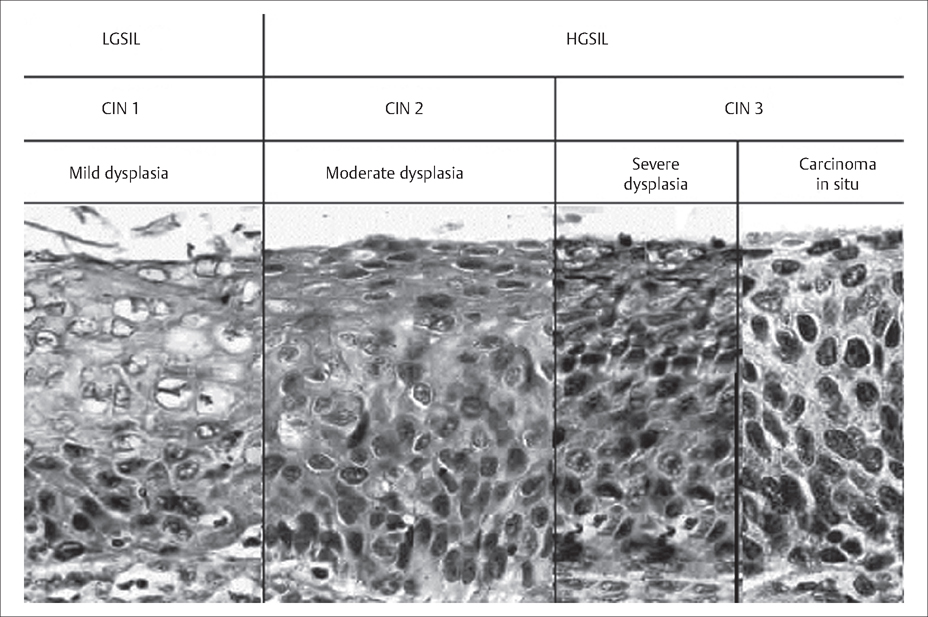
48 Cervical Neoplasia and Cancer Robert L. Barbieri Cervical neoplasia represents a disease continuum from low-grade cervical neoplastic lesions to invasive and metastatic cancer (Fig. 48.1). Most cases of cervical neoplasia are caused by infection with human papilloma virus (HPV). For women infected with oncogenic HPV subtypes, it may take 5 to 10 years or more for the disease to progress from low-grade cervical neoplasia to invasive cancer. This relatively long disease progression interval makes cervical neoplasia an optimal target for population screening. Unique nomenclature is used to describe cervical neoplasia based on cytology assessment (Papanicolaou [Pap] smear test) and histological assessment (cervical tissue biopsy) (Table 48.1). Cervical cancer is staged using clinical examination (Table 48.2). Unlike ovarian and endometrial cancer, cervical cancer is not surgically staged. Fig. 48.1 Histology of cervical intra-epithelial lesions For nomenclature key, see Table 48.1. Modifed with permission from Holschnei der, CH. Cervical intraepithelial neoplasia: Definition, incidence and pathogenesis. In: UpToDate, Basow DS (Ed), UpToDate, Waltham, MA, 2009 (www.uptodate.com)
Cervical Neoplasia
Definition
| Cytology assessment—Pap smear | |
| Negative for intra-epithelial lesion or malignancy | |
| Atypical squamous cells (ASC) of undetermined significance (ASC-US) | |
| Low-grade squamous intra-epithelial lesion (LSIL) | |
| High-grade squamous intra-epithelial lesion (HSIL) | |
| Squamous cell carcinoma | |
| Histological assessment—cervical tissue biopsy | |
| Normal | |
| Cervical intra-epithelial neoplasia 1 (CIN 1) | Dysplastic cellular changes are confined to the basal third of the epithelium |
| Cervical intra-epithelial neoplasia 2 (CIN 2) | Dysplastic cellular changes are confined to the basal two-thirds of the epithelium |
| Cervical intra-epithelial neoplasia 3 (CIN 3) | Dysplastic cellular changes are greater than two-thirds of the epithelial thickness |
| Cancer | |
Prevalence and Epidemiology
HPV infection is endemic, and the majority of sexually active men and women will have had an HPV infection by the time they are 50 years of age. In a recent survey of almost 2000 females, the prevalence of HPV infection— as detected by a polymerase chain reaction technique performed on cellular material obtained from a vaginal swab—was about 27%. Most HPV infections are cleared within 18 months of infection. There are over 100 different subtypes of HPV, each with a unique biology and oncogenic potential, of which 40 subtypes are specific for the anogenital epithelium. HPV-6 and HPV-11 cause about 90% of condylomatous genital warts. These subtypes are unlikely to cause cervical cancer, because they do not integrate into the genome and disrupt cell turnover. HPV subtypes 6 and 11 may cause low-grade cervical lesions such as LSIL or CIN 1. HPV subtypes 16 and 18 cause about 75% of all cervical cancers. These two high-risk subtypes persist in cells and may integrate into the genome, increasing cell mitosis. HPV-16 and HPV-18 cause both low-grade and high-grade lesions. The other HPV subtypes that may cause cervical cancer are 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, and 82.
| FIGO stage | Characteristics |
| 0 | Carcinoma in situ |
| I | Cervical carcinoma confined to the cervix/uterus |
| IA Invasive carcinoma diagnosed by microscopy | |
| IA1 Stromal invasion less than 3 mm in depth and 7 mm in lateral spread | |
| IA2 Stromal invasion between 3 mm and 5 mm in depth | |
| IB Clinically visible lesion confined to the cervix | |
| IB1 Clinically visible lesion 4 cm or less in greatest dimension | |
| IB2 Clinically visible lesion greater than 4 cm in greatest dimension | |
| II | Cervical cancer invades beyond the cervix/uterus but not to pelvic side-wall or to the lower third of the vagina |
| IIA No parametrial invasion | |
| IIB Parametrial invasion | |
| III | Cervical cancer extends to the pelvic side-wall and/or involves the lower third of the vagina and/or causes hydronephrosis |
| IIIA Tumor involves lower third of vagina, no extension to pelvic side-wall | |
| IIIB Tumor extends to pelvic side-wall or causes hydronephrosis | |
| IV | Cervical cancer extends beyond the pelvis and/or involves the bladder or rectal epithelium |
| IVA Spread to adjacent organs such as the bladder and/or rectum | |
| IVB Distant metastases |
* International Federation of Gynecology and Obstetrics
Etiology
HPV infection of a cervical or vaginal cell causes cellular changes including nuclear enlargement, multinucleation, hyperchromasia, and perinuclear cytoplasmic halos that can be detected by cytological or histological assessment. The HPV infection may be reversed by the recruitment of macrophages and lymphocytes and the production of anti-HPV antibodies. Immunosuppression increases the risk of an HPV infection progressing to high-grade lesions. Alternatively, certain HPV subtypes may integrate into the genome causing high-grade lesions and eventually cervical cancer. HPV infection also causes many cases of vaginal, vulvar (see Chapter 47), anal, and penile cancer. Integration of the HPV virus into the genome results in the overexpression of two viral genes, E6 and E7. HPV E6 increases the degradation of p53 and E7 inactivates the retinoblastoma protein (Rb), which are the products of two important tumor suppressor genes. Reduced expression of these two tumor suppressor genes results in increased mitotic activity of the infected cell with the potential for additional mutations leading to cancer.
Prevention
L1 and L2 are the encapsulating proteins for HPV. L1 molecules, which are specific for each HPV subtype, self-assemble into hollow viruslike particles with high immunogenicity. Two HPV vaccines are currently available: a bivalent vaccine for HPV subtypes 16 and 18; and a quadrivalent vaccine for HPV subtypes 6, 11, 16, and 18. The quadrivalent vaccine is parenterally administered in three doses at 0, 2, and 6 months. In one trial of the quadrivalent vaccine, 552 women with a mean age of 20 years were randomized to vaccination with the active vaccine or a placebo with 3 years of follow-up. Protection against cervical intra-epithelial neoplasia (CIN) caused by HPV subtypes 6, 11, 16, and 18 occurred in 100% of the women in the study. In the women treated with placebo injections, 7 cases of CIN infection caused by these four subtypes were detected. No genital warts due to HPV-6 or HPV-11 occurred in the treated group. In the women in the placebo group 4 cases of genital warts occurred. This trial stimulated the completion of very large-scale clinical trials, which have confirmed the protective effect of HPV vaccination (Evidence Box 48.1).
Data from multiple clinical trials indicate that HPV vaccination does not significantly alter the course of HPV infection in those women already infected prior to vaccination. However, if a woman is infected with one specific HPV subtype, for example HPV-11, at the time of vaccination, the vaccine is protective against the other subtypes, for example, 6, 16, and 18, to which the patient has not yet been infected. Consequently, HPV vaccination is most cost-effective when administered prior to exposure to the virus, which typically occurs after sexual debut. The current quadrivalent vaccine is recommended for females from 9 to 26 years of age. It is likely that studies will demonstrate that it is also effective for older women who have not yet been exposed to all four subtypes contained in the vaccine. Vaccination of males would reduce the prevalence of HPV infection, but in some economic models vaccination of males is less cost-effective than vaccination of females. Males in high-risk groups may be candidates for vaccination. In resource-limited settings, it may be very difficult to provide the vaccine to either females or males because of the expense.
Screening
The primary method of screening for cervical neoplasia caused by HPV infection is cervical cytology (Pap smear). Molecular testing for HPV nucleotides may be performed in conjunction with cervical cytology, or as a follow-up test for certain cervical cytology results. Screening for cervical neoplasia should begin about 3 years after the onset of sexual activity and no later than 21 years of age. From 21 to 30 years of age screening using cervical cytology should be performed every year. After 30 years of age, if recent screening has been normal, cervical cytology can be performed every 2–3 years.
Cervical—Vaginal Cytology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


