INTRODUCTION
Polycystic ovarian syndrome (PCOS) is a common endocrinopathy typified by oligoovulation or anovulation, signs of androgen excess, and multiple small ovarian cysts. These signs and symptoms vary widely between women and within individuals over time. Women with this endocrine disorder also have higher rates of dyslipidemia and insulin resistance, which increase long-term health risks. As a result, women with PCOS may first present to various medical specialists, including pediatricians, gynecologists, internists, endocrinologists, or dermatologists.
DEFINITION
In Rotterdam, The Netherlands, PCOS was redefined in a consensus meeting between the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine (ESHRE/ASRM)—The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004. As shown in Table 17-1, affected individuals must meet two out of three criteria. Importantly, because other etiologies, such as congenital adrenal hyperplasia, androgen-secreting tumors, and hyperprolactinemia, may also lead to oligoovulation and/or androgen excess, these must be excluded. Thus, PCOS currently is a diagnosis of exclusion.
| ESHRE/ASRM (Rotterdam) 2003 |
| Two of the three: |
| Clinical and/or biochemical hyperandrogenism |
| Oligo-/anovulation |
| Polycystic ovaries |
| NIH (1990) |
| To include both: |
| Clinical and/or biochemical hyperandrogenism |
| Oligo-/anovulation |
| AE-PCOS (2006) |
| To include both: |
| Clinical and/or biochemical hyperandrogenism |
| Oligo-/anovulation and/or polycystic ovaries |
The Rotterdam criteria constitute a broader spectrum than that formerly put forward by the National Institutes of Health (NIH) Conference in 1990 (Zawadzki, 1990). The prominent difference is that the NIH Conference defined PCOS without regard to ovarian sonographic appearance. Last, a third organization—The Androgen Excess and PCOS Society (AE-PCOS)—has also defined criteria for PCOS (Azziz, 2006). As is shown in Table 17-1, criteria are similar among these three groups, and controversy exists as to which is most appropriate.
Ovarian hyperthecosis, often considered a more severe form of PCOS, is a rare condition characterized by nests of luteinized theca cells distributed throughout the ovarian stroma. Affected women exhibit severe hyperandrogenism and may occasionally display frank virilization signs such as clitoromegaly, temporal balding, and voice deepening (Culiner, 1949). In addition, a much greater degree of insulin resistance and acanthosis nigricans typically is found (Nagamani, 1986).
The hyperandrogenic-insulin resistant-acanthosis nigricans (HAIRAN) syndrome is also uncommon and consists of marked hyperandrogenism, severe insulin resistance, and acanthosis nigricans (Barbieri, 1983). The etiology of this disorder is unclear, and HAIRAN syndrome may represent either a PCOS variant or a distinct genetic syndrome. Both ovarian hyperthecosis and HAIRAN are exaggerated phenotypes of PCOS, and their treatment mirrors that for PCOS described later in this chapter.
INCIDENCE AND ETIOLOGY
PCOS is the most common endocrine disorder of reproductive-aged women and affects approximately 4 to 12 percent in general population studies (Asunción, 2000; Knochenhauer, 1998; Lauritsen, 2014). Although symptoms of androgen excess may vary among ethnicities, PCOS appears to affect all races and nationalities equally.
The underlying cause of PCOS is unknown. However, a genetic basis that is both multifactorial and polygenic is suspected, as there is a well-documented aggregation of the syndrome within families (Franks, 1997). Specifically, an increased prevalence is noted between affected individuals and their sisters (32 to 66 percent) and mothers (24 to 52 percent) (Govind, 1999; Kahsar-Miller, 2001; Yildiz, 2003). Twin studies also suggest a prominent heritable influence (Vink, 2006).
Some have suggested an autosomal dominant inheritance with expression in both females and males. For example, first-degree male relatives of women with PCOS have been shown to have significantly higher rates of elevated circulating dehydroepiandrosterone sulfate (DHEAS) levels, early balding, and insulin resistance compared with male controls (Legro, 2000, 2002).
Identification of candidate genes linked to PCOS is a major research focus, given the large potential benefit for both diagnosis and management. In general, putative genes include those involved in androgen synthesis and those associated with insulin resistance. Genome-wide association studies in Chinese women have identified variants in 11 genomic regions as potential risk factors for PCOS (Chen, 2011; Shi, 2012).
In addition, epigenetic modification of genetic susceptibility within the maternal-fetal environment may influence adult PCOS development (Dumesic, 2014). Further investigation, however, is needed to determine the roles of these gene products in PCOS pathogenesis.
PATHOPHYSIOLOGY
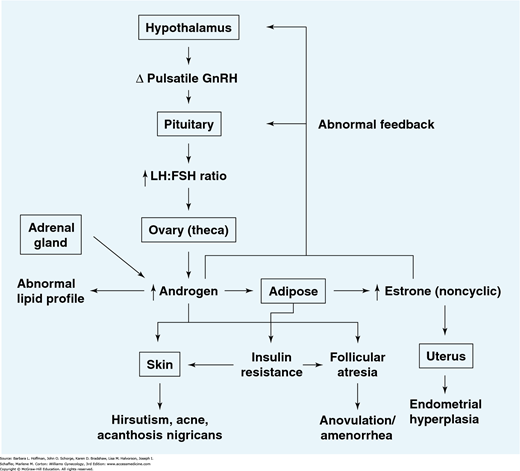
Anovulation in women with PCOS is characterized by inappropriate gonadotropin secretion (Fig. 17-1). Specifically, altered gonadotropin-releasing hormone (GnRH) pulsatility leads to preferential production of luteinizing hormone (LH) compared with follicle-stimulating hormone (FSH) (Hayes, 1998; Waldstreicher, 1988). It is currently unknown whether hypothalamic dysfunction is a primary cause of PCOS or is secondary to abnormal steroid feedback. In either case, serum LH levels rise, and increased levels are observed clinically in approximately 50 percent of affected women (van Santbrink, 1997). Similarly, LH:FSH ratios are elevated and rise above 2:1 in approximately 60 percent of patients (Rebar, 1976).
FIGURE 17-1
Model for the initiation and maintenance of polycystic ovarian syndrome (PCOS). Alterations in pulsatile gonadotropin-releasing hormone (GnRH) release may lead to a relative increase in luteinizing hormone (LH) versus follicle-stimulating hormone (FSH) biosynthesis and secretion. LH stimulates ovarian androgen production, while the relative paucity of FSH prevents adequate stimulation of aromatase activity within the granulosa cells, thereby decreasing androgen conversion to the potent estrogen estradiol.
Increased intrafollicular androgen levels result in follicular atresia. Increased circulating androgen levels contribute to abnormalities in patient lipid profiles and the development of hirsutism and acne. Increased circulating androgens can also be derived from the adrenal gland.
Elevated serum androgens (primarily androstenedione) are converted in the periphery to estrogens (primarily estrone). As conversion occurs primarily in the stromal cells of adipose tissue, estrogen production will be augmented in obese PCOS patients. This conversion results in chronic feedback at the hypothalamus and pituitary gland, in contrast to the normal fluctuations in feedback observed in the presence of a growing follicle and rapidly changing levels of estradiol. Unopposed estrogen stimulation of the endometrium may lead to endometrial hyperplasia.
Insulin resistance due to genetic abnormalities and/or increased adipose tissue contributes to follicular atresia in the ovaries as well as the development of acanthosis nigricans in the skin.
Lack of follicular development results in anovulation and subsequent oligo- or amenorrhea.
Note that this syndrome may develop from primary dysfunction of any one of a number of organ systems. For example, elevated ovarian androgen production may be due to either an intrinsic abnormality in enzymatic function and/or abnormal hypothalamic-pituitary stimulation with LH and FSH.
The common denominator is development of a self-perpetuating noncyclic hormonal pattern.
Women with PCOS also display greater degrees of insulin resistance and compensatory hyperinsulinemia than nonaffected women. Insulin resistance is defined as a reduced glucose-uptake response to a given amount of insulin. This decreased insulin sensitivity appears to stem from a postbinding abnormality in insulin receptor-mediated signal transduction (Dunaif, 1997). Both lean and obese women with PCOS are found to be more insulin resistant than nonaffected weight-matched controls (Dunaif, 1989, 1992).
Insulin resistance has been associated with an increase in several disorders including type 2 diabetes mellitus (DM), hypertension, dyslipidemia, and cardiovascular disease. Therefore, PCOS is not simply a disorder of short-term consequences such as irregular periods and hirsutism, but also one of potential long-term health consequences (Table 17-2).
| Short-term consequences |
| Obesity |
| Infertility |
| Depression |
| Sleep apnea |
| Irregular menses |
| Abnormal lipid levels |
| Non-alcoholic fatty liver disease |
| Hirsutism/acne/androgenic alopecia |
| Insulin resistance/acanthosis nigricans |
| Long-term consequences |
| Diabetes mellitus |
| Endometrial cancer |
| Cardiovascular disease |
Both insulin and LH stimulate androgen production by the ovarian theca cell (Dunaif, 1992). As a result, affected ovaries secrete elevated levels of testosterone and androstenedione. Specifically, elevated free testosterone levels are noted in 70 to 80 percent of women with PCOS, and 25 to 65 percent exhibit elevated levels of DHEAS (Moran, 1994, 1999; O’Driscoll, 1994). In turn, elevated androstenedione levels contribute to an increase in estrone levels through peripheral conversion of androgens to estrogens by aromatase.
Women with PCOS display decreased levels of sex hormone-binding globulin (SHBG). This glycoprotein, produced in the liver, binds most sex steroids. Only approximately 1 percent of these steroids are unbound and thus free and bioavailable. The synthesis of SHBG is suppressed by insulin as well as androgens, corticoids, progestins, and growth hormone (Bergh, 1993). Because of suppressed SHBG production, less circulating androgen is bound and thus more remains available to bind with end-organ receptors. Accordingly, some women with PCOS will have total testosterone levels in the normal range, but will be clinically hyperandrogenic due to elevated free testosterone levels.
In addition to hyperandrogenism, low SHBG levels have also been linked to impaired glucose control and a risk for developing type 2 DM (Ding, 2009). The mechanism of this association is not fully understood and may reflect a role for SHBG in glucose homeostasis. For example, Veltman-Verhulst (2010) evaluated SHBG levels in women with PCOS and found an association between low SHBG levels and subsequent development of gestational diabetes mellitus.
The precise mechanism leading to anovulation is unclear, but altered GnRH pulsatility and inappropriate gonadotropin secretion have been implicated in menstrual irregularity.
Moreover, anovulation may result from insulin resistance, as a substantial number of anovulatory patients with PCOS may resume ovulatory cycles when treated with metformin, an insulin sensitizer (Nestler, 1998). It has been suggested that oligoovulatory women with PCOS exhibit a milder phenotype of ovarian dysfunction than anovulatory PCOS patients and have a more favorable response to ovulation induction agents (Burgers, 2010).
Finally, the large antral follicle cohort with increased intraovarian androgens seen in PCOS may contribute to anovulation. This is supported by the fact that some patients who have undergone ovarian wedge resection or laparoscopic ovarian drilling have improved menstrual regularity. One study demonstrated that 67 percent of PCOS patients developed regular menses following such surgery compared with only 8 percent prior to surgery (Amer, 2002).
SIGNS AND SYMPTOMS
In women with PCOS, symptoms may include menstrual irregularities, infertility, manifestations of androgen excess, or other endocrine dysfunction. Symptoms classically become apparent within a few years of puberty.
In women with PCOS, menstrual dysfunction may range from amenorrhea to oligomenorrhea to episodic menometrorrhagia with associated iron-deficiency anemia. In most cases, amenorrhea and oligomenorrhea result from anovulation. Namely, without ovulation and endogenous progesterone production from the corpus luteum, a normal menstrual period is not triggered. Alternatively, amenorrhea can stem from elevated androgen levels. Specifically, androgens can counteract estrogen to produce an atrophic endometrium. Thus, with markedly elevated androgen levels, amenorrhea and a thin endometrial stripe can be seen.
In contrast to amenorrhea, women with PCOS may have heavy and unpredictable bleeding. In these cases, progesterone is absent due to anovulation, and chronic estrogen exposure results. This produces constant mitogenic stimulation of the endometrium. The instability of the thickened endometrium leads to unpredictable bleeding.
Characteristically, oligomenorrhea (fewer than eight menstrual periods in 1 year) or amenorrhea (absence of menses for 3 or more consecutive months) with PCOS begins with menarche. Those with PCOS fail to establish monthly ovulatory menstrual cycles by midadolescence, and they often continue to have irregularity. However, approximately 50 percent of all postmenarchal girls have irregular periods for up to 2 to 4 years because of hypothalamic-pituitary-ovarian axis immaturity. Thus, due to the frequency of both irregular cycles and acne in unaffected adolescents, some advocate delaying the diagnosis of PCOS until after age 18 (Shayya, 2011).
Last, some evidence suggests that PCOS patients with prior irregular cycle intervals may develop regular cycle patterns as they age. A decreasing antral follicle cohort as women enter their 30s and 40s may lead to a concurrent decrease in androgen production (Elting, 2000).
This condition is usually manifested clinically by hirsutism, acne, and/or androgenic alopecia. In contrast, signs of virilization such as increased muscle mass, voice deepening, and clitoromegaly are not typical of PCOS. Virilization reflects higher androgen levels and should prompt investigation for an androgen-producing tumor of the ovary or adrenal gland.
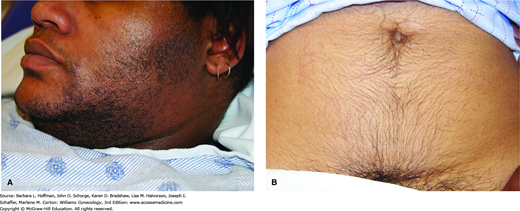
In a female, hirsutism is defined as coarse, dark, terminal hairs distributed in a male pattern (Fig. 17-2). This is distinguished from hypertrichosis, which is a generalized increase in lanugo, that is, the soft, lightly pigmented hair associated with some medications and malignancies. PCOS accounts for 70 to 80 percent of cases of hirsutism, which typically begins in late adolescence or the early 20s. Idiopathic hirsutism is the second most frequent cause (Azziz, 2003). Additionally, various drugs may also lead to hirsutism, and their use should be investigated (Table 17-3).
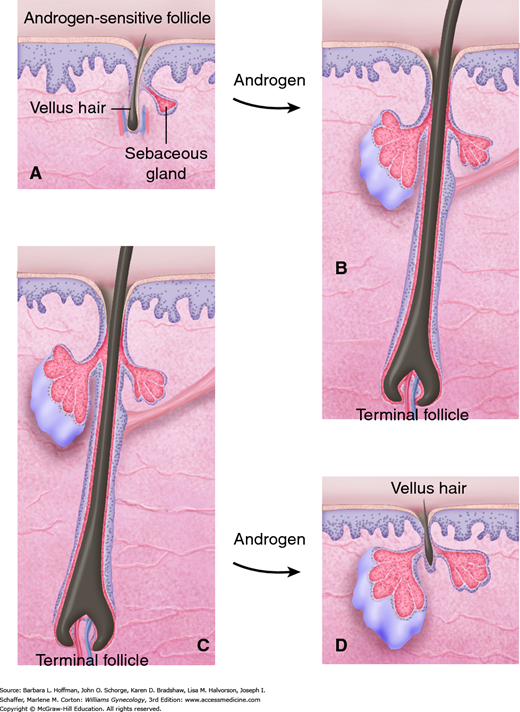
Elevated androgen levels play a major role in determining the type and distribution of hair. Within a hair follicle, testosterone is converted by the enzyme 5α-reductase to dihydrotestosterone (DHT) (Fig. 17-3). Although both testosterone and DHT convert short, soft vellus hair to coarse terminal hair, DHT is markedly more effective than testosterone. Conversion is irreversible, and only hairs in androgen-sensitive areas are changed in this manner to terminal hairs. As a result the most common areas affected with excess hair growth include the upper lip, chin, sideburns, chest, and linea alba of the lower abdomen. Specifically, escutcheon is the term used to describe the hair pattern of the lower abdomen. In women, this pattern is triangular and overlies the mons pubis, whereas in men it extends up the linea alba to form a diamond shape.
FIGURE 17-3
Androgenic effects on the pilosebaceous unit. In some hair-bearing areas, androgens stimulate sebaceous glands, and vellus follicles (A) are converted to terminal follicles (B), leading to hirsutism. Under the influence of androgens, terminal hairs that were not previously dependent on androgens (C) revert to a vellus form and balding results (D).
The concentration of hair follicles per unit area does not differ between men and women, however, racial and ethnic differences do exist. Individuals of Mediterranean descent have a higher concentration of hair follicles than Northern Europeans, and a much higher concentration than Asians. For this reason, Asians with PCOS are much less likely to present with overt hirsutism than other ethnic groups. Additionally, the familial tendency for the hirsutism development is strong and stems from genetic differences in 5α-reductase activity and in target tissue sensitivity to androgens.
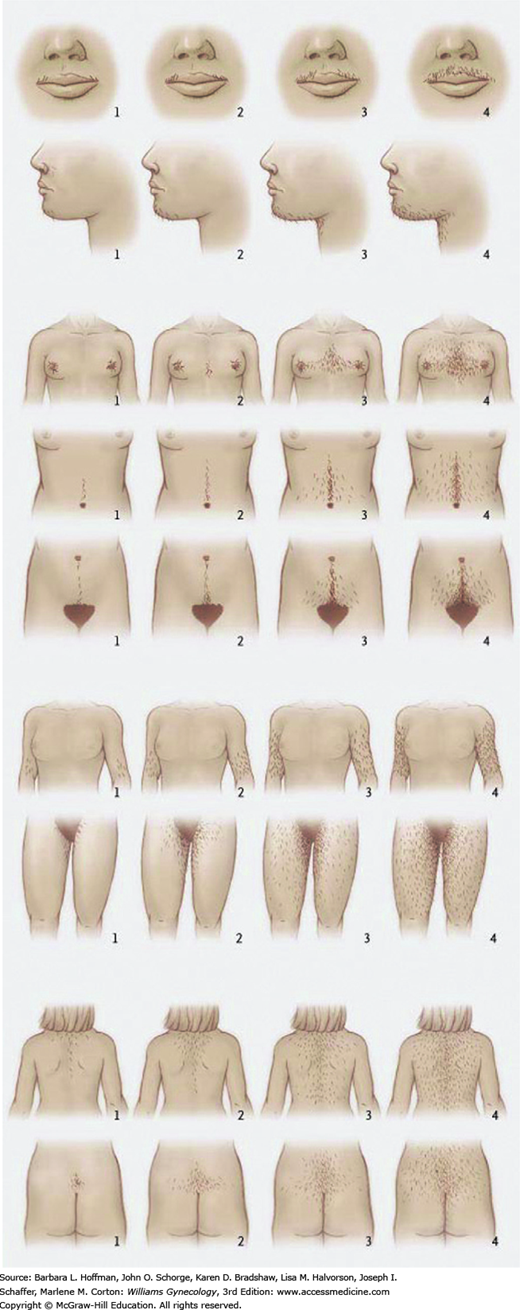
To quantify the degree of hirsutism for research purposes, the Ferriman-Gallwey scoring system was developed in 1961 and later modified in 1981 (Ferriman, 1961; Hatch, 1981). Within this system, abnormal hair distribution is assessed in nine body areas and scored from 0 to 4 (Fig. 17-4). Increasing numeric scores correspond to greater hair density within a given area. Many investigators define hirsutism as a score ≥8 using the modified scoring. Because of the lower follicle concentration in Far East Asians, the AE-PCOS Society suggests a threshold value ≥3 for this group (Escobar-Morreale, 2011).
The Ferriman-Gallwey scoring system is cumbersome and thus is not used frequently in clinical settings. Nevertheless, it may be useful for following treatment responses in individual patients. Alternatively, an abbreviated score that combines only the upper and lower abdomen and chin scores may be a suitable surrogate (Cook, 2011). Also, many specialists choose to classify hirsutism more generally as mild, moderate, or severe depending on the location and density of hair growth.
Acne vulgaris is a frequent clinical finding in adolescents. However, acne that is particularly persistent or late onset suggests PCOS (Homburg, 2004). The prevalence of acne in women with PCOS is unknown, although one study found that 50 percent of adolescents with PCOS have moderate acne (Dramusic, 1997). In addition, androgen level elevation has been reported in 80 percent of women with severe acne, 50 percent with moderate acne, and 33 percent with mild acne (Bunker, 1989). Women with moderate to severe acne have an increased prevalence (52 to 83 percent) of polycystic ovaries identified during sonographic examination (Betti, 1990; Bunker, 1989).
The pathogenesis of acne vulgaris involves four factors: blockage of the follicular opening by hyperkeratosis, sebum overproduction, proliferation of commensal Propionibacterium acnes, and inflammation. As in the hair follicle, testosterone is converted within sebaceous glands to its more active metabolite, DHT, by 5α-reductase. In women with androgen excess, overstimulation of androgen receptors in the pilosebaceous unit increases sebum production that eventually leads to inflammation and comedone formation (see Fig. 17-3). Inflammation leads to the main long-term side effect of acne—scarring. Accordingly, treatment is directed at minimizing inflammation, decreasing keratin production, lowering colonization of P acnes, and reducing androgen levels to diminish sebum production.
Female androgenic alopecia is a less common finding in women with PCOS. Hair loss progresses slowly and is characterized by diffuse thinning at the crown with preservation of the frontal hairline (Quinn, 2014). Its pathogenesis involves an excess of 5α-reductase activity in the hair follicle leading to a rise in DHT levels. Moreover, androgen receptor expression in these individuals is increased (Chen, 2002).
Alopecia, however, may reflect other serious disease. For this reason, affected women are also evaluated to exclude thyroid dysfunction, anemia, or other chronic illness.
Although not well characterized, the association among insulin resistance, hyperandrogenism, and PCOS has long been recognized. The precise incidence of insulin resistance in women with PCOS has been difficult to ascertain for lack of a simple method to determine insulin sensitivity in an office setting. Although obesity is known to exacerbate insulin resistance, one classic study demonstrated that both lean and obese women with PCOS have increased rates of insulin resistance and type 2 DM compared with weight-matched controls without PCOS (Fig. 17-5) (Dunaif, 1989, 1992).
FIGURE 17-5
Insulin sensitivity is decreased in obese women with polycystic ovarian syndrome. NL = normal (those without PCOS); PCOS = polycystic ovarian syndrome. (Reproduced with permission from Dunaif A, Segal KR, Futterweit W, et al: Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome, Diabetes 1989 Sep;38(9):1165–1174.)
This skin condition is characterized by thickened, gray-brown velvety plaques seen in flexure areas such as the back of the neck, axillae, inframammary creases, waist, and groin (Fig. 17-6) (Panidis, 1995). Thought to be a cutaneous marker of insulin resistance, acanthosis nigricans may be found in individuals with or without PCOS. Insulin resistance leads to hyperinsulinemia, which is believed to stimulate keratinocyte and dermal fibroblast growth, producing the characteristic skin changes (Cruz, 1992). Acanthosis nigricans develops more frequently in obese women with PCOS (50 percent incidence) than in those with PCOS and normal weight (5 to 10 percent). As part of its differential diagnosis, acanthosis nigricans rarely can be seen with genetic syndromes or gastrointestinal tract malignancy, such as adenocarcinoma of the stomach or pancreas. To differentiate, acanthosis nigricans associated with malignancy usually has a more abrupt onset, and skin involvement is more extensive (Moore, 2008).
Women with PCOS are at increased risk for impaired glucose tolerance (IGT) and type 2 DM. Based on oral glucose tolerance testing of obese women with PCOS, the prevalence of IGT and DM is approximately 30 percent and 7 percent, respectively (Legro, 1999). Even after adjusting for body mass index (BMI), women with PCOS remained more likely to have DM (Lo, 2006). Specifically, β-cell dysfunction that is independent of obesity has been reported in patients with PCOS (Dunaif, 1996a). Similar findings are reported in groups of obese and normal-weight adolescent girls with PCOS (Flannery, 2013; Palmert, 2002).
The classic atherogenic lipoprotein profile seen in PCOS shows increased low-density lipoprotein (LDL) and triglyceride levels, elevated total cholesterol:high-density lipoprotein (HDL) ratios, but depressed HDL levels (Banaszewska, 2006). Independent of total cholesterol levels, these changes may increase the cardiovascular disease risk in women with PCOS. The prevalence of dyslipidemia in PCOS approaches 70 percent (Legro, 2001; Talbott, 1998).
Compared with age-matched controls, women with PCOS are more likely to be obese, as reflected by elevated BMIs and waist:hip ratios (Talbott, 1995). This ratio reflects an android or central pattern of obesity, which itself is an independent risk factor for cardiovascular disease and predicts insulin resistance. As noted earlier, insulin resistance is believed to play a large role in the pathogenesis of PCOS and is often exacerbated by obesity (Dunaif, 1989). For example, obesity can worsen hyperandrogenism by lowering SHBG and therefore increasing bioavailable testosterone (Lim, 2013). Thus, obesity can have a synergistic effect on PCOS and can worsen ovulatory dysfunction, hyperandrogenism, and acanthosis nigricans.
This disorder is likely related to central obesity and insulin resistance (Fogel, 2001; Vgontzas, 2001). Women with PCOS have a 30- to 40-fold higher risk of sleep apnea compared with weight-matched controls. This suggests a link between obstructive sleep apnea and the metabolic and hormonal abnormalities associated with PCOS. Moreover, some theorize two subtypes of PCOS, that is, PCOS with or without obstructive sleep apnea. PCOS patients with this condition may be at much higher risk for DM and cardiovascular disease than women with PCOS but without obstructive sleep apnea (Nitsche, 2010).
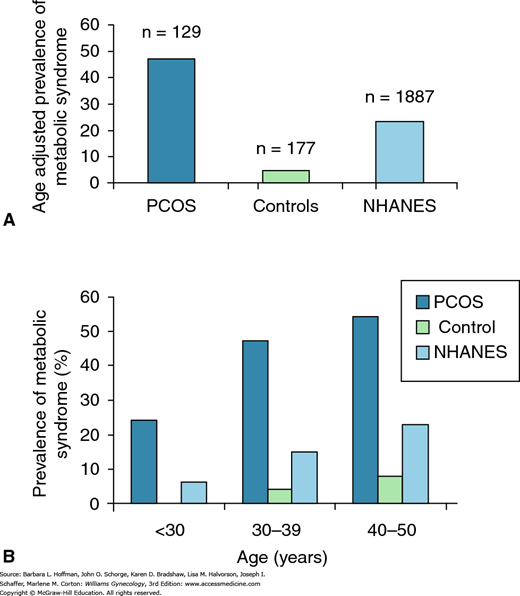
The metabolic syndrome is characterized by insulin resistance, obesity, atherogenic dyslipidemia, and hypertension. It is associated with an increased risk of cardiovascular disease (CVD) and type 2 DM (Schneider, 2006). The prevalence of metabolic syndrome approximates 45 percent in women with PCOS compared with 4 percent in age-adjusted controls (Fig. 17-7) (Dokras, 2005). PCOS shares several endocrine features with the metabolic syndrome, although definitive evidence for an increased incidence of CVD in women with PCOS is lacking (Legro, 1999; Rebuffe-Scrive, 1989; Talbott, 1998). However, in a small group of women with PCOS, Dahlgren and colleagues (1992) predicted a relative risk of 7.4 for myocardial infarction. Another 10-year surveillance study showed an odds ratio of 5.91 for CVD in overweight white women with PCOS (Talbott, 1995). Thus, evidence suggests that women with PCOS should have CVD factors identified and treated (Table 1-8) (Wild, 2010).
FIGURE 17-7
A. Women with polycystic ovarian syndrome (PCOS) have an increased risk of metabolic syndrome compared with age-adjusted controls and with women from the Third National Health and Nutrition Survey (NHANES III). B. In women with PCOS, the risk of metabolic syndrome begins earlier than in controls or those from NHANES III. NHANES III collected data from a representative sample of the noninstitutionalized civilian U.S. population from 1988 through 1994. (Reproduced with permission from Dokras A, Bochner M, Hollinrake E: Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol 2005 Jul;106(1):131–137.)
In addition to components of the metabolic syndrome, other markers of subclinical disease link PCOS and CVD. Women with PCOS have been found to have a greater incidence of left ventricular diastolic dysfunction and increased internal and external carotid artery stiffness (Lakhani, 2000; Tiras, 1999
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree