought to replace müllerian epithelium (Ulfelder, 1976). This process usually terminates near the external cervical os, forming the original (congenital) squamocolumnar junction (SCJ). When visible on the ectocervix, the SCJ is a pink, smooth squamous epithelium juxtaposed against the red, velvety columnar epithelium surrounding the external cervical os. Rarely, this migration is incomplete resulting in an SCJ in the upper vaginal fornices. This is a normal variant and also seen with in utero DES exposure.
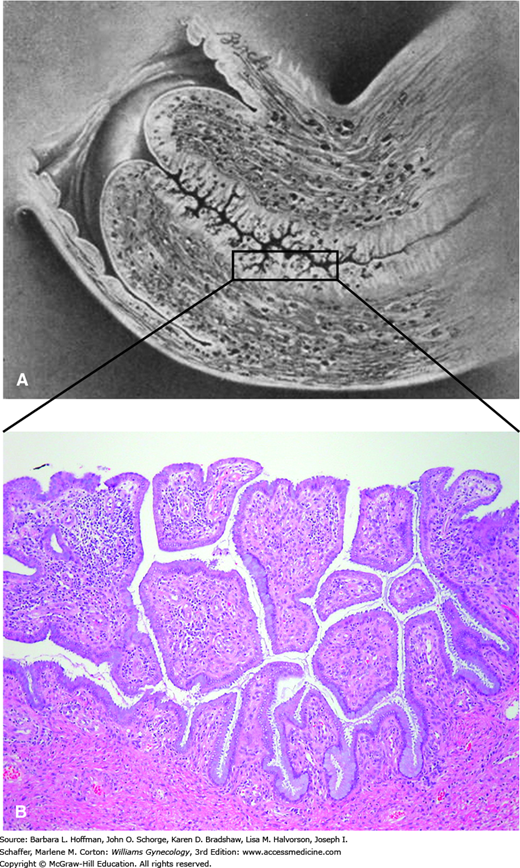
The columnar epithelium is commonly referred to as “glandular.” This is because it produces mucus, and its deep infoldings appear histologically similar to glandular tissue (Fig. 29-3). However, true glands, consisting of acini and ducts, are not present on the cervix (Ulfelder, 1976).
FIGURE 29-3
Endocervical anatomy. A. Sagittal view of the cervix. In this drawing, a portion of the endocervical canal is boxed. (Modified with permission from Eastman NJ, Hellman LM: Williams Obstetrics 12th ed., New York: Appleton-Century-Crofts, Inc; 1961.) B. The endocervix is lined by a simple columnar, mucin-secreting epithelium. Crypts and small exophytic projections appear pseudopapillary when viewed in cross section. (Used with permission from Dr. Kelley Carrick.)
The location of the SCJ varies with age and hormonal status (Fig. 29-4). During the reproductive years, it everts outward onto the ectocervix, especially during adolescence, pregnancy, and with combination hormonal contraceptive use. It regresses into the endocervical canal during the natural process of squamous metaplasia and in low-estrogen states such as menopause, prolonged lactation, and long-term progestin-only contraceptive use.
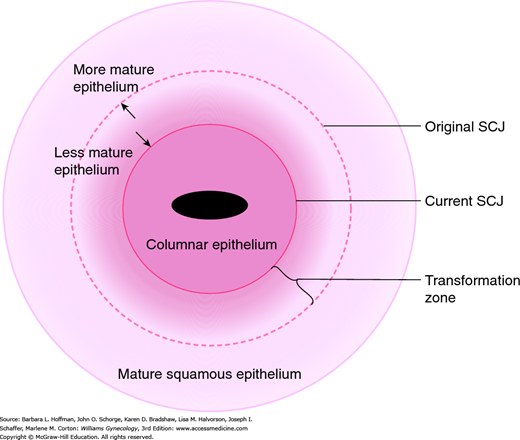
At puberty, the rise in estrogen levels leads to increased glycogenation of the LGT nonkeratinized squamous epithelium. In providing a carbohydrate source, glycogen allows vaginal flora to be dominated by lactobacilli, which produce lactic acid. The resultant acidic vaginal pH is the suspected stimulus for squamous metaplasia, which is the normal replacement of columnar by squamous epithelium on the cervix. Relatively undifferentiated reserve cells underlying the cervical epithelia are the apparent precursors of the new metaplastic cells, which differentiate further into squamous epithelium. This normal process creates a progressively widening band of metaplastic and maturing squamous epithelium, termed the transformation zone (TZ), between the congenital (original) columnar epithelium and the squamous epithelium (Fig. 29-5).
FIGURE 29-5
Schematic describing relevant cervical landmarks. The original squamocolumnar junction (SCJ) marks the terminal site of the upward migration of squamous epithelium during embryonic development. The SCJ location moves with age and hormonal status. With higher estrogen states, the SCJ everts outward. With low-estrogen states and with squamous metaplasia, the SCJ moves closer to the cervical os. The transformation zone consists of the band of squamous metaplasia lying between the original SCJ and new (current) SCJ. As the metaplastic epithelium matures, it moves outward relative to the newer, less mature areas of metaplasia and can become indistinguishable from the original squamous epithelium.
Nearly all cervical neoplasia, both squamous and columnar, develops within the TZ, usually adjacent to the new or current SCJ. Cervical reserve and immature metaplastic cells appear particularly vulnerable to the oncogenic effects of HPV and cocarcinogens (Stanley, 2010a). Squamous metaplasia is most active during adolescence and pregnancy. This may explain why early ages of first sexual activity and of first pregnancy are cervical cancer risk factors.
HUMAN PAPILLOMAVIRUS
The causative role of HPV in nearly all cervical neoplasia and a significant proportion of vulvar, vaginal, and anal neoplasia is firmly established. HPV primarily infects human squamous or metaplastic epithelial cells. It is a double-stranded DNA virus with a protein capsid unique to each viral type. More than 150 genetically distinct HPV types have been identified, and of these, approximately 40 types infect the LGT (Doorbar, 2012).
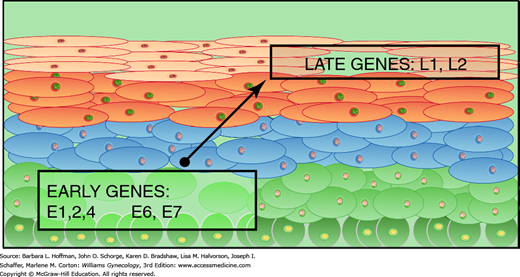
The circular HPV genome consists of only nine identified open reading frames (Stanley, 2010a). In addition to one regulatory region, the six “early” (E) genes govern functions early in the viral life cycle, including DNA maintenance, replication, and transcription. Early genes are expressed in the lower squamous epithelial layers (Fig. 29-6). The two “late” genes encode the major (L1) and minor (L2) capsid proteins. These proteins are expressed in the superficial epithelial layers late in the viral life cycle and during the assemblage of new, infectious viral particles. Sequential HPV gene expression is synchronous with and dependent on squamous epithelial differentiation. Thus, completion of the viral life cycle takes place only within an intact, fully differentiating squamous epithelium (Doorbar, 2012). This makes it nearly impossible to culture HPV in vitro. HPV is a nonlytic virus, and therefore infectiousness depends on normal desquamation of infected epithelial cells. A new infection is initiated when the L1 and L2 capsid proteins bind to the epithelial basement membrane and/or basal cells, permitting entry of HPV viral particles into cells of a new host (Sapp, 2009).
FIGURE 29-6
The human papillomavirus life cycle is completed in synchrony with squamous epithelium differentiation. Early genes, including the E6 and E7 oncogenes, are expressed most strongly within the basal and parabasal layers. The late genes encoding capsid proteins are expressed later in the superficial layers. Intact virus is shed during normal desquamation of superficial squames. Late genes are not strongly expressed in high-grade neoplastic lesions.
Genital HPV is the most common sexually transmitted disease (STD) in the United States, and most sexually active adults are infected at some time (Dunne, 2014). Most incident HPV infections develop in women younger than 25 years. The point prevalence in U.S. females aged 14 to 59 years is 27 percent. It is highest in those aged 20 to 24 years (45 percent) and becomes less prevalent with increasing age (Dunne, 2007). Clinically, HPV types are classified as high-risk (HR) or low-risk (LR) based on their strength of association with cervical cancer. LR HPV types 6 and 11 cause nearly all genital warts, laryngeal papillomas, and a minority of subclinical HPV infections. LR HPV infections are rarely, if ever, oncogenic.
In contrast, persistent HR HPV infection is now viewed as required for the development of cervical cancer. HR HPV types, including 16, 18, 31, 33, 35, 45, and 58, along with a few less common types, account for approximately 95 percent of cervical cancer cases worldwide (Muñoz, 2003). HPV 16 is the most oncogenic, accounting for the largest percentage of CIN 3 lesions (45 percent) and cervical cancers (55 percent) worldwide. It is also the dominant type in other HPV-related anogenital and oropharyngeal cancers (Schiffman, 2010; Smith, 2007). Although the prevalence of HPV 18 is much lower than that of HPV 16 in the general population, it is found in 13 percent of cervical squamous cell carcinomas, and in an even higher proportion of cervical adenocarcinomas and adenosquamous carcinomas (approximately 40 percent) (Bruni, 2010; Smith, 2007). Together, HPVs 16 and 18 account for approximately 70 percent of cervical cancers worldwide, 68 percent of squamous cell carcinomas, and 85 percent of adenocarcinomas (Bosch, 2008). HPV type 45 is the third most common found in cervical cancers (de Sanjose, 2010). HPV 16 accounts for more than 1 in 5 cervical HPV infections and is the most common HPV found among low-grade lesions and in women without neoplasia (Bruni, 2010; Herrero, 2000). Thus, HR HPV infection does not cause neoplasia in most infected women, and additional host, viral, and environmental factors determine progression to LGT neoplasia.
The most important risk factors for the acquisition of genital HPV infection are the number of lifetime and recent sexual partners and early age at first sexual intercourse (Burk, 1996; Fairley, 1994; Franco, 1995). Genital HPV is transmitted by direct, usually sexual, contact with the genital skin, mucous membranes, or body fluids of an individual with either warts or subclinical HPV infection. The infectivity of inapparent (subclinical) HPV is assumed to be high. HPV is thought to access to the basal cell layer and basement membrane through microabrasions of the genital epithelium during sexual contact. Once infected, these basal cells may become a viral reservoir (Stanley, 2010b).
Cervical HR HPV infection generally requires penetrative intercourse. Oral-genital and hand-genital HPV transmissions are possible but are much less common than with genital-genital transmission (Winer, 2003). Women who have sex with women have rates of HR HPV positivity, abnormal cervical cytology, and high-grade cervical neoplasia similar to those of heterosexual women, but undergo cervical cancer screening less often. Women with or without past sexual experiences with men have a similar risk, implying that digital, oral, and perhaps object contact places them at risk of HR HPV infection (Marrazzo, 2000). Thus, all women who are sexually active should undergo cervical cancer screening according to current recommendations regardless of sexual orientation.
Genital HPV detection, including HR HPV, has been reported in apparently sexually naïve girls and young women (Doerfler, 2009; Winer, 2003). Nonetheless, genital warts that develop in children after infancy are always reason to consider the possibility of sexual abuse. HPV infection by nonsexual contact, autoinoculation, or fomite transfer appears possible. This is supported by reports of nongenital HPV types in a significant minority of pediatric and adolescent genital wart cases (Cohen, 1990; Obalek, 1990; Siegfried, 1997).
Congenital HPV infection from vertical transmission (mother to fetus or newborn) beyond transient skin colonization is rare. Conjunctival, laryngeal, vulvar, or perianal warts present at birth or that develop within 1 to 3 years of birth are most likely due to perinatal exposure to maternal HPV (Cohen, 1990). Infection is not linked to maternal genital warts or route of delivery (Silverberg, 2003; Syrjänen, 2010). Accordingly, cesarean delivery generally is not recommended for maternal HPV infection. Exceptions include cases of large genital warts that would obstruct delivery or might avulse and bleed with cervical dilation or vaginal delivery.
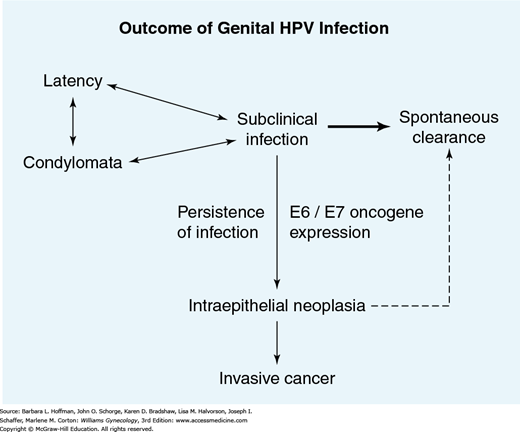
Genital HPV infection causes variable outcomes (Fig. 29-7). These can be broadly grouped as latent or expressed infections. Infection expression may be productive, that is, creating infectious viral particles, or it may be neoplastic, causing preinvasive disease or malignancy. Most productive infections are subclinical, but a smaller percentage yields clinically apparent genital warts. Last, HPV infection can be transient or can become persistent. High-grade neoplasia (CIN 3 or worse) is the least common outcome of genital HPV infection, requiring HPV persistence.
FIGURE 29-7
The natural history of genital human papillomavirus (HPV) infection varies between individuals and over time. Most infections are subclinical. Spontaneous resolution is the most common outcome. Neoplasia is the least common manifestation of HPV infection, developing as the result of persistent infection with integration of HPV DNA.
Latent infection refers to that in which cells are infected, but HPV remains quiescent. There are no detectable tissue effects, as the virus is not actively replicating. The virus is present below detectable levels. Thus, it is uncertain whether apparent clearance of the HPV constitutes true eradication of HPV from infected tissues or whether it reflects latency.
Productive infections are characterized by viral life-cycle completion and plentiful production of infectious viral particles (Stanley, 2010a). Viral gene expression and assemblage are completed in synchrony with terminal squamous differentiation, concluding with desquamation of infected squames. These infections have little or no malignant potential because the HPV genome remains episomal and its oncogenes are expressed at very low levels (Durst, 1985; Stoler, 1996).
In both female and male genital tracts, productive HPV infections cause either visible genital warts (condyloma acuminata) or much more commonly, subclinical infections. Subclinical infections may be indirectly identified as low-grade cytologic, colposcopic, or histologic abnormalities. However, all these observational diagnoses are subjective and poorly reproducible. HPV testing more accurately reflects HPV infection but is limited to specific HPV types and viral loads.
With neoplastic infection (CIN 3 and cervical cancer), the circular HPV genome is disrupted and integrates at random locations into a host chromosome (Fig. 30-1). Unrestrained transcription of the E6 and E7 oncogenes follows (Durst, 1985; Stoler, 1996). The E6 and E7 oncoproteins produced interfere with and accelerate degradation of p53 and pRb, which are key tumor suppressor proteins produced by the host (Fig. 30-2). This leaves the infected cell vulnerable to malignant transformation by loss of cell-cycle control, cellular proliferation, and accumulation of DNA mutations over time (Doorbar, 2012).
In resultant preinvasive lesions, normal epithelial differentiation is disrupted and incomplete. The degree of disruption is used to grade histology as low-grade (encompassing HPV changes and CIN 1) or high-grade (CIN 2, CIN 3, and CIS). The average age at diagnosis of low-grade cervical disease is younger than that of high-grade lesions and invasive cancers. Thus, disease was thought to progress from milder- to higher-grade lesions over time. An alternative theory now proposes that low-grade lesions are generally acute, transient, and not oncogenic. High-grade lesions and cancers are monoclonal and arise de novo rather than from preexistent low-grade disease (Baseman, 2005; Kiviat, 1996).
The pathogenesis of HPV-related neoplasia at other anogenital sires is thought to be similar to that of the cervix. Genital HPV infection is usually multifocal and involves the cervix most often. Neoplasia at one site increases the risk of neoplasia elsewhere in the LGT (Spitzer, 1989).
Infection with HPV, predominantly HR types, is very common soon after initiation of sexual activity (Brown, 2005; Winer, 2003). This infection often accompanies sexual debut and is not evidence of promiscuity (Collins, 2002).
Most HPV infection and related lesions, whether clinical or subclinical, spontaneously resolve, especially in adolescents and young women (Ho, 1998; Moscicki, 1998). Questions have been raised as to whether apparent clearance reflects true resolution or limited testing sensitivity (Winer, 2011). Several studies show that LR HPV infections resolve faster than those involving HR HPV (Moscicki, 2004; Schlecht, 2003; Woodman, 2001). Younger women frequently change HPV types, reflecting transience of infection and sequential reinfection by new partners rather than persistence (Ho, 1998; Rosenfeld, 1992). Simultaneous or sequential infection with multiple HPV types is common (Schiffman, 2010).
Persistent HR HPV infection is necessary for the development of cervical neoplasia. A minority of HPV infections become persistent, but most young women (65 percent) with HPV 16/18 infections lasting more than 6 months will develop SIL (Trottier, 2009). The risk of progression to high-grade neoplasia increases with age, as HPV infection in older women is more likely to reflect persistence (Hildesheim, 1999). Cell-mediated immunity likely plays the largest role in HPV infection persistence and in progression or regression of benign and neoplastic lesions.
HPV infection is suspected based on clinical lesions or results of cytology, histology, and colposcopy, all of which are subjective and often inaccurate. Moreover, serology is unreliable and cannot distinguish past from current infection (Dillner, 1999). As noted, culture of HPV is not feasible. Thus, diagnosis is confirmed only by the direct detection of HPV nucleic acids by methods that include in situ hybridization, nucleic acid amplification testing (NAAT), polymerase chain reaction (PCR), or others (Molijn, 2005). Currently, four HR HPV tests are approved by the Food and Drug Administration (FDA) for clinical use, and all use NAAT to detect any of 13 or 14 HR HPV types. Two of these tests report specifically the presence of HPV 16 or HPV 18 to aid risk stratification and customized management. Due to clinical test limitations, a negative test result does not exclude HPV infection. Therefore, these tests are not indicated or useful for routine STD screening. LR HPV testing has no indication and can lead to inappropriate expense, further evaluation, and unnecessary treatment.
The clinical role of HR HPV testing for cervical cancer screening and for surveillance of SIL continues to evolve. It is not offered as a screen for HPV infection outside of current guidelines. Namely, appropriate clinical uses for HR HPV testing include: cotesting with cervical cytology screening in women aged 30 years or older, triage or surveillance of certain abnormal cytology results and untreated CIN, and posttreatment surveillance (Davey, 2014). One HR HPV test (cobas HPV Test) was recently FDA approved as a stand-alone screening test for cervical cancer for women 25 years of age and older. If typical genital warts are found in a young woman or if high-grade cervical neoplasia or invasive cancer is identified by cytology or histology, then HPV infection is assumed, and HPV testing is unnecessary. Because of high HPV prevalence in young women (less than age 25), HR HPV testing for cervical cancer screening is not recommended. HPV testing is not FDA approved for use in women after complete hysterectomy, and there are no guidelines for managing HPV test results in these women.
The indications to treat HPV-related LGT disease are symptomatic warts, high-grade neoplasia, or invasive cancer. No effective treatment resolves subclinical or latent HPV infection. Needless physical LGT damage may result from unrealistic attempts to eradicate HPV infections, which are usually self-limited. Encouragement of positive health behaviors and optimal management of immune compromise seems sensible. Treatment of cervical LSIL (HPV changes or CIN 1) is not necessary and is considered only after observation for at least 2 years.
Various treatment modalities are available for genital warts and are chosen according to lesion size, location, and number (Rosales, 2014). Mechanical removal or destruction, topical immunomodulators, and chemical or thermal coagulation can be used (Table 3-15). Examination of a male partner does not benefit a female partner either by influencing reinfection or by altering the clinical course or treatment outcome for genital warts or LGT neoplasia (Centers for Disease Control and Prevention, 2010).
Sexual abstinence, delaying coitarche, and limiting the number of sexual partners are logical strategies to avoid genital HPV infection and its adverse effects. However, evidence is lacking from trials of counseling and sexual practice modification. Condoms do not cover all potentially HPV-infected anogenital skin. Therefore, condoms may not be completely protective but are likely to reduce acquisition and transmission of HPV. Winer and associates (2003) conducted the first prospective study of male condom use and showed reductions in HPV infection rates in young women even if condoms were used inconsistently.
These offer the greatest promise for prevention and possibly reversal of its sequelae in those already infected. Local and humoral immunity likely protect against incident infection, and prophylactic vaccines elicit type-specific humoral antibody production that prevents new HPV infection by blocking its entry into host cells (Stanley, 2010b). They do not prevent transient HPV positivity or resolve preexistent infection. HPV vaccines have the potential to prevent malignancies at least six body sites that include cervix, vulva, vagina, penis, anal canal, and oropharynx.
Currently, three HPV vaccines are FDA approved for prevention of incident HPV infection and cervical neoplasia. They use recombinant technologies for the synthetic production of the L1 capsid proteins of each HPV type included in the vaccine. The resultant virus-like particles are highly immunogenic but are not infectious as they lack viral DNA (Stanley, 2010b).
Cervarix (HPV2) is a bivalent vaccine against HPVs 16 and 18. Gardasil (HPV4) is a quadrivalent vaccine against HPV types 6, 11, 16, and 18. HPV4 is being replaced by Gardasil 9 (HPV9), a nonavalent vaccine. HPV9 protects against all HPV types in HPV4 plus types 31, 33, 45, 52, and 58. Coverage of these additional HR HPV types will bring the theoretical percentage of cervical cancers prevented from 65 percent to approximately 80 percent. All three vaccines contain adjuvants that boost the immune response to vaccine antigens. They are administered in three intramuscular doses during a 6-month period. Specifically, the second dose is given 1 to 2 months after the first dose, and the third dose is given 6 months after the first dose. Prolongation of the dosing schedule does not appear to diminish immunogenicity. Optimal vaccination strategies administer these prior to sexual activity initiation, when the potential benefit is greatest. However, a history of prior sexual activity, HPV-related disease, or HPV-test positivity should not deter vaccine administration. Testing for HPV is not recommended prior to vaccination (American College of Obstetricians and Gynecologists, 2014a). The Advisory Committee on Immunization Practices (ACIP) currently recommends that HPV vaccine be administered routinely to girls aged 11 to 12 years (as early as age 9 years). Vaccination is also recommended for 13- to 26-year-old women not previously vaccinated (Markowitz, 2014; Petrosky, 2015). Vaccination can be given during lactation but is avoided during pregnancy (American College of Obstetricians and Gynecologists, 2014a). Immune compromised women are candidates to receive the vaccine and show high seroconversion rates despite the theoretical risk of a blunted immune response.
All three vaccines show nearly 100-percent efficacy in preventing incident infection and high-grade cervical neoplasia from HPV types included in the vaccines (Future II Study Group, 2007; Paavonen, 2009; Joura, 2015). HPV4 and HPV9 additionally protect against HPV types 6 and 11, which cause nearly all genital warts, laryngeal papillomatosis, and many low-grade cytologic abnormalities. HPV4 and HPV9 are approved for genital wart prevention in both males and females. They are also FDA approved for the prevention of vaginal, vulvar, and anal neoplasia. HPV2 does not prevent genital warts. It is not approved for extracervical LGT disease prevention, although theoretically it should.
HPV vaccines are highly immunogenic with maintenance of protection for at least 5 to 8 years after vaccination (Ferris, 2014; Harper, 2006). No evidence supports the need for later booster dosing. They have excellent safety profiles, are well-tolerated, and can be administered along with other recommended vaccinations.
Because HPV vaccines prevent most, but not all, HPV-related cervical cancers, cervical cancer screening should continue per current guidelines. Countries with high vaccination rates have seen dramatic reductions in anogenital warts, and reductions in Pap abnormalities and cervical neoplasia are expected (Ali, 2013). Despite suboptimal vaccination rates in the United States, HPV4 vaccine-type infections among U.S. adolescents have decreased by 56 percent since vaccine introduction in 2006 (Stokley, 2014).
CERVICAL INTRAEPITHELIAL NEOPLASIA
Henk and associates (2010) estimated that 412,000 cases of CIN are diagnosed in the United State annually. Risk factors are similar to those of invasive cervical cancer, and CIN is most strongly related to persistent genital HR HPV infection and increasing age (Table 29-1) (Ho, 1995; Kjaer, 2002; Remmink, 1995).
| Demographic risk factors |
|
| Behavioral risk factors |
|
| Medical risk factors |
|
The median age of cervical cancer diagnosis in the United States (late fifth decade) is approximately a decade later than for CIN. In an older woman, HPV infection is more likely to persist than resolve. Older age is linked with waning immune competence and also allows accumulation of genetic mutations over time that can lead to malignant cellular transformation. Additionally, adverse socioeconomic factors and decreased need for prenatal care and contraception cause older women to be screened less often.
Behavioral risk factors for CIN mirror those for HPV acquisition and include early onset of sexual activity, multiple sexual partners, and male partner promiscuity (Buckley, 1981; de Vet, 1994; Kjaer, 1991). After adjustments for HPV positivity and lower socioeconomic status, tobacco use also increases the preinvasive disease risk (Castle, 2004; Plummer, 2003).
Dietary deficiencies of certain vitamins such as A, C, E, beta carotene, and folic acid may alter cellular immunity to HPV infection. This may promote viral infection persistence and cervical neoplasia (Paavonen, 1990). However, in the United States, lack of association between dietary deficiencies and cervical disease may reflect the relatively sufficient nutritional status of even lower-income women (Amburgey, 1993).
Combination oral contraceptives (COCs) have been linked with an increased risk of cervical cancer in current users (International Collaboration of Epidemiological studies of Cervical Cancer, 2007). Possible mechanisms include increased persistence of HPV infection and oncogene expression (de Villiers, 2003). Conversely, multiple studies have failed to find an increased CIN risk in users of hormonal contraceptives or postmenopausal hormone therapy (Castle, 2005; Harris, 2009; Yasmeen, 2006). DES exposure in utero appears to double the risk of developing high-grade cervical disease in addition to an increased risk of cervical and vaginal clear cell adenocarcinoma (Hoover, 2011).
Increasing parity has been correlated with cervical cancer risk, but it is unclear if this is related to earlier sexual activity, a progestin-exposure effect, or other factors. Immune suppression during pregnancy, hormonal influences on cervical epithelium, and physical trauma related to vaginal deliveries have all been suggested (Brinton, 1989; Muñoz, 2002).
Immunosuppressed women in general show increased risks for CIN and for greater lesion severity, multifocal lesion pattern, and lesions at multiple LGT sites. They also experience higher rates of treatment failure, persistence, and recurrence of LGT disease compared with those who are immunocompetent. Specifically, human immunodeficiency virus (HIV)-positive women have higher rates of abnormal Pap results and CIN compared with HIV-negative women. Transplant recipients have an increased risk of developing a malignancy after transplantation, including neoplasms of the LGT and anal canal (Gomez-Lobo, 2009). Women on immunosuppressive medications for other disorders have higher rates of LGT neoplasia.
Inadequate screening is another risk factor. Of women diagnosed with cervical cancer in the United States, approximately 60 percent either have never been screened (50 percent) or have not had a Pap test during the previous 5 years (10 percent) (American College of Obstetricians and Gynecologists, 2012b). Lack of screening is a major contributor to higher rates of cervical cancer in socioeconomically disadvantaged women, particularly those of minority ethnicity, rural residence, or older age, and those who are recent immigrants (Benard, 2007).
Preinvasive lesions can spontaneously regress, remain stable, or progress. The risk of progression to invasive cancer increases with the severity of CIN. Estimates of CIN progression, persistence, and regression are provided in a review by Ostor (1993) and shown in Table 29-2. Low-grade lesions are thought to be manifestations of acute HPV infection, and most spontaneously regress within a few years. High-grade lesions are less likely to do so. Castle and coworkers (2009b) calculated that approximately 40 percent of CIN 2 regresses spontaneously within 2 years. This is even more frequent (greater than 60 percent) in young, healthy women (Moscicki, 2010). CIN 2 is thought to be a mixture of low- and high-grade lesions that are difficult to distinguish histologically, rather than an intermediate step in the progression from CIN 1 to CIN 3. The risk of progression to invasive cancer of biopsied but otherwise untreated CIN 3 lesions approximates 30 percent over 30 years (McCredie, 2008).
CERVICAL NEOPLASIA DIAGNOSIS
Cervical cancer screening ideally finds preinvasive lesions that can be eradicated or finds early-stage cervical cancer that can be treated successfully. Cervical cancer screening was previously limited to cervical cytology. But, during the past decade, HR HPV testing has also become an important screening tool.
In general, LGT preinvasive lesions are visible only with aided inspection. One exception is VIN, which is generally visible, palpable, or both. Only cervical lesions at either end of the neoplastic disease spectrum are grossly visible, namely, condylomata and invasive cancers. Accordingly, all symptoms suspicious for cervical neoplasia and grossly visible cervical lesions require prompt biopsy.
Cervical cytologic screening is one of modern medicine’s great success stories. It detects most cervical neoplasia during the typically prolonged premalignant or early occult invasive phases, when treatment outcomes are optimal. Conventional glass slides (traditionally called the Pap smear) and liquid-based Pap tests are considered equally acceptable for screening by all current guidelines (American College of Obstetricians and Gynecologists, 2012b; Saslow, 2012; U.S. Preventive Services Task Force, 2012).
Introduced in the 1940s, cervical cytology has never been evaluated in a randomized, controlled, or masked trial (Koss, 1989). However, countries with organized screening programs have consistently realized dramatic declines in both cervical cancer incidence and mortality rates. The Pap test’s specificity is consistently high, approximating 98 percent. However, estimates of its sensitivity for detection of CIN 2 or worse are lower, are more variable, and range from 45 to 65 percent (Whitlock, 2011). This imperfect sensitivity is balanced by recommendations for repetitive screening throughout a woman’s life. Although the incidence of cervical squamous carcinoma continues to decline, both the relative and absolute incidences of adenocarcinoma have increased, particularly in women younger than 50 (Herzog, 2007). Adenocarcinoma and adenosquamous carcinoma now account for at least 20 percent of cervical cancers. This increase may be due in part to the Pap test’s lower sensitivity for detection of adenocarcinoma than for squamous cancers and their precursor lesions.
False-negative Pap test results may follow sampling error, in which abnormal cells are not present in the Pap test, or by screening error, in which the cells are present but missed or misclassified by the screener (Wilkinson, 1990). Mandated quality assurance measures and computerized slide-screening technologies address screening errors. Suboptimal management of abnormal results by providers and failure of patient follow up also contribute to avoidable cases of cervical cancer. Clinicians can maximize the benefit of screening by obtaining an optimal cytologic specimen and by adhering to current evidence-based guidelines for the management of abnormal test results.
Ideally, Pap tests are scheduled to avoid menstruation. Patients should abstain from vaginal intercourse, douching, vaginal tampon use, and intravaginal medicinal or contraceptive creams for a minimum of 24 to 48 hours before a test. Treatment of cervicitis or vaginitis prior to Pap testing is optimal. However, Pap testing is not deferred due to unexplained discharge or unscheduled bleeding, as these may be signs of cervical or other genital tract cancers.
As shown in Figure 21-9, the appearance of cervical squamous cells varies throughout the menstrual cycle and with hormonal status. Thus, clinical information aids accurate Pap interpretation and often includes: date of last menstrual period, current pregnancy, exogenous hormone use, menopausal status, complaints of abnormal bleeding, and prior abnormal Pap test results, CIN, or other LGT neoplasia. Additionally, intrauterine devices (IUDs) can cause reactive cellular changes, and their presence is noted. Full visualization of the cervix is essential for detection of gross lesions and SCJ identification. Speculum placement should be as comfortable as possible. A thin coating of water-based lubricant can be used on the outside of the speculum blades without compromising Pap test quality or interpretation (Griffith, 2005; Harmanli, 2010). Touching the cervix prior to performing a Pap test is avoided, as dysplastic epithelium may be inadvertently removed with minimal trauma. Discharge covering the cervix may be carefully absorbed by a large swab, with care not to contact the cervix. Vigorous blotting or rubbing may cause scant cellularity or a false-negative Pap test result. When indicated, additional sampling to detect other cervical or vaginal infection may follow Pap test collection.
Sampling of the transformation zone at the SCJ is paramount to the sensitivity of the Pap test. Techniques are adapted and sampling devices chosen according to SCJ location, which varies widely with age, obstetric trauma, and hormonal status. Women known or suspected of in utero DES exposure may also benefit from a separate Pap test of the upper vagina, as these women carry an additional risk for vaginal cancer.
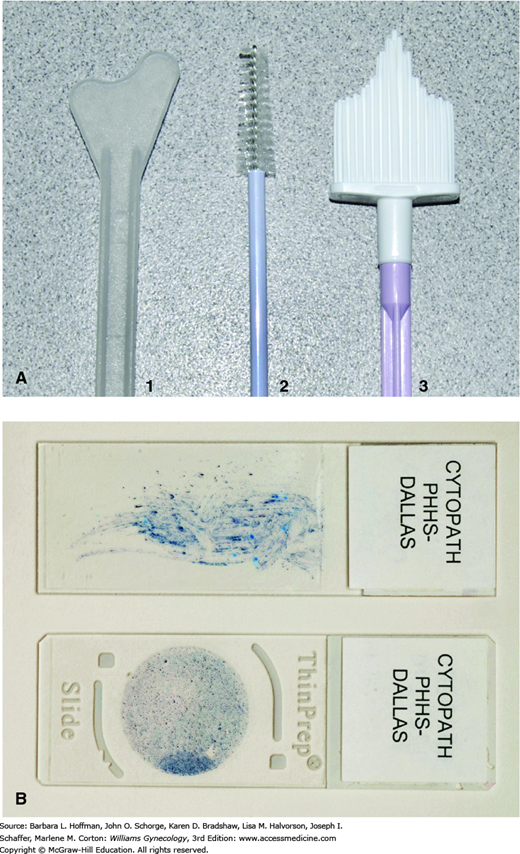
Three types of plastic devices are commonly used to sample the cervix: the spatula, broom, and endocervical brush (also known as a cytobrush) (Fig. 29-8). A spatula predominantly samples the ectocervix. An endocervical brush samples the endocervical canal and is used in combination with a spatula. A broom samples both endo- and ectocervical epithelia simultaneously but can be supplemented by an endocervical brush. Wooden collection devices and cotton swabs are no longer recommended due to their inferior collection and release of cells.
FIGURE 29-8
A. Cervical cytology collection devices: (1) Plastic spatula. (2) Endocervical brush. (3) Plastic broom. B. Pap preparations. Conventional cervical cytology is prepared by smearing collected cells directly onto a glass slide with the collection device followed by immediate fixation (upper slide). Liquid-based cytology involves transfer of collected cells from the collection device into a liquid transport medium with subsequent processing and transfer onto a glass slide. Cells are distributed over a smaller area, and debris, mucus, blood, and cell overlap are largely eliminated, allowing computer-assisted screening (lower slide). (Used with permission from Dr. Raheela Ashfaq.)
A spatula is oriented to best fit the cervical contour, straddle the squamocolumnar junction, and sample the distal endocervical canal. A clinician firmly scrapes the cervical surface, completing at least one full rotation. After the spatula sample is obtained, the endocervical brush, with its conical shape and plastic bristles, is inserted into the endocervical canal only until the outermost bristles remain visible just within the external os. This prevents inadvertent sampling of lower uterine segment cells, which can be mistaken for atypical cervical cells. To avoid obscuring blood, the brush is rotated only one-quarter to one-half turn and is used after the ectocervix has been sampled. If the cervical canal is very wide, the brush is moved so as to contact all surfaces of the endocervical canal.
Broom devices have longer central bristles that are inserted into the endocervical canal. These longer bristles are flanked by shorter bristles that splay out over the ectocervix during rotation. Five rotations in the same direction are recommended. Reversing direction may cause loss of cellular material. Broom devices are favored for liquid-based Pap testing.
Conventional slide collection requires special care to avoid air drying artifact, a leading cause of poor slide quality. The spatula sample is held while the endocervical brush sampling immediately follows. The spatula sample is then quickly spread as evenly as possible over one half to two thirds of a glass slide (see Fig. 29-8). The endocervical brush is firmly rolled over the remaining area of the slide, after which fixation is quickly carried out by spraying from a distance of 10 to 12 inches or immersing the slide in fixative.
Currently, two liquid-based cytology (LBC) Pap tests are FDA approved. Sampling and cell transfer to a liquid medium is performed according to manufacturer specifications. SurePath allows for the use of all three device types but uses modified tips that are broken off and sent to the laboratory in the liquid medium. ThinPrep requires immediate and vigorous agitation of the chosen collection device(s) in the liquid medium, after which the device is discarded.
A role for HR HPV testing in cervical cancer screening is attractive due to its improved sensitivity for CIN 3 or cervical cancer and the objectivity of its results. However, strategies for incorporation of HPV testing must compensate for a decreased specificity, particularly in young women.
In 2003, the FDA first approved an HPV test for use with cytology for cervical cancer screening in women 30 years and older. The combination of HR HPV testing with cytology is referred to as cotesting. This strategy is not currently endorsed for women younger than 30 due to the high prevalence of HR HPV infection in this age group and the resultant lack of test specificity. HPV testing is usually performed from the residual LBC specimen after the cytology slide is prepared. Alternatively, a cervical sampling for HPV can be sent in a specific collection device separate from the cytology specimen. Testing is performed only for HR HPV types. As noted earlier, there is no clinical role for LR HPV testing (Castle, 2014; Thomsen, 2014).
The combination of HPV testing with cytology increases the sensitivity of a single screening test for high-grade neoplasia to nearly 100 percent and leads to earlier detection and management of HSIL (Ronco, 2010). The lack of sensitivity for cervical adenocarcinoma seen with traditional cytology testing also supports HPV testing use for primary screening (Castellsagué, 2006).
Due to a high negative predictive value for high-grade neoplasia, slow progression of new HPV infection to neoplasia, and increased cost, cotesting is repeated at 5-year intervals if both cytology and HPV test are negative. Clinical guidelines have been developed for management of abnormal cotest results (Saslow, 2012). If cytology is abnormal, current cytology management guidelines are followed. Cytology-negative and HPV-positive test results will occur in less than 10 percent of screened patients (Castle, 2009a; Datta, 2008). In such cases, cotesting is repeated 12 months later. This is because the risk of high-grade neoplasia is less than that of a Pap test with an atypical squamous cell of undetermined significance (ASC-US) result, and most HPV infections will resolve during this time (Saslow, 2012). Colposcopy is recommended for persistently positive HPV DNA test results. An abnormal repeat cytology result is managed according to current guidelines regardless of concurrent HPV status.
An alternative strategy is now available for management of a negative cytology but a positive HR HPV test result. A reflex test specifically for HPVs 16 and 18, called genotyping, can be performed. If positive, immediate colposcopy is recommended (American College of Obstetricians and Gynecologists, 2012b; Saslow, 2012). This approach targets those at highest risk for significant disease, and evidence provides a sound basis for this strategy (Khan, 2005; Wright, 2015).
A growing body of evidence supports HR HPV testing alone without initial cytology as an option for primary cervical cancer screening (Castle, 2011; Cuzick, 2006; Dillner, 2013). In late 2014, the cobas HPV test was the first HPV test approved by the FDA for primary cervical cancer screening in women 25 years and older. This test gives simultaneous results for the presence or absence of HPVs 16 and 18 and for a group of 12 other oncogenic HPV types. This represents a profound paradigm shift in cervical cancer screening, in which Pap testing assumes a secondary role for the triage of HPV positive results.
HPV testing alone is approximately twice as sensitive (>90 percent) as a single Pap test and leads to earlier detection of high-grade neoplasias. The very high negative-predictive value of a single negative HPV test was shown by Sankaranarayanan (2009). During this 8-year study, a single round of HPV testing outperformed cytology, with no cervical cancer deaths within 8 years of a negative HR HPV test result. Recent evaluations show that cotesting does not perform any better than HPV testing alone (Dillner, 2013; Whitlock, 2011). However, specificity declines with HPV testing, particularly in younger women (Mayrand, 2007; Ronco, 2006, 2010). This could lead to excessive numbers of colposcopies, biopsies, and treatments. Triaging women with positive non-HPV16/18 HPV test results to reflex cytology is a viable counterbalance to the decreased specificity. Overall, this strategy is expected to result in more colposcopic referrals but yield higher and earlier HSIL detection rates.
Suggested interim guidelines for primary HPV test screening are recently published and will no doubt be debated and revised in coming years (Huh, 2015). These propose that screening with a HR HPV test can be used as an alternative to cytology alone or cotesting in women 25 years and older and at intervals no less than 3 years. Immediate colposcopy is recommended if HPV 16/18 is identified. If other HR HPV types are found, then triage to reflex cytology is proposed. Colposcopy is recommended for any cytologic abnormality. Importantly, as of mid-2015, major cervical cancer screening guidelines do not include this screening option.
Notably, all approved cervical cancer screening strategies, including the use of periodic cytology alone, dramatically reduce a woman’s lifetime risk of developing or dying from cervical cancer. Since most women with positive HR HPV test results will not develop significant disease, the choice of screening strategy should be a shared decision by the provider and individual patient. Reviewed by the National Cancer Institute (2015a), the balance of benefits and harms of each screening strategy warrants careful consideration by both health care providers and health care policy agencies. With that perspective, guidelines are subsequently presented.
Evidence-based cervical cancer screening guidelines continue to evolve. In 2012, all major professional societies updated these. The American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology (ACS/ASCCP/ACP) jointly issued guidelines (Saslow, 2012). The U.S. Preventive Services Task Force (USPSTF) (Moyer, 2012) and the American College of Obstetricians and Gynecologists (2012b) each published guidelines as well. These three sets of guidelines agree with few exceptions and pertain only to average-risk women, that is, immunocompetent women with no history of HSIL or cervical cancer. All agree on the acceptability of both conventional and liquid-based Paps, the age of initiation and cessation of screening, screening intervals, and continued screening after HPV vaccination. Adherence to current guidelines should not preclude or delay other indicated gynecologic care. In particular, provision of contraception is never contingent on compliance with cervical cancer screening recommendations or the evaluation of cytologic abnormalities.
Cervical cancer screening ideally begins at age 21 in average-risk women. This is true regardless of sexual history, sexual orientation, or other risks. In young women, most Pap abnormalities represent transient HPV infection, and the spontaneous regression of even high-grade lesions is common (Moscicki, 2005). Most high-grade lesions are CIN 2 rather than CIN 3 in young women (Moscicki, 2008). Cervical cancer is exceedingly rare in adolescents and not as preventable by screening as for older women (Saslow, 2012). Additionally, treatment of high-grade CIN in adolescents is often followed by persistence of Pap abnormalities, and theoretically may have adverse reproductive consequences (Case, 2006; Moore, 2007).
Whether to begin screening earlier in the presence of significant immune compromise, as with HIV infection, use of immunosuppressive medications, and organ transplantation, is uncertain and not addressed by current guidelines. The Centers for Disease Control and Prevention (CDC) (2015) recommends initiation of screening soon after HIV diagnosis, even if before age 21, and repeat Pap testing in 6 months. As for other such conditions, clinician judgment is exercised, taking into consideration age and severity of immune compromise. In general, initial screening at age 21 seems reasonable (American College of Obstetricians and Gynecologists, 2012a).
Between ages 21 and 29, all guidelines recommend screening with cytology alone at 3-year intervals. Women aged 30 to 65 can continue screening with cytology alone at 3-year intervals or can begin cotesting at 5-year intervals. The risk of cancer is approximately the same using either strategy. USPSTF sees both strategies as equally effective, and cotesting is available to women wishing to extend their screening interval. However, both the ACS/ASCCP/ACP (Saslow, 2012) and American College of Obstetricians and Gynecologists (2012b) have deemed cotesting the preferred screening strategy in women 30 years and older. Women with HIV infection and other immune suppression should receive annual cytology screening (Centers for Disease Control and Prevention, 2015; American College of Obstetricians and Gynecologists, 2012a).
Screening may be stopped in women older than 65 if they have an average risk for cervical cancer and have undergone adequate screening, regardless of sexual history. Adequate screening is three consecutive, negative Pap results or two consecutive, negative cotest results in the prior 10 years, with the most recent within the past 5 years. Women with prior treatment for CIN 2, CIN 3, AIS, or cervical cancer should continue routine screening for at least 20 years, as they remain at increased long-term risk of cervical cancer (Saslow, 2012; Strander, 2007). It is uncertain when HIV-positive women can discontinue screening or whether this should continue annually and indefinitely for as long as there is reasonable life expectancy.
Vaginal cancers are rare and account for less than 2 percent of cancers in women. All guidelines recommend against Pap screening in women who have undergone total hysterectomy for benign disease if there is no past history of high-grade CIN or cervical cancer. The absence of a cervix should be confirmed by examination or pathology report as many women are inaccurate in their reporting of hysterectomy type. Women who have undergone supracervical hysterectomy should continue routine screening. Recommendations for vaginal cytology after hysterectomy in women with histories of high-grade cervical neoplasia or cancer are less clear, as vaginal cancer is still rare, and screening is of uncertain benefit (Saslow, 2012). The American College of Obstetricians and Gynecologists (2012b) recommends cytology of the vaginal cuff every 3 years for 20 years after the initial posttreatment surveillance, which is generally a schedule of three Pap tests in the first 2 years posthysterectomy. HPV testing is not FDA-approved in the absence of a cervix but remains common. Evidence-based recommendations for managing vaginal HPV test results are nonexistent (Chappell, 2010). Such testing should be avoided.
Cervical cytology reporting is standardized by the Bethesda System nomenclature (National Cancer Institute Workshop, 1989; Nayar, 2015; Solomon, 2002). Clinically, the key elements reported are specimen adequacy and epithelial cell abnormalities (Tables 29-3 and 29-4). An overview of evidence-based guidelines for the initial management of cervical cytology abnormalities for nonpregnant women follows in the next paragraphs (American College of Obstetricians and Gynecologists, 2013; Massad, 2013). Full guidelines should be reviewed and applied on an individualized basis. Guidelines cannot address all clinical situations or prevent all cervical cancers.
| Specimen type |
|
| Specimen adequacy |
|
| General categorization (optional) |
|
| Interpretation/Results |
|