INTRODUCTION
Endometriosis is a common benign disorder defined as the presence of endometrial glands and stroma outside of the normal location. Implants of endometriosis are most often found on the pelvic peritoneum, but other frequent sites include the ovaries and uterosacral ligaments. Endometrial tissue located within the myometrium is termed adenomyosis and discussed in Chapter 9. Women with endometriosis may be asymptomatic, subfertile, or suffer varying degrees of pelvic pain. This is an estrogen-dependent disease and thus lends itself to hormone-based treatment. However, in those with disease refractory to medical management, surgery may be required.
INCIDENCE
The incidence of endometriosis is difficult to quantify, as women with the disease are often asymptomatic. Moreover, imaging modalities have low sensitivities for small implants (Wall, 2015). The primary method of diagnosis is laparoscopy, with or without biopsy for histologic diagnosis (Dunselman, 2014). Using this standard, the annual incidence of surgically diagnosed endometriosis was 1.6 cases per 1000 women aged between 15 and 49 years (Houston, 1987). In asymptomatic women, the prevalence of endometriosis ranges from 6 to 11 percent, depending on the population studied and mode of diagnosis (Buck Louis, 2011; Mahmood, 1991). However, because of its link with infertility and pelvic pain, endometriosis is notably more prevalent in subpopulations of women with these complaints. From studies, the prevalence lies between 20 to 50 percent in infertile women, and in those with pelvic pain, it ranges from 40 to 50 percent (Balasch, 1996; Eskenazi, 2001; Meuleman, 2009). In adolescents, Janssen and coworkers (2013) reported that nearly two thirds of adolescents undergoing diagnostic laparoscopy for pelvic pain had evidence of endometriosis.
Previously, white women were thought to be disproportionately affected. More recent studies have provided variable results. Some show greater rates for whites and Asians, whereas others have found no statistically significant differences in endometriosis prevalence among any racial or ethnic groups (Jacoby, 2010). Of other patient characteristics, lower body mass appears to positively correlate with endometriosis risk (Peterson, 2013; Shah, 2013).
PATHOPHYSIOLOGY
The definitive cause of endometriosis remains unknown, but theories have been proposed. A more favored one describes retrograde menstruation through the fallopian tubes (Sampson, 1927). These refluxed endometrial fragments invade the peritoneal mesothelium and develop a blood supply for implant survival and growth. Supporting data include a report that surgical obliteration of the outflow tract in baboons induces endometriosis (D’Hooghe, 1997). In correlation, women with outflow tract obstruction also have a high incidence of endometriosis, which often resolves following obstruction relief (Sanfilippo, 1986; Williams, 2014). Importantly however, most women have retrograde menstruation (Halme, 1984). Thus, other factors, such as immunologic and angiogenic components, likely aid implant persistence.
Another hypothesis, the stem cell theory, implicates undifferentiated endometrial cells that initially reside in the endometrium’s basalis layer. These cells differentiate into epithelial, stromal, and vascular cells as the endometrium is routinely regenerated each cycle. If displaced to an ectopic location, such as by retrograde menstruation, these stem cells may give rise to endometriosis (Valentijn, 2013).
Aberrant lymphatic or vascular spread of endometrial tissue has also been implicated (Jerman, 2015). Lymphatic spread of endometriosis to pelvic sentinel lymph nodes is noted in affected women (Mechsner, 2008; Tempfer, 2011). Findings of endometriosis in unusual locations, such as the groin, also bolster this theory (Mourra, 2015). Last, cases in which no peritoneal implants are found, but solely isolated retroperitoneal lesions are noted, implicate lymphatic spread (Moore, 1988).
Another theory concerns coelomic metaplasia and suggests that the parietal peritoneum is pluripotent and can undergo metaplastic transformation to tissue histologically identical to normal endometrium. Because the ovary and the progenitor of the endometrium, the müllerian ducts, are both derived from coelomic epithelium, such metaplasia may help explain endometriosis involving the ovary. This process may also underlie cases of endometriosis in those without menstruation, such as premenarchal girls and males treated with estrogen and orchiectomy for prostate cancer (Marsh, 2005; Taguchi, 2012). Last, a theory purports that müllerian remnants left along their embryonic path undergo abnormal differentiation (Batt, 2013; Signorile, 2012).
Endometriosis may develop anywhere within the pelvis and on other extrapelvic peritoneal surfaces. Most commonly, endometriosis is found in the dependent areas of the pelvis. As such, the anterior and posterior cul-de-sacs, other pelvic peritoneum, the ovary, and uterosacral ligaments are frequently involved. Additionally, the rectovaginal septum, ureter, and bladder and rarely, pericardium, surgical scars, and pleura may be affected. One pathologic review revealed that endometriosis has been identified on all organs except the spleen (Markham, 1989). Implants may be superficial or they may be deep infiltrating endometriosis (DIE), that is, infiltrative forms that involve vital structures such as bowel, bladder, and ureters (Koninckx, 2012; Vercellini, 2004). Some definitions of DIE also quantify invasion as >5 mm (Koninckx, 1994).
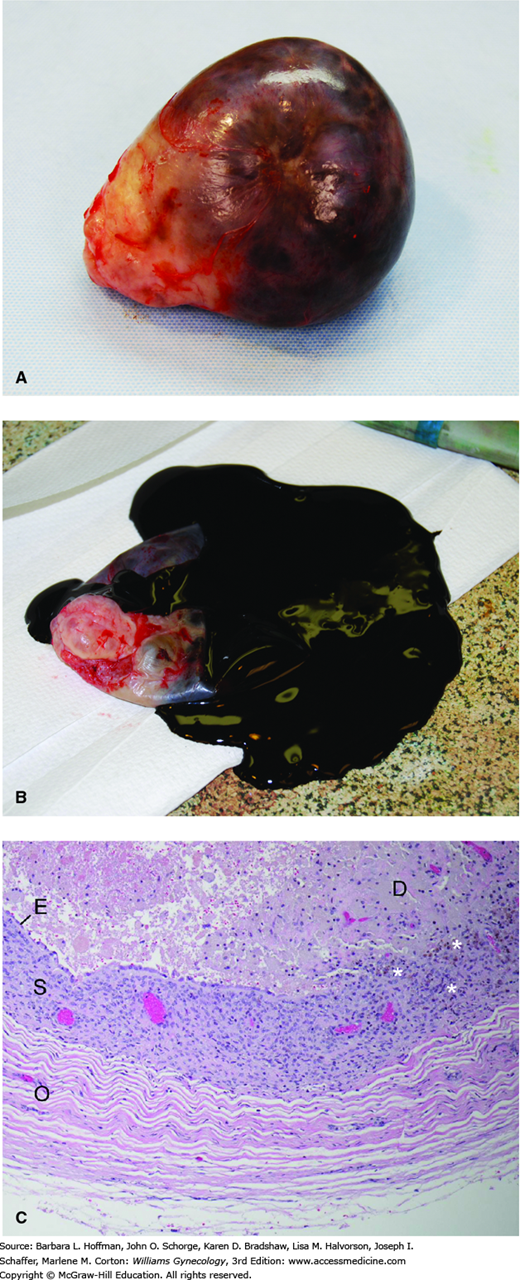
As just noted, ovarian endometriomas are frequent manifestations of endometriosis (Fig. 10-1). These smooth-walled, dark-brown ovarian cysts are filled with a chocolate-appearing fluid and may be unilocular or, when larger, multilocular. Their pathogenesis is unclear, yet three theories include invagination of ovarian cortex implants, coelomic metaplasia, and secondary involvement of functional ovarian cysts by endometrial implants located on the ovarian surface (Vignali, 2002).
FIGURE 10-1
Endometrioma. A. Surgical specimen of an ovary containing an endometrioma. B. Dark, chocolate-like fluid had filled this cyst. (Used with permission from Dr. Roxanne Pero.) C. In ovarian endometriomas, endometrial-type epithelium (E) and subjacent stroma (S) line the cyst, and are bordered peripherally by ovarian stroma (O). The golden brown pigment in the cyst wall (asterisks) is hemosiderin, indicating remote hemorrhage. Debris composed of necrotic and degenerating cells and remote hemorrhage occupies the interior of the cyst (D). It is the remote hemorrhage that confers the chocolate-like color to cyst fluid.
Endometriosis is an estrogen-dependent, chronic inflammatory disease with aberrant growth of ectopic endometrial tissue. In this discussion, eutopic endometrium is that which lines the uterine cavity, whereas ectopic endometrium describes that outside the cavity. In affected patients, ectopic endometrial implants show molecular differences from the eutopic endometrium of unaffected women. The disturbed molecular mechanisms in this disease are yet to be completely defined. However, suspected underpinnings include an environment of estrogen dominance, estrogen dependence, and progesterone resistance within implants; inflammation; escape from immune clearance; local invasion and neurovascularity development; and genetic predisposition.
Estrogen plays a causative role in endometriosis formation and is derived from multiple sources. First, most estrogen in women is produced directly by the ovaries. Second, peripheral tissues also produce estrogens through conversion of ovarian and adrenal androgens by the enzyme aromatase. Endometriotic implants express aromatase and 17β-hydroxysteroid dehydrogenase type 1, which are the enzymes responsible for conversion of androstenedione to estrone and of estrone to estradiol, respectively. Implants, however, are deficient in 17β-hydroxysteroid dehydrogenase type 2, which inactivates estrogen (Kitawaki, 1997; Zeitoun, 1998). This enzymatic combination ensures that implants create an estrogenic environment. Moreover, it provides the rationale for aromatase inhibitor use to diminish aromatase activity in refractory clinical cases. Last, the endometriotic stromal cell uniquely expresses the full complement of genes in the steroidogenic cascade, which is sufficient to convert cholesterol to estradiol itself (Bulun, 2012).
In addition to an estrogenic environment, normal progesterone effects are attenuated in endometriosis. This progesterone resistance is thought to stem from an overall low concentration of progesterone receptors within implants (Attia, 2000). Specifically, pathological overexpression of estrogen receptor β in endometriosis suppresses estrogen receptor α expression. This diminishes estradiol-mediated induction of the progesterone receptor in endometriotic cells (Xue, 2007).
As one consequence of this resistance, survival of refluxed endometrium in affected women may be bolstered. Namely, normal endometrium does not express aromatase and has elevated levels of 17β-hydroxysteroid dehydrogenase type 2 in response to progesterone (Satyaswaroop, 1982). As a result, progesterone antagonizes the estrogen effects in normal endometrium during the luteal phase. Endometriosis, however, manifests a relative progesterone-resistant state, which prevents this antagonism in its implants.
Progesterone resistance may also enhance implantation of refluxed endometrium. Invasion of the mesothelium can be aided by matrix metalloproteinases (MMPs). These are a group of collagenase proteins that can digest and remodel extracellular matrix and are implicated in endometrial turnover during normal menstruation. Of the various MMPs, MMP-3 expression is significantly increased in women with endometriosis compared with healthy controls, and its expression is significantly elevated during the luteal phase (Kyama, 2006). Progesterone represses MMP activity (Itoh, 2012). Thus, in affected patients, progesterone resistance within these implants may augment the MMP activity necessary for implant invasion.
Prostaglandin E2 (PGE2) is the most potent inducer of aromatase activity in endometrial stromal cells (Noble, 1997). Estradiol produced in response to the increased aromatase activity subsequently augments PGE2 production by stimulating the cyclooxygenase type 2 (COX-2) enzyme in uterine endothelial cells (Gurates, 2003). This creates a positive feedback loop and potentiates the estrogenic effects on endometriosis proliferation. As discussed on page 239, nonsteroidal antiinflammatory drugs (NSAIDs) are used clinically to reduce prostaglandin formation and thereby decrease endometriosis-linked pain.
With retrograde menstruation, refluxed menstrual tissue in most women is usually cleared by macrophages, natural killer (NK) cells, and lymphocytes. For this reason, immune system dysfunction is one likely mechanism for endometriosis establishment (Seli, 2003). Of these immune cells, macrophages serve as scavengers, and increased numbers are found in the peritoneal cavity of women with endometriosis (Haney, 1981; Olive, 1985b). Although this increased population might logically act to suppress endometrial proliferation, macrophages in these affected women actually stimulate endometriotic tissue (Braun, 1994).
Of other immune system players, NK cells have cytotoxic activity against foreign cells. Although NK cell numbers are unaltered in the peritoneal fluid of affected women, the NK cell cytotoxicity against endometrium is decreased (Ho, 1995; Wilson, 1994).
Cellular immunity may also be disordered in women with endometriosis, and T lymphocytes are implicated. For example, in patients with endometriosis compared with unaffected individuals, total lymphocyte numbers or helper/suppressor subpopulation ratios do not differ in peripheral blood. However, peritoneal fluid lymphocyte numbers are increased (Steele, 1984). Also, the cytotoxic activity of T lymphocytes against autologous endometrium in affected women is impaired (Gleicher, 1984).
Humoral immunity is also altered in affected women and is thought to play a role. Endometrial antibodies of the IgG class are more frequently detected in the sera of women with endometriosis (Odukoya, 1995). One study also identified IgG and IgA autoantibodies against endometrial and ovarian tissues in the sera and in cervical and vaginal secretions of affected women (Mathur, 1982). These results suggest that endometriosis may be, in part, an autoimmune disease.
Cytokines are small, soluble immune factors involved in signaling of other immune cells. Numerous cytokines, especially interleukins, are suspected in endometriosis pathogenesis. Of specific interest, increased levels of interleukin-1β (IL-1β), IL-6, and IL-8 have been identified in relevant tissues and fluids (Arici, 1998; Mori, 1991; Tseng, 1996).
Other cytokines and growth factors are associated with endometriosis establishment. For example, both monocyte chemoattractant protein-1 (MCP-1) and RANTES (regulated on activation, normal T-cell expressed and secreted) can attract monocytes. Levels of these cytokines are increased in the peritoneal fluid of those with endometriosis and positively correlate with disease severity (Arici, 1997; Khorram, 1993). In addition, vascular endothelial growth factor (VEGF) is an angiogenic growth factor, which is upregulated by estradiol in endometrial stromal cells and peritoneal fluid macrophages. Levels of this factor are increased in the peritoneal fluid of affected women (McLaren, 1996). Although the exact role of these cytokines is unclear, perturbations in their expression and activity further support an immunologic role in endometriosis development.
No mendelian genetic inheritance pattern has been identified for endometriosis. But, the increased incidence in first-degree relatives suggests a polygenic/multifactorial pattern. For example, in population studies, 4 to 8 percent of the female siblings or mothers of affected women had endometriosis (Dalsgaard, 2013). Other research revealed that women with endometriosis and an affected first-degree relative were more likely to have severe endometriosis (61 percent) than women without an affected first-degree relative (24 percent) (Malinak, 1980). Studies also demonstrate concordance for endometriosis in monozygotic twin pairs (Saha, 2015; Treloar, 1999).
To assist with identifying candidate genes, population-based genome-wide association studies (GWASs) have been performed. These studies are founded on the principle that common diseases, such as endometriosis, are caused by genetic variants that are common themselves. With GWAS, a set of several 100,000 common single nucleotide polymorphisms (SNPs or single DNA base-pair changes) are selected to provide the maximum coverage of the genome. Their frequencies are then compared between affected and unaffected groups. From GWASs of endometriosis, several candidate genes and chromosomes have been identified for further study (Burney, 2013).
CLASSIFICATION
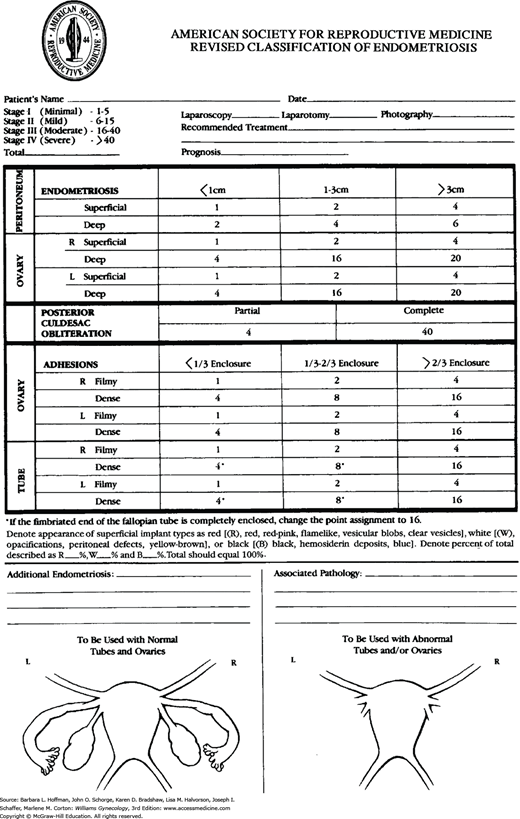
The primary method of endometriosis diagnosis is visualization of endometriotic lesions by laparoscopy, with or without biopsy for histologic confirmation. The extent of endometriosis can vary widely between individuals, and thus, one classification by the American Society for Reproductive Medicine (1997) allows disease to be quantified (Fig. 10-2). With this, endometriosis on the peritoneum, ovaries, fallopian tubes, and cul-de-sac is scored at surgery. At these sites, points are assigned for disease surface area, degree of invasion, morphology, and extent of associated adhesions. Also, endometriotic lesions are morphologically categorized as white, red, or black. In this system, endometriosis is classified as stage I (minimal), stage II (mild), stage III (moderate), and stage IV (severe).
Advantages of this system are its widespread implementation, its ease of use, and its four simple-to-comprehend stages. However, the system has limitations. It correlates poorly with infertility and pain symptoms (Guzick, 1997; Vercellini, 1996). For example, women with extensive disease (stage IV) may note few complaints, whereas those with minimal disease (stage I) may have significant pain or subfertility or both. This poor predictive ability stems in part from scores that are derived from subjective visual examination. Moreover, disease involving ureter, bowel, or other extrapelvic sites is not scored (Adamson, 2013). To address these shortcomings, other systems have been developed but are yet to be widely used. These include the ENZIAN staging system to better represent DIE and the Endometrial Fertility Index (Adamson, 2010; Haas, 2011).
SYMPTOMS
As noted, women with endometriosis may be asymptomatic, but chronic pelvic pain (CPP) or subfertility is common (Ballard, 2008). Of endometriosis-associated CPP, dysmenorrhea, dyspareunia, and noncyclic pain are frequent types. Less often and described on page 234, affected women may also complain of dyschezia (pain with defecation), dysuria, or abdominal wall pain.
At the molecular level, the underlying cause of pain is unclear, but proinflammatory cytokines and prostaglandins released by endometriotic implants may be one source (Bulun, 2009). Other investigations implicate nerve growth into endometriotic implants (Barcena de Arellano, 2011; McKinnon, 2012). Once established, continued exposure of these sensory nerves to the inflammatory environment within the implants can lead to central sensitization and CPP, as described in Chapter 11 (As-Sanie, 2013; Bajaj, 2003). The variability of implant location and these chemical influences help explain the differing pain manifestations experienced by women with endometriosis. That said, typical pain scoring tools such as the visual analogue scale and the numerical rating scale are suitable for initial assessment and for evaluation of treatment efficacy (Fig. 11-3) (Bourdel, 2015).
Of pain types, endometriosis-associated dysmenorrhea typically precedes menses by 24 to 48 hours. Compared with primary dysmenorrhea, this pain is thought to be more severe and is less responsive to NSAIDs and combination oral contraceptives (Allen, 2009; Opoku-Anane, 2012). Presence of DIE also positively correlates with dysmenorrhea severity (Lafay Pillet, 2014).
Endometriosis-associated dyspareunia is often related to rectovaginal septum, uterosacral ligament, or posterior cul-de-sac disease, although other involved sites can cause painful intercourse (Vercellini, 2007, 2012). Tension on diseased uterosacral ligaments during intercourse may trigger this pain (Fauconnier, 2002). Although some women with endometriosis describe a history of dyspareunia since coitarche, endometriosis-associated dyspareunia is suspected if pain develops after years of pain-free intercourse (Ferrero, 2005).
Noncyclic chronic pelvic pain (CPP) is another frequent symptom of endometriosis. Approximately 33 percent of women with CPP are found to have endometriosis at the time of laparoscopy (Howard, 2003). This percentage is higher in adolescents with CPP (Janssen, 2013). Some studies correlate pain severity with advanced-stage disease, whereas other studies do not (Fedele, 1992; Hsu, 2011).
The focus of chronic pain may vary. If the rectovaginal septum or uterosacral ligaments are involved with disease, pain may radiate to the rectum or lower back. Alternatively, pain radiating down the leg and causing cyclic sciatica may reflect sciatic nerve involvement (Possover, 2011). That said, pain may correlate poorly with pelvic disease location (Hsu, 2011).
The incidence of endometriosis in women with subfertility is 20 to 30 percent (Waller, 1993). In addition, although wide variability is reported, patients with infertility appear to have a greater incidence of endometriosis than fertile controls (13 to 33 percent versus 4 to 8 percent) (D’Hooghe, 2003; Strathy, 1982). Furthermore, Matorras and colleagues (2001) noted an increased prevalence of more severe stages of endometriosis in women with infertility.
Adhesions are one intuitive explanation for endometriosis-related infertility. These may impair normal oocyte pick-up and transport by the fallopian tube. Beyond mechanical impairment, numerous subtle defects also appear to be involved. Such defects include perturbations in follicle development, ovulation, sperm function, embryo quality and development, and implantation (Macer, 2012; Stilley, 2012).
A link between infertility and milder forms of endometriosis is less well supported (D’Hooghe, 1996; Schenken, 1980). An association is suggested by the differing prevalence of endometriosis between infertile and fertile women. For example, Rodriguez-Escudero and associates (1988) reported that women with minimal endometriosis had a 12-month cumulative pregnancy rate of 47 percent, which is below that of normal fertile women. Furthermore, a prospective cohort study demonstrated that women with minimal or mild endometriosis had a fecundity similar to that of those with unexplained infertility.
In moderate to severe endometriosis (stage III to IV), tubal and ovarian architecture are often distorted. As a result, impaired fertility would be expected. Few studies report fecundity rates in women with severe endometriosis. One investigation comparing mild, moderate, and severe endometriosis revealed a monthly fecundity rate of 8.7 percent in those with mild disease, 3.2 percent with moderate disease, and no pregnancies with severe disease (Olive, 1985a). In another, women with severe endometriosis undergoing in vitro fertilization (IVF) had poorer implantation and pregnancy rates compared with those with mild disease (Harb, 2013).
Defecatory pain develops much less often than other types of CPP in affected patients. Complaints may be chronic or cyclic, and they can be associated with constipation, diarrhea, or cyclic hematochezia (Roman, 2013). Thus, gastrointestinal causes of CPP are also entertained during evaluation (Chap. 11). The origin of symptoms can be fixation of the rectum to adjacent anatomic structures or rectal wall inflammation.
Symptoms may also stem from DIE of the gastrointestinal tract, which complicates 5 to 12 percent of proven endometriosis cases. Bowel DIE predominantly involves rectosigmoid colon and much less so the small bowel, cecum, or appendix (Ruffo, 2014b). Lesions are usually confined to the subserosa and muscularis propria. Thus, colonoscopy offers poor diagnostic sensitivity (Milone, 2015). Rarely, more severe cases may involve the bowel wall transmurally and lead to intestinal obstruction or a clinical picture suggesting malignancy (Kaufman, 2011; Ruffo, 2014a).
For diagnosis, rectal DIE can be imaged by transvaginal sonography (TVS), and sensitivity approximates 80 percent. However, TVS techniques used to diagnose DIE have a learning curve, and these are predominantly performed at tertiary care centers (Tammaa, 2014). Magnetic resonance (MR) imaging can clarify anatomy and degree of invasion, especially preoperatively (Bazot, 2009; Wall, 2015). Laparoscopy typically provides the definitive diagnosis.
Without obstructing symptoms, women may be considered for conservative management with hormonal therapy. However, treatment is often surgical, and cases often warrant a surgeon skilled in bowel surgery. Variables such as anatomic site, DIE depth, lesion size, and number of foci influence surgery. Colorectal segment resection may be needed. Less invasive techniques that shave down the lesion without opening the rectum or that excise discrete nodules are also described (Alabiso, 2015).
Logically, endometriosis should be considered if urinary tract symptoms persist despite negative urine culture results. Symptoms, if present, are more common with bladder disease. These include dysuria, suprapubic pain, urinary frequency, urgency, and hematuria (Gabriel, 2011; Seracchioli, 2010). Costovertebral angle pain may reflect ureteral endometriosis with obstruction and hydronephrosis that can progress eventually to kidney function loss (Knabben, 2015).
In a large series by Antonelli and coworkers (2006), the prevalence of urinary tract DIE was 2.6 percent. In this series of 31 patients, 12 had bladder endometriosis, 15 had ureteral endometriosis, and four had both ureteral and bladder involvement. TVS has suitable accuracy for bladder DIE but is less sensitive for ureteral disease (Exacoustos, 2014a). In unclear cases, MR imaging can add additional anatomic information. In light of associated symptoms with urinary tract DIE, cystoscopy with biopsy can also help clarify the diagnosis.
Treatment is either medical or surgical. If elected, surgery for bladder invasion is typically partial cystectomy. Surgeries for ureteral involvement vary by disease severity and include: (1) freeing the tethered ureter by ureterolysis, (2) segmental resection and reanastomosis, or (3) ureter reimplantation into the bladder, that is, ureteroneocystotomy (Seracchioli, 2010).
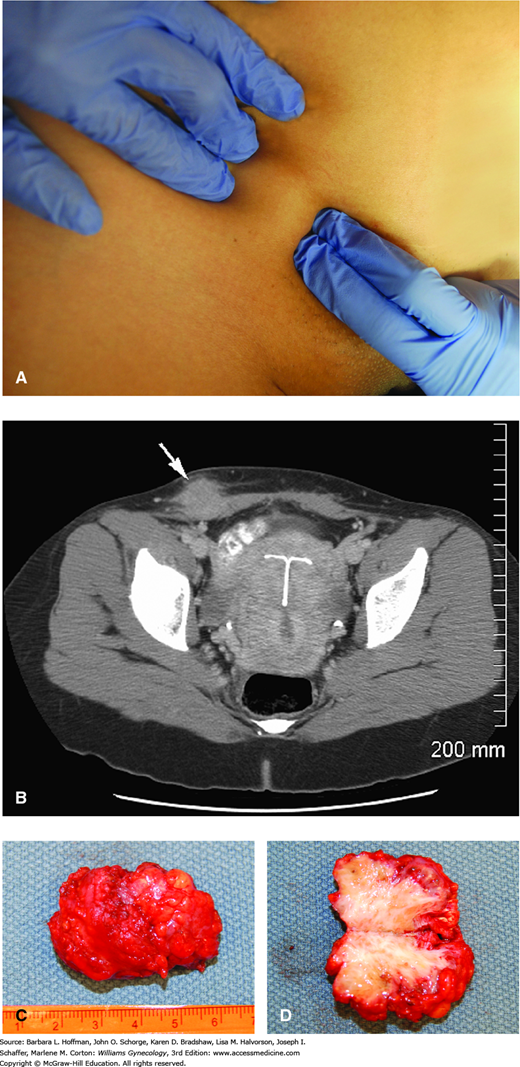
Some individuals with abdominal pain can have anterior abdominal wall endometriomas. Most of these lesions develop in the abdominal scar after uterine surgery or cesarean delivery, whereas others form unrelated to prior operations (Fig. 10-3) (Ding, 2013). Implants usually are found within the subcutaneous layer, are palpable, and may involve the adjacent fascia. Less often, the rectus abdominis muscle is infiltrated (Mostafa, 2013). Diagnostic tools are variably employed, and abdominal wall sonography, computed tomography (CT), MR imaging, and fine-needle aspiration are options. The decision to perform concurrent TVS is typically guided by whether CPP symptoms coexist.
FIGURE 10-3
Endometriosis
within a Pfannenstiel incision scar. A. Preoperative photograph delineates the borders of the mass. B. Computed tomography image shows a subcutaneous mass (arrow) extending down to the anterior abdominal wall fascia on the left. C. Excised mass. D. Bisected mass shows white fibrous scarring within yellow subcutaneous fat. Pathologic evaluation confirmed endometriosis. (Used with permission from Dr. Christi Capet.)
In most instances, implants are surgically excised for pain relief and diagnosis. For small implants, preoperative imaging may not be needed. But with larger implants and concerns for fascial or rectus abdominis muscle involvement, CT or MR imaging can aid surgery planning (Ecker, 2014). Large fascial defects following excision may require mesh to close the defect.
Thoracic endometriosis defines implants inside the thoracic cavity that lead to symptoms described as menstrual or synonymously called “catemenial.” These include cyclic chest or shoulder pain, hemoptysis, or pneumothorax, which predominantly occurs on the right (Haga, 2014; Rousset-Jablonski, 2011). Chest CT is preferred imaging (Rousset, 2014). For pneumothorax, minimally invasive thorascopic surgery is usually indicated. This is often coupled with several months of postoperative gonadotropin-releasing hormone (GnRH) or of progestin therapy identical to that for pelvic endometriosis treatment (Alifano, 2010). Hemoptysis, depending on findings, may be treated hormonally or surgically.
DIAGNOSTIC EVALUATION
For the most part, endometriosis is a disease confined to the pelvis. Accordingly, visual cues are often lacking. Some exceptions include endometriosis within an episiotomy scar or surgical scar, most often within a Pfannenstiel incision (Koger, 1993; Zhu, 2002). Rarely, endometriosis may develop spontaneously within the perineum or perianal region (Watanabe, 2003). Occasionally, blue or red powder-burn lesions are seen on the cervix or the posterior vaginal fornix. These lesions can be tender or bleed with contact. One study found that speculum examination displayed endometriosis in 14 percent of patients diagnosed with DIE (Chapron, 2002).
During bimanual examination, pelvic organ palpation often reveals suggestive anatomic abnormalities. Uterosacral ligament nodularity and tenderness may reflect active disease or scarring along the ligament. An enlarged, cystic adnexal mass may represent an ovarian endometrioma, which can be mobile or adhered to other pelvic structures. A retroverted, fixed, tender uterus and a firm, fixed posterior cul-de-sac are among other findings. Pelvic nodularities secondary to endometriosis may be more easily detected by bimanual examination during menses (Koninckx, 1996). However, examination is generally inaccurate in assessing the extent of endometriosis, especially if the lesions are extragenital. Last, rectal examination may reveal rectovaginal septum nodularity or tenderness.
Laboratory investigations are often undertaken to exclude other causes of pelvic pain that are listed in Table 11-1. Initially, a complete blood count (CBC), human chorionic gonadotropin assay, urinalysis and urine cultures, vaginal cultures, and cervical swabs may be collected to exclude infections or pregnancy complications. If urinary tract endometriosis is suspected, then renal function can also be assessed by creatinine levels.
Numerous serum markers have been studied as possible diagnostic tools. Cancer antigen 125 (CA125) is a glycoprotein that is found in fallopian tube epithelium, endometrium, endocervix, pleura, and peritoneum. As discussed in Chapter 35, this marker is used in ovarian cancer evaluation and surveillance. Recognized by monoclonal antibody assays, elevated CA125 levels positively correlate with endometriosis severity (Hornstein, 1995a). Unfortunately, the assay has poor sensitivity in detecting mild endometriosis and appears to be a better diagnostic test for stage III or IV endometriosis (Mol, 1998; Santulli, 2015). Although the role of this assay in clinical practice is uncertain, it may be useful in the presence of a sonographically detected ovarian cyst suggestive of an endometrioma.
As for other serum markers, May and colleagues (2010) completed a systematic review of more than 100 putative biomarkers. They were unable to identify a single biomarker or biomarker panel that they felt was clinically useful.
Many women with endometriosis present with CPP, and TVS is an initial imaging tool. It is accurate in detecting endometriomas and aids exclusion of other causes of pelvic pain. However, imaging of superficial endometriosis or endometriotic adhesions is inadequate. Small endometriotic plaques or nodules may occasionally be seen, but these findings are inconsistent (Wall, 2015).
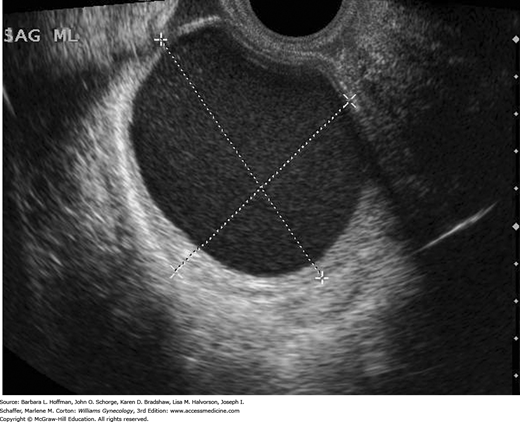
Endometriomas can be diagnosed by TVS with adequate sensitivity in most settings if they are 20 mm in diameter or greater. Sensitivity and specificity of TVS to diagnose endometriomas range from 64 to 90 percent and from 22 to 100 percent, respectively (Moore, 2002). An endometrioma classically is cystic with homogeneous, low-level internal echoes, often described as “ground glass” echogenicity. There is normal surrounding ovarian tissue (Fig. 10-4). As such, these may have an identical appearance to hemorrhagic corpus luteum cysts. Although endometriomas are most often unilocular, one to four thin septations can be found (Van Holsbeke, 2010). Less typically, these cysts can display thick septations or walls. Also less often, echogenic wall foci that lack flow when color Doppler is applied can be seen and are typically depositions of blood or blood components (Bhatt, 2006). Color Doppler TVS often demonstrates pericystic, but not intracystic, flow. Although endometriomas can be found in postmenopausal women, they are less common and more often are multilocular compared with those in reproductive-aged women.
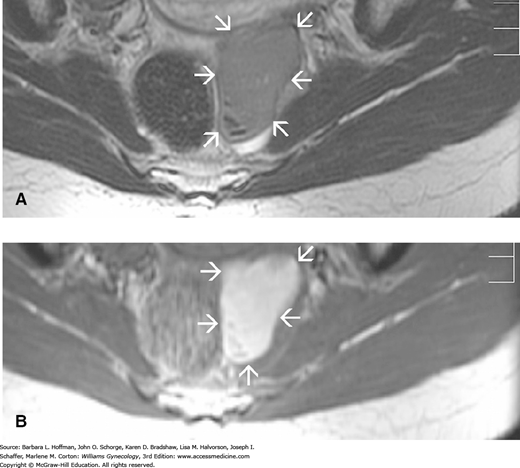
As noted previously, TVS for DIE involving the bowel and bladder has suitable accuracy (Exacoustos, 2014a; Hudelist, 2011). That said, for the diagnosis of rectal endometriosis, TVS is highly operator dependent, and experience is often lacking (Dunselman, 2014). Thus, MR imaging may clarify anatomy for equivocal sonographic findings and offers superior resolution at soft tissue interfaces (Fig. 10-5). In some cases with DIE, MR imaging can assist preoperative planning.
CT scanning plays a limited role in the evaluation of endometriosis. This is because TVS images endometriomas well, and CT has poor sensitivity for small implants and plaques. That said, chest CT is preferred for thoracic endometriosis. CT is suitable for abdominal wall endometrioma evaluation. Also, in selected cases, CT may have a role to evaluate bowel or ureteral endometriosis (Exacoustos, 2014b).
Although imaging can add clinical information, laparoscopy is the primary method used for diagnosing endometriosis (American College of Obstetricians and Gynecologists, 2014b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree