INTRODUCTION
Breast disease in women encompasses a spectrum of benign and malignant disorders, which present most commonly as breast pain, nipple discharge, or palpable mass. The specific causes of these symptoms vary with patient age. Benign disorders predominate in young premenopausal women, whereas malignancy rates increase with advancing age. Evaluation of breast disorders usually requires the combination of a careful history, physical examination, imaging, and, when indicated, biopsy.
ANATOMY
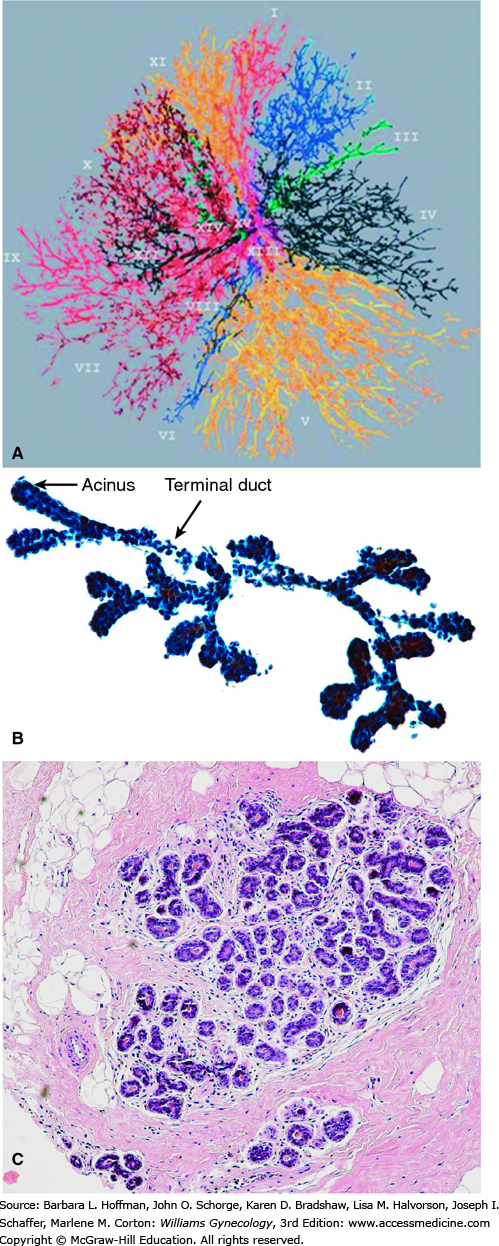
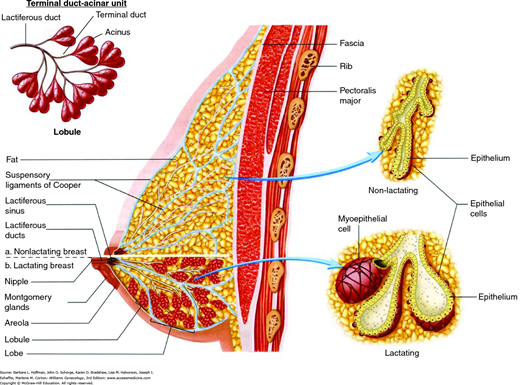
The glandular portion of the breast is composed of 12 to 15 independent ductal systems that each drain approximately 40 lobules (Fig. 12-1). Each lobule consists of 10 to 100 milk-producing acini that empty into small terminal ducts (Parks, 1959). Terminal ducts drain into larger collecting ducts that merge into even larger ducts, which exhibit a saccular dilation just below the nipple called a lactiferous sinus (Fig. 12-2).
FIGURE 12-1
A. Ductal anatomy of the breast. (Reproduced with permission from Going JJ, Moffat DF: Escaping from Flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions, J Pathology 2004 May;203(1):538–544.) B. Terminal duct—acinar structure from a fine-needle aspiration biopsy. C. Histology of a normal breast lobule. The terminal duct lobular units are surrounded by loosely cellular intralobular stroma, which consists of dense fibrous tissue admixed with adipocytes.
In general, only six to eight openings are visible on the nipple surface. These drain the dominant ductal systems, which account for approximately 80 percent of the breast’s glandular volume (Going, 2004). Minor ducts either terminate just below the nipple surface or open on the areola near the base of the nipple. The areola itself contains numerous lubricating sebaceous glands, called Montgomery glands, which are often visible as punctate prominences.
In addition to epithelial structures, the breast is composed of varying proportions of collagenous stroma and fat. The distribution and abundance of these stromal components accounts for a breast’s consistency when palpated and for its imaging characteristics.
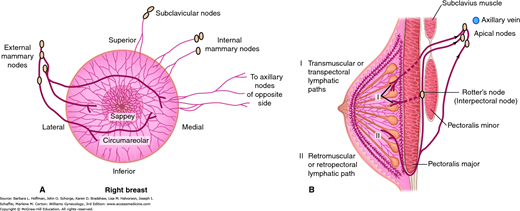
Afferent lymphatic drainage of the breast is provided by dermal, subdermal, interlobar, and prepectoral systems (Fig. 12-3)(Grant, 1953). Each of these may be viewed as a lattice of valveless channels that interconnect with every other system and that ultimately drain into one or two axillary lymph nodes (the sentinel nodes). Because all of these systems are interconnected, the breast drains as a unit, and injection of colloidal dyes in any part of the breast at any level will result in accumulation of dye in the same one or two axillary sentinel lymph nodes. The axillary lymph nodes receive most of the lymphatic drainage of the breast and consequently are the nodes most frequently involved with breast cancer metastases (Hultborn, 1955). However, there are also alternate drainage pathways that do not appear to interconnect with other networks and that drain directly into internal mammary, supraclavicular, contralateral axillary, or abdominal lymph node basins.
DEVELOPMENT AND PHYSIOLOGY
During fetal development, the primordial breast arises from the basal layer of the epidermis. Before puberty, the breast is a rudimentary bud composed of a few branching ducts capped with alveolar buds, end buds, or small lobules (Osin, 1998). At puberty, usually between the ages of 10 and 13 years, ovarian estrogen and progesterone cooperate to direct organized communication between breast epithelial cells and mesenchymal cells, resulting in extensive branching of the ductal system and development of lobules (Ismail, 2003). Specific disorders of this development are discussed in Chapter 14. Final differentiation of the breast is mediated by progesterone and prolactin and is not completed until the first full-term pregnancy (Grimm, 2002; Ismail, 2003).
During the reproductive years, terminal ducts near the acini and the acini themselves are most sensitive to ovarian hormones and prolactin. Most forms of benign and malignant breast disease arise in these terminal duct-acinar structures. Breast epithelial cells proliferate during the luteal phase of the menstrual cycle when estrogen and progesterone levels are increased, and then undergo programmed cell death at the end of the luteal phase, when levels of these hormones decline (Anderson, 1982; Soderqvist, 1997). This effect is mediated by paracrine signaling induced by estrogen-receptor activation and is associated with an increase in the water content of the extracellular matrix (Stoeckelhuber, 2002). This is often recognized as breast fullness and tenderness in the week preceding menses.
At menopause, when ovarian estrogen production ceases, breast lobules involute, and the collagenous stroma is replaced by fat. Because estrogen receptor expression is negatively regulated by estrogen, there is an increase in estrogen receptor expression after menopause (Khan, 1997). Despite a decline in ovarian estrogen production, postmenopausal women continue to produce estrogen through the action of the enzyme aromatase, which converts adrenal androgens to estrogen (Bulun, 1994). Aromatase is found in fat, muscle, and breast tissue.
EVALUATION OF A BREAST LUMP
It is not possible to distinguish benign from malignant or cystic from solid breast masses by clinical examination. However, findings from clinical examination, interpreted in conjunction with imaging and pathology (the triple test), contribute significantly to management decisions (Hermansen, 1987).
The breast is comma shaped, and the comma’s tail corresponds to the axillary tail of Spence. This extension can be large, especially during pregnancy and lactation, and is frequently mistaken for an axillary mass.
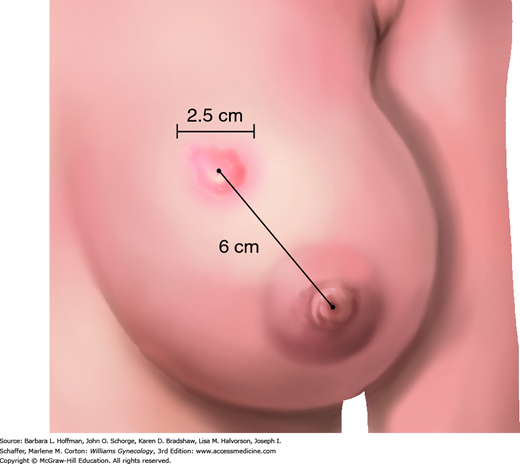
Clinical examination of the breast begins with inspection of the breast to determine whether there is dimpling, nipple retraction, or skin changes. This examination is described in Chapter 1. The presence and character of expressible nipple discharge is recorded. In addition, the location of a mass is specifically documented according to its clock position and then measured along the long axis using a ruler or caliper (Fig. 12-4). The distance from the center of the nipple to the center of the mass is specified. Since numerous health care providers are typically involved in the evaluation and management of the same breast mass, the most useful entry in the clinical record will define the location and size of the mass (e.g., right breast, 2-cm mass, 3:00, 4 cm from the nipple). Although clinical examination alone can never exclude malignancy, noting that a mass has benign features such as smoothness, roundness, and mobility will factor into the ultimate decision to excise or observe a lesion. Evaluation also includes careful examination of the axillae, infraclavicular fossa, and supraclavicular fossa to identify lymphadenopathy.
Imaging of a suspected mass may begin with mammography that includes magnification, extra compression, or extra views beyond the usual medial lateral oblique and cranial caudal views that are typically used for screening. Unlike screening mammography, diagnostic mammography may be appropriate for women of any age. In addition, sonography is invaluable for determining whether a mass is cystic or solid and is a component of most diagnostic imaging algorithms. Certain features of solid masses, such as irregular margins, internal echoes, or a width-to-height ratio <1.7 may suggest malignancy (Stavros, 1995).
Diagnostic imaging results should be summarized according to the Breast Imaging Reporting and Data System (BI-RADS) classification (Table 12-1) (D’Orsi, 2013). Lesions that are graded BI-RADS 5 are highly suggestive of malignancy, and ≥95 percent of these are ultimately proven to be cancerous. Decreasing numerical grades are associated with diminishing probability of malignancy.
| BI-RADS Category | Description | Examples |
| 0 | Additional views or sonography required | Focal asymmetry, microcalcifications, or a mass identified on a screening mammogram |
| 1 | No abnormalities identified | Normal fat and fibroglandular tissue |
| 2 | Not entirely normal, but definitely benign | Fat necrosis from a prior excision, stable biopsy-proven fibroadenoma, stable cyst |
| 3 | Probably benign | Circumscribed mass that has been followed for <2 years |
| 4A | Low suspicion for malignancy, but intervention required | Probable fibroadenoma, complicated cyst |
| 4B | Intermediate suspicion for malignancy, intervention required | Partially indistinctly marginated mass otherwise consistent with a fibroadenoma |
| 4C | Moderate suspicion, but not classic for carcinoma | New cluster of fine pleomorphic calcifications, ill-defined irregular solid mass |
| 5 | Almost certainly malignant | Spiculated mass, fine linear and branching calcifications |
| 6 | Biopsy-proven carcinoma | Biopsy-proven carcinoma |
Evaluation of a solid breast mass is completed by needle biopsy. These biopsies should be performed after an imaging test or a minimum of 2 weeks prior to an imaging test. This is because resulting tissue trauma can produce image artifacts that simulate malignancy (Sickles, 1983). Options include fine-needle aspiration (FNA) biopsy or core-needle biopsy. The trend in recent years favors core-needle biopsy (Tabbara, 2000). Although FNA takes less time to perform and is less expensive than core-needle biopsy, it is less likely to provide a specific diagnosis and has a higher insufficient sample rate (Shannon, 2001). FNA retrieves clusters of epithelial cells that may be interpreted as benign or malignant, but it cannot reliably differentiate between benign proliferative lesions and fibroepithelial neoplasms or between ductal carcinoma in situ and invasive cancer (Boerner, 1999; Ringberg, 2001).
In contrast, core-needle biopsy is performed using an automated device that takes one core at a time or is completed using a vacuum-assisted device that, once initially positioned, delivers multiple cores. Needle biopsy of solid masses should be done prior to excision, as the biopsy results contribute significantly to surgical planning (Cox, 1995).
The combination of clinical examination, imaging, and needle biopsy is called the triple test (Wai, 2013). When all three assessments suggest a benign lesion or all three suggest a breast cancer, the triple test is said to be concordant. A concordant benign triple test is >99-percent accurate, and breast lumps in this category can be followed by clinical examination alone at 6-month intervals. If any of the three assessments suggests malignancy, the lump should be excised regardless of results from the other two. It is always appropriate to offer excision of a fully evaluated breast lump, even after a benign concordant triple test result, as breast lumps can be a source of significant anxiety.
BENIGN LUMPS AND FIBROEPITHELIAL NEOPLASMS
Most breast cysts arise from apocrine metaplasia of lobular acini. They are generally lined by a single layer of epithelium that ranges from flattened to columnar. One autopsy series that included 725 women reported microcysts in 58 percent and cysts >1 cm in 21 percent (Davies, 1964). The incidence of breast cysts peaks between 40 and 50 years, and the lifetime incidence of palpable breast cysts is estimated to be 7 percent (Haagensen, 1986).
Breast cysts are diagnosed and classified by sonography. There are three types of cysts: simple, complicated, and complex (Berg, 2003). Simple cysts are sonolucent, have a smooth margin, and show enhanced through-transmission (Fig. 12-5). These lesions do not require special management or monitoring, but they may be aspirated if painful. Recurrent cysts can be reimaged and reaspirated, but recurrent symptomatic cysts are best managed by excision.
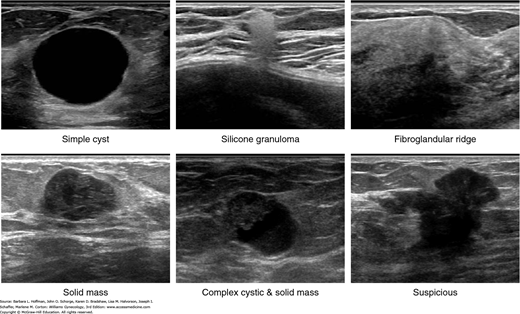
FIGURE 12-5
Sonographic appearance of palpable breast masses. A. Simple cyst. B. Silicone granuloma. C. Fibroglandular ridge. D. Solid mass (benign phyllodes tumor). E. Complex cystic and solid mass (intracystic papillary carcinoma with low-grade ductal carcinoma in situ). F. Suspicious (invasive ductal carcinoma). (Used with permission from Stephen J. Seiler, MD.)
Complicated cysts show internal echoes during sonography and can sometimes be indistinguishable from solid masses. Internal echoes are usually caused by proteinaceous debris. Consideration is given to aspirating complicated cysts. The aspirated material may be submitted for culture, if it is purulent, or for cytology, if there are worrisome clinical or imaging features. If the sonographic abnormality does not resolve completely with aspiration, a core-needle biopsy is usually performed.
Complex cysts show septa or intracystic masses during sonographic evaluation. An intracystic mass usually represents a papilloma, but medullary carcinoma, papillary carcinoma, and some infiltrating ductal carcinomas can present as complex cysts. Although some advocate core-needle biopsy for the evaluation of complex cysts, this procedure can decompress a cyst, making it difficult to localize at the time of surgery. Additionally, papillary lesions diagnosed by needle biopsy will require excision. Thus, it seems reasonable to recommend excision of all complex cysts.
This represents a focal developmental abnormality of a breast lobule and as such is not a true neoplasm. Histologically, fibroadenomas are composed of glandular and cystic epithelial structures surrounded by a cellular stroma. Fibroadenomas account for 7 to 13 percent of breast clinic visits and had a prevalence of 9 percent in one autopsy series (Dent, 1988; Franyz, 1951). They often present in adolescence, are recognized most frequently in premenopausal women, and usually spontaneously involute at menopause.
Fibroadenomas classified as benign concordant by the triple test can be safely followed without excision. Because some fibroadenomas may grow large, and because benign phyllodes tumors are often indistinguishable from fibroadenomas by imaging and needle biopsy, a fibroadenoma that is growing should be excised.
These are true biphasic neoplasms characterized by epithelial-lined spaces surrounded by cellular stroma. Both the epithelial and stromal components can be monoclonal and clonally related (Karim, 2013). Phyllodes tumors are classified as benign, intermediate, or malignant, based on the degree of stromal cell atypia, number of mitoses, tumor margin characteristics, and abundance of stromal cells (Oberman, 1965). Phyllodes tumors account for less than 1 percent of breast neoplasms, and the median age at diagnosis is 40 years (Kim, 2013; Reinfuss, 1996).
Malignant phyllodes tumors can metastasize to distant organs, with lung being the primary site. Chest radiographs or chest computed-tomography (CT) scanning are appropriate staging tests for malignant cases. Phyllodes tumors rarely metastasize to lymph nodes, thus axillary staging is not required unless nodes clinically appear involved (Chaney, 2000).
Treatment consists of wide local excision with a minimum 1-cm margin. Mastectomy may be required to achieve this margin, as the median tumor size at presentation is 5 cm. Local recurrence rates for completely excised tumors range from 8 percent for benign lesions to 36 percent for malignant ones (Barth, 1999). Fibroproliferation in the surrounding breast tissue and necrosis are the strongest predictors of recurrence (Barrio, 2007). Postoperative adjuvant radiation therapy may be indicated for high-risk cases (Barth, 2009).
NIPPLE DISCHARGE
Fluid can be expressed from the nipple ducts of at least 40 percent of premenopausal women, 55 percent of parous women, and 74 percent of women who have lactated within 2 years (Wrensch, 1990). The fluid generally issues from more than one duct and may range from milky white to dark green or brown. Green coloration is related to the content of cholesterol diepoxides and does not suggest underlying infection or malignancy (Petrakis, 1988).
Multiduct discharges that are elicited only following manual expression are considered physiologic and do not require additional evaluation. However, spontaneous discharges merit evaluation (Fig. 12-6). Spontaneous milky nipple discharge, also called galactorrhea, results from various causes (Table 12-2) (Chap. 15). Of these, pregnancy is a frequent cause of new-onset spontaneous discharge, and a bloody multiduct discharge during pregnancy is not uncommon.
| Idiopathic | Systemic disorders Chronic renal failure |
| PhysiologicLactation Breast stimulation Stress | Hypothyroidism Cirrhosis Pseudocyesis Seizures |
| Hypothalamic lesions | Ectopic tumor production |
| Tumors Infiltrative disorders Irradiation Trauma, surgery Rathke cleft cyst | Pharmacologic Dopamine-blocking agents:
|
| Pituitary lesions Prolactinoma Other tumors Infiltrative disorders Lymphocytic hypophysitis Empty sella |
Dopamine depletors: reserpine, opiates, α-methyldopa H2 antagonists: cimetidine, ranitidine Serotonergic pathway stimulation: amphetamines |
| Intercostal nerve stimulation Chest wall lesions Chest surgery Spinal cord injury | Calcium-channel blockers: verapamil Antidepressants: MAOI, TCA, SSRI Estrogen |
Pathologic nipple discharge is defined as a spontaneous single-duct discharge that is serous or bloody. The rate of underlying malignancy ranges from approximately 2 percent for young women with no associated findings on imaging or physical examination to 20 percent for older women with associated findings (Cabioglu, 2003; Lau, 2005). Most pathologic nipple discharges are caused by benign intraductal papillomas, which are simple milk duct polyps (Urban, 1978). They arise in the major milk ducts, generally within 2 cm of the nipple, and contain a velvety papillary epithelium on a central fibrovascular stalk.
Evaluation of a pathologic nipple discharge begins with breast examination. Careful evaluation can frequently locate a trigger point on the areolar edge that elicits the discharge when pressed. Occult-blood testing and microscopic examination of the discharge can provide additional information. A glass slide that has been touched to the discharge and immediately fixed in 95-percent alcohol may be used for cytologic assessment. Nipple fluid samples are acellular in 25 percent of cases and thus cannot exclude an underlying malignancy (Papanicolaou, 1958). However, malignant cells, if found, are highly correlated with an underlying cancer (Gupta, 2004).
Following these examinations, diagnostic mammography and an assessment of the subareolar ducts by sonography or ductography is indicated. Diagnostic mammography is usually negative, but it may occasionally identify an underlying ductal carcinoma in situ (DCIS). Mammary ductography, also known as galactography, requires cannulating the affected duct, injecting radiocontrast, and then performing mammography (Fig. 12-7).
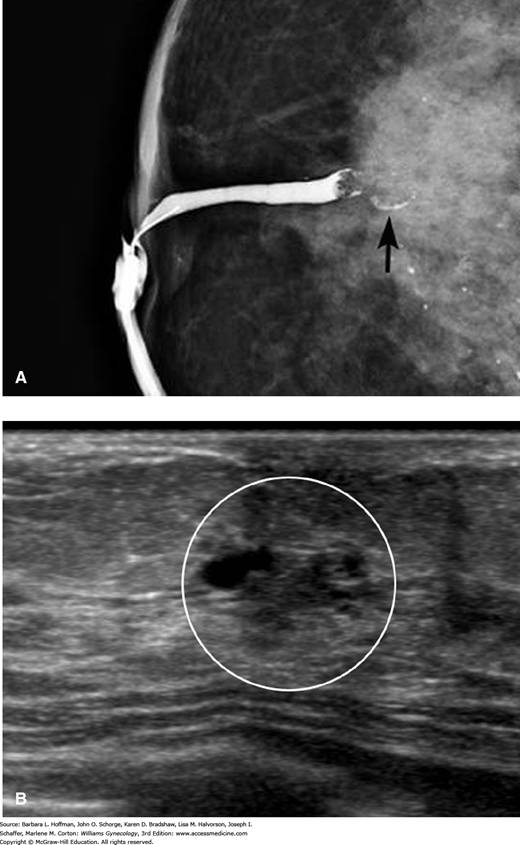
FIGURE 12-7
Imaging for a pathologic nipple discharge. A. Ductography shows a single dilated duct with an irregular filling defect (arrow). B. Periareolar sonogram demonstrates an irregular intraductal mass with microlobulated margins within the white circle. An excisional biopsy revealed a benign intraductal papilloma. (Used with permission from Stephen J. Seiler, MD.)
An evaluation of the subareolar ducts, as described above, is required to localize an intraductal lesion for subsequent excision. However, pathologic nipple discharge is definitively diagnosed and treated by subareolar duct excision, which is also known as microductectomy (Locker, 1988). Subareolar duct excision can also be used to treat bothersome multiduct discharges not associated with pituitary prolactinoma.
BREAST INFECTIONS
Breast infections are generally divided into puerperal, which develop during pregnancy and lactation, and nonpuerperal. Of these, pregnancy-related breast infection is characterized by warm, tender, diffuse breast erythema, associated with systemic signs of infection such as fever, malaise, myalgias, and leukocytosis. The most common organism is staphylococcus, and it is successfully treated with oral or intravenous antibiotics, depending on the severity. However, infection may also progress to form deep parenchymal abscesses that require surgical drainage (Branch-Elliman, 2012). Sonographic examination is highly sensitive for identifying underlying abscesses if mastitis does not improve rapidly with antibiotics or if an abscess is suggested clinically. Women with puerperal mastitis should continue to breast feed or breast pump during treatment to prevent milk stasis, which may contribute to infection progression (Thomsen, 1983). Cracked or excoriated nipples may provide entry for bacteria and are treated with lanolin-based lotions or ointments.
Appropriate antibiotics for puerperal mastitis include those covering staphylococcal species. Group A and B Streptococcus, Corynebacterium, and Bacteroides species and Escherichia coli are less frequently isolated. Commonly, cephalexin (Keflex) or dicloxacillin (Dynapen), each given at dosages of 500 mg orally four times daily, or the combination of amoxicillin and clavulanate (Augmentin), 500 mg orally three times daily, may be prescribed for 7 days. Erythromycin, 500 mg orally four times daily, will provide adequate coverage for those with a penicillin allergy. Methicillin-resistant Staphylococcus aureus (MRSA) is becoming a more prevalent community-acquired pathogen causing mastitis in pregnancy and the puerperium (Laibl, 2005; Stafford, 2008). If MRSA is suspected or if a patient fails to improve on an initial regimen, then trimethoprim-sulfamethoxazole double strength (Bactrim DS, Septra DS), one or two tablets orally twice daily, or clindamycin, 300 mg orally three times daily, is a suitable choice. In ill patients with extensive infection, hospitalization and intravenous (IV) antibiotics are typically required. In these complicated cases, MRSA coverage may be prudent, and clindamycin, 600 mg IV every 8 hours, or vancomycin, 1 g IV every 12 hours, can be administered. Intravenous antibiotics are typically given until the woman is afebrile for 24 to 48 hours. Oral antibiotics are then continued to complete a 7- to 10-day course.
Focal mastitis may result from an infected galactocele. A tender mass will usually be palpable at the site of skin erythema. Needle aspiration of the galactocele and antibiotics are frequently all that is required, but recurrence or progression may mandate surgical drainage.
Uncomplicated cellulitis in a nonirradiated breast and in a nonpuerperal setting is uncommon. Accordingly, its presence prompts imaging and biopsies to exclude inflammatory breast cancer, described on page 291.
Nonpuerperal breast abscesses are generally classified as peripheral or subareolar. Peripheral abscesses usually are skin infections such as folliculitis or infection of epidermal inclusion cysts or Montgomery glands. These abscesses are all adequately treated by drainage and antibiotics discussed in the previous section. In contrast, subareolar abscesses arise from keratin-plugged milk ducts directly behind the nipple. The abscess itself usually presents under the areola, and fistulous communications between multiple abscesses are common (Kasales, 2014). Simple drainage is associated with a recurrence rate of nearly 40 percent, thus effective treatment requires subareolar duct excision and complete removal of sinus tracts. In general, surgical drainage of nonpuerperal breast abscesses is usually always accompanied by biopsy of the abscess wall, as breast cancer occasionally presents as an abscess (Benson, 1989; Watt-Boolsen, 1987).
Idiopathic granulomatous mastitis (IGM) is not a true infection. It is included in this section because the painful masses, fluid collections, skin erythema, ulceration, and draining sinus tracts are often confused with infection. Core biopsy will show noncaseating granulomas, and fluid aspirated from apparent “abscesses” is nearly always sterile. Tissue stains can be used to exclude tuberculosis or mycotic infection. Wegener granulomatosis and sarcoidosis are considered in the initial differential diagnosis. This is a self-limiting condition that may take years to resolve. Procedures should be minimized as they will often result in painful draining sinuses. High-dose corticosteroids or methotrexate have been used for treatment, but it is not clear whether they are effective (Mohammed, 2013; Pandey, 2014).
MASTALGIA
The prevalence of breast pain is 66 percent and is higher for women nearing menopause than for younger women (Euhus, 1997; Maddox, 1989). The precise etiology of mastalgia is unknown, but it is likely related to estrogen- and progesterone-mediated changes in interstitial water content and therefore in interstitial pressure.
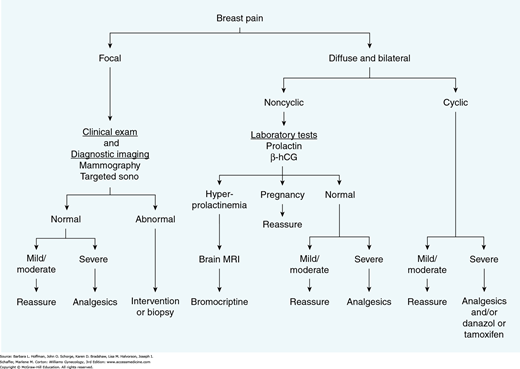
Mastalgia is generally classified as cyclic or noncyclic. Noncyclic mastalgia is often focal and shows no relationship to the menstrual cycle. Although focal mastalgia is frequently caused by a simple cyst, breast cancer occasionally presents as focal breast pain. Therefore, this complaint is evaluated by careful clinical examination, targeted imaging, and needle biopsy of any palpable or imaging abnormalities.
In contrast, cyclic mastalgia is usually bilateral, diffuse, and most severe during the late luteal phase of the menstrual cycle (Gateley, 1990). It remits with the onset of menstruation. Cyclic mastalgia requires no specific evaluation and is generally managed symptomatically with nonsteroidal antiinflammatory agents (Fig. 12-8). Various other treatments have been proposed including bromocriptine, vitamin E, or oil of evening primrose. However, outcomes are no better than placebo in the best randomized clinical trials, except for bromocriptine in the subset of women with elevated prolactin levels (Kumar, 1989; Mansel, 1990). For the most severe cases, several agents are effective when administered during the last 2 weeks of the menstrual cycle. These include: (1) danazol, 200 mg orally daily; (2) the selective estrogen-receptor modifier toremifene (Fareston), 20 mg orally daily; or (3) tamoxifen (Nolvadex), 20 mg orally daily. Pregnancy must first be excluded and then avoided if these medications are used.
BENIGN BREAST DISEASE
The primary tissue components of the breast are fat, fibrous stroma, and epithelial structures. The hormonally responsive component is the epithelium, but considerable paracrine communication exists between the epithelium and stroma. The natural hormonal changes of puberty, pregnancy, lactation, and menopause drive considerable physiologic remodeling of breast tissue during a woman’s lifetime, but pathologic remodeling is observed in some. This is initially characterized by acinar dilation and fibrosis, termed nonproliferative benign breast disease. Depending on the extent and pattern of these changes, a breast may appear mammographically dense, feel nodular to palpation, or both. The term “fibrocystic change” is often used to refer to palpably nodular breast tissue or to the histologic pattern of dilated ducts and acini invested with dense collagenous stroma. This is not a significant breast cancer risk factor and does not require any special management.
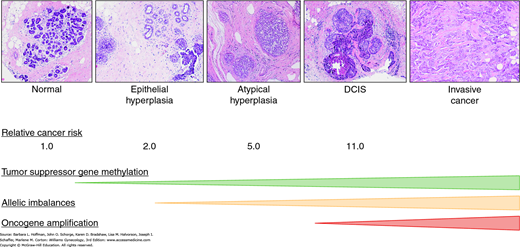
When this change is accompanied by accumulation of luminal epithelial cells (e.g., epithelial hyperplasia), it is called benign proliferative disease (Fig. 12-9). This change has been linked to higher levels of estrogen, insulin, and certain inflammatory cytokines, as well as reduced levels of the beneficial adipokine adiponectin (Catsburg, 2014). Benign proliferative breast disease without atypia is a modest breast cancer risk factor with a relative risk of 1.5 to 1.9 (Dupont, 1993; Hartmann, 2005; Sneige, 2002).
Atypia refers to specific alterations in the size, shape, or nuclear features of individual epithelial cells in combination with the way groups of cells are organized. Atypical proliferation of ductal cells is termed atypical ductal hyperplasia (ADH), whereas similar changes in acinar cells are termed atypical lobular hyperplasia (ALH). As more and more terminal ducts or acini become involved, the condition is recognized as ductal carcinoma in situ or lobular carcinoma in situ, respectively, which are discussed in later sections (Ringberg, 2001).
Benign proliferative disease with atypia historically accounts for 4 percent of benign breast lesions (Hartmann, 2005). However, the incidence has recently decreased coincident to reductions in hormone replacement therapy use (Menes, 2009).
It is important to recognize that the difference between ADH and low-grade ductal carcinoma in situ is based on the area occupied by the proliferative epithelial cells (Vandenbussche, 2013). Accordingly, surgical excision is usually recommended when ADH is diagnosed by core biopsy, as 4 to 38 percent of cases will be upgraded to in situ or invasive cancer.
Chemoprevention is an excellent option for high-risk women with atypical hyperplasia. These lesions are estrogen-driven, and tamoxifen has been shown to reduce breast cancer risk by 52 to 86 percent for these women (Coopey, 2012; Fisher, 1999).
Benign proliferative disease with atypia is a marker of increased breast cancer risk. Relative risks are 4.5 to 5.0, and absolute risks approximate 1 percent per year for 20 to 30 years (Degnim, 2007; Dupont, 1993). This risk is higher for more extensive lesions. Risk does not appear to increase further with hormone replacement use.
LOBULAR CARCINOMA IN SITU
Similar to proliferative ductal lesion, lobular carcinoma in situ (LCIS) differs from ALH by the greater extent of lobular cell proliferation with LCIS and increased acini distention. LCIS is not associated with any specific mammographic or palpable features and thus is only diagnosed incidentally. Classic LCIS has not traditionally been viewed as a direct precursor of breast cancer, but this view is changing. For example, although LCIS is associated with increased risk for both breasts, women with LCIS most commonly develop carcinoma in the ipsilateral breast (Fisher, 2004b; Ottesen, 1993; Salvadori, 1991). Moreover, infiltrating lobular cancers frequently show associated LCIS, and a clonal relationship between LCIS and subsequent invasive cancer has been demonstrated (Abner, 2000; Andrade, 2012; Sasson, 2001).
As noted, LCIS is also a marker of increased breast cancer risk. The risk of subsequent breast cancer approximates 1 percent per year but is modified upward by early age at diagnosis, family history of breast cancer, and extensive disease (Bodian, 1996).
Surgical excision is recommended if LCIS is diagnosed by needle biopsy, as an associated cancer will be identified in 2 to 25 percent of cases (Buckley, 2014). Excising to clear margins is not required (Sadek, 2013). These lesions are strongly estrogen receptor positive, and tamoxifen has been shown to reduce breast cancer risk by 56 percent in this setting (Fisher, 1999).
DUCTAL CARCINOMA IN SITU
DCIS can be understood as a condition in which cancer cells fill portions of a mammary ductal system without invading beyond the duct’s basement membrane (Ringberg, 2001). Although DCIS cells have accumulated many of the DNA changes common to invasive breast cancer, they lack certain critical changes that would permit them to persist outside of the duct (Aubele, 2002). Ductal carcinoma in situ is classified as stage 0 breast cancer.
The U.S. incidence of DCIS has increased in parallel with that of invasive breast cancer during the past two decades. But, as with invasive breast cancer, the incidence has plateaued during the past several years (Virnig, 2010). DCIS currently accounts for 25 to 30 percent of all breast cancers in the United States. It is most commonly diagnosed by screening mammography as it is frequently associated with pleomorphic, linear, or branching calcifications (Fig. 12-10).
DCIS is classified by morphologic type, the presence or absence of comedonecrosis, and nuclear grade. The common morphologic types include cribriform, solid, micropapillary, and comedo (Fig. 12-11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree