Cell Structure and Function
Antonio De Maio
Virginia L. Vega
Quira Zeidan
Division of Pediatric Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205.
Division of Pediatric Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205.
Division of Pediatric Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205.
CELLS HAVE SAME CAPACITY FOR BUILDING AND DESTROYING
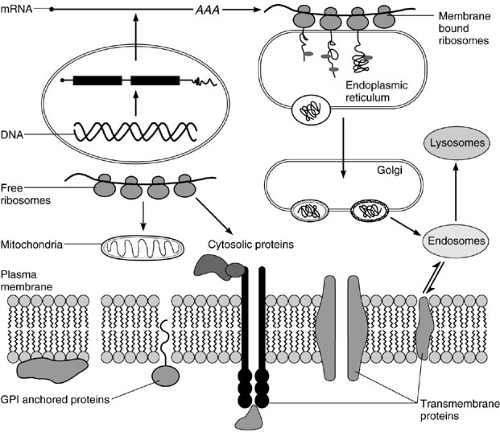
The cell contains the information necessary for its function and survival. This information is stored in the chromosomes as genetic material named genes. For each particular cellular function, a specific set of genes is expressed to carry out specific tasks (Fig. 1-1). The expression of these genes is modulated by the cellular state and the milieu in which the cell resides. The cellular environment is constantly modified by many factors, including the availability of nutrients, oxygen tension, the occurrence of harmful substances, and the presence of other cells. Thus, the particular physiological state of the cell is the response to a unique environment, resulting in a particular pattern of gene expression. When the cell moves from one physiological state to another, macromolecules that define the prior condition are degraded and replaced by a different set of molecules. When environmental conditions are not optimal for function or survival (e.g., high temperatures), damaged cellular components (e.g., proteins) are repaired or destroyed. In multicellular organisms, such as humans, cells do not exist in isolation, but rather in a complex environment modified by the presence of other cells. Organs are comprised of a combination of different cell types, each serving a different function. For example, the liver is composed of hepatocytes, endothelial cells, Kuppfer cells, and stella (ito) cells. Each cell type performs a different role and consequently expresses different genes. These different cell populations also need to communicate with each other to maintain proper tissue function. Even the same cell type within an organ could be metabolically different, thus increasing organ heterogeneity. Therefore, the pattern of gene expression of the same cell type within an organ could be different, qualitatively and/or quantitatively. A typical example is again the liver. Hepatocytes within the same hepatic acinus are metabolically different because the concentrations of nutrients and oxygen to which they are exposed are not identical. Thus, the concentrations of oxygen and glucose (Glc) in the periportal area are much higher than in the pericentral region. Cells in the periportal area are more glycolytic than in the pericentral zone.
Communication among cells within an organ is accomplished via extracellular factors (e.g., hormones) or direct cell–cell communication (e.g., gap junctions). Moreover, the physical integrity of an organ is generated by the interaction between cells (tight junctions) and the presence of an appropriate attachment surface (extracellular matrix). When organ homeostasis is altered, such as in a pathological condition, every cell within the tissue responds according to the new condition. The change in gene expression within a particular organ has an impact on the function of distant tissues. When the function of a particular organ is compromised, it is likely to disrupt the function of distant tissues, resulting in the subsequent collapse of the organism. With these ideas in mind, the study of a human condition, such as a disease, is only completely relevant in the context of a multiorgan system. The cell, however, is still the central player.
NUCLEUS: WHERE GENETIC INFORMATION IS STORED
Genetic information, stored in the form of genes, resides within the nucleus. This information contains the blueprint of the cell. Genes encode for the proteins necessary for cellular function, including metabolic activity, interaction with the environment, cell division, and response to harmful conditions. In addition, genomic material contains segments that give rise to different RNA molecules, which are necessary for the biosynthesis of cellular
macromolecules. RNA is an important component of the ribosomes, which are particles responsible for protein synthesis. The material inside the nucleus is separated from the cytosol, or the rest of the cell, by a membrane called the nuclear envelope. This membrane contains pores that allow the exchange of substances between nucleus and cytosol. The passage of material through these pores is actively monitored, contributing to the regulation of nuclear activity. The number of genes in the human genome is small; approximately 30,000 genes are distributed among several chromosomes. The size of individual genes, which is usually referred to in base pairs (bps), is variable as is the density of genes within a chromosome. The encoding region on the gene (exons) is usually disrupted by DNA sequences (introns), which do not form part of the final transcriptional product, the messenger RNA (mRNA). There are several discrete regions in a mature mRNA. The open reading frame is the region that gives rise to the polypeptide. This area is flanked by untranslated regions (UTRs), which play an important role in message stability and translation. The region upstream from the transcriptional initiation site is involved in gene transcriptional regulation. This region is known as the promoter and contains regulatory elements (groups of nucleic acids), which are binding sites for transcriptional factors and enhancers that participate in the selective transcription of the gene. There is a common sequence very close to the transcription initiation site, the TATA box (TATAAAA), which is recognized by RNA polymerase II, the enzyme responsible for gene transcription. Finally, genes within a chromosome are separated by large stretches of DNA. Because no function has been associated with these regions, scientists have called them “junk DNA.”
macromolecules. RNA is an important component of the ribosomes, which are particles responsible for protein synthesis. The material inside the nucleus is separated from the cytosol, or the rest of the cell, by a membrane called the nuclear envelope. This membrane contains pores that allow the exchange of substances between nucleus and cytosol. The passage of material through these pores is actively monitored, contributing to the regulation of nuclear activity. The number of genes in the human genome is small; approximately 30,000 genes are distributed among several chromosomes. The size of individual genes, which is usually referred to in base pairs (bps), is variable as is the density of genes within a chromosome. The encoding region on the gene (exons) is usually disrupted by DNA sequences (introns), which do not form part of the final transcriptional product, the messenger RNA (mRNA). There are several discrete regions in a mature mRNA. The open reading frame is the region that gives rise to the polypeptide. This area is flanked by untranslated regions (UTRs), which play an important role in message stability and translation. The region upstream from the transcriptional initiation site is involved in gene transcriptional regulation. This region is known as the promoter and contains regulatory elements (groups of nucleic acids), which are binding sites for transcriptional factors and enhancers that participate in the selective transcription of the gene. There is a common sequence very close to the transcription initiation site, the TATA box (TATAAAA), which is recognized by RNA polymerase II, the enzyme responsible for gene transcription. Finally, genes within a chromosome are separated by large stretches of DNA. Because no function has been associated with these regions, scientists have called them “junk DNA.”
The transcription of a particular gene is regulated by cellular proteins that activate and/or modulate the rate of RNA synthesis. These proteins are known as transcriptional factors, and they recognize specific sequences within the promoter region. There are basic transcriptional factors that cooperate with RNA polymerase II to transcribe a gene. Some of these factors contain activities necessary to unwind (helicase) a short stretch of the DNA to gain access for the transcriptional system. The second set of transcriptional factors gives specificity to the process of RNA synthesis, such as tissue specificity. Other factors are responsible for activating the transcription of genes necessary for the cellular response to an external stimulus. For example, heat shock factor 1 is activated to drive the expression of heat shock genes in conditions of physiological stress. These genes encode proteins that are necessary to preserve cell function during harmful conditions. Many transcriptional factors are activated by phosphorylation, which results in a conformational change of the protein. More recently, acetylglucosamine moieties, which are attached to serine, threonine, or tyrosine via the hydroxyl
group of these amino acids (O-linked), have been found to activate transcriptional factors. In other circumstances, transcriptional factors are associated with an inhibitory protein arresting their potential transcriptional activity. This is the case of NF-κB, which drives the expression of many genes, particularly those involved in the inflammatory process. NF-κB is normally present in the cytosol associated with Iκ-Bα, which is an inhibitory protein. When the cell is activated by an external stimulus, such as an inflammatory mediator, Iκ-Bα is phosphorylated and dissociates from NF-κB. Then, NF-κB is free to translocate into the nucleus and activate transcription. To maintain the activated stage of the cell, Iκ-Bα is degraded by the proteosome system. Consequently, the attenuation of NF-κB activation can only be accomplished by new synthesis of Iκ-Bα. Transcription of the gene encoding for Iκ-Bα is stimulated by NF-κB. Thus, this system has a built-in mechanism to regulate its own expression, like many other cellular systems.
group of these amino acids (O-linked), have been found to activate transcriptional factors. In other circumstances, transcriptional factors are associated with an inhibitory protein arresting their potential transcriptional activity. This is the case of NF-κB, which drives the expression of many genes, particularly those involved in the inflammatory process. NF-κB is normally present in the cytosol associated with Iκ-Bα, which is an inhibitory protein. When the cell is activated by an external stimulus, such as an inflammatory mediator, Iκ-Bα is phosphorylated and dissociates from NF-κB. Then, NF-κB is free to translocate into the nucleus and activate transcription. To maintain the activated stage of the cell, Iκ-Bα is degraded by the proteosome system. Consequently, the attenuation of NF-κB activation can only be accomplished by new synthesis of Iκ-Bα. Transcription of the gene encoding for Iκ-Bα is stimulated by NF-κB. Thus, this system has a built-in mechanism to regulate its own expression, like many other cellular systems.
Transcription is composed of three steps: initiation, elongation, and termination. Each step is regulated by a particular set of factors. During elongation, RNA polymerase does not proceed at a constant rate, but rather in bursts between pauses. Reversible pausing is the rate-limiting step of transcription, and it depends on the gene being transcribed. During pausing, RNA polymerase can move forward to add more nucleotides to the nascent transcript, or move in reverse excluding the nascent transcript from the site of transcription, which can be removed by a ribonuclease activity present at the active site of the polymerase. This process is important to correct possible errors that occur during transcription. Transcription of genes is carried out by RNA polymerase II. Ribosomal RNA (rRNA) is synthesized by RNA polymerase I, while transfer RNA (tRNA), SSrRNA, and 7S RNA (part of the signal recognition particle) are synthesized by RNA polymerase III. The first product of gene transcription, heterogeneous nuclear RNA (hnRNA), contains both exons and introns; the latter are removed by a process known as splicing. This process occurs within the nucleus by a large RNA-protein complex named spliceosome. For some genes, splicing results in the production of several mRNAs, which encode related, but different, polypeptides (alternative splicing). Therefore, a single gene could encode different polypeptides with different specificities. The configuration of alternative splicing for a particular gene could be identical or different between various cell types. Thus, alternative splicing could contribute to the divergent pattern of gene expression observed in different tissues. About 50% of the total rate of transcription is due to synthesis of mRNA precursors by RNA polymerase II, which could be blocked by the addition of α-amantin, a fungal toxin. Total transcriptional activity could be inhibited by administration of actinomycin D. Although mRNA precursors comprise half of the normal transcriptional rate, the cellular abundance of RNAs is different. Thus, the composition of total cellular RNA is divided into rRNA (approx. 75%); tRNA, small RNA, which is involved in splicing, and 5S rRNA (approx. 15%); and hnRNA and mRNA (less than 10%). The discrepancy between transcription rate and abundance of different RNAs is due to their particular stability within the cell. There are about 500,000 copies of different mRNAs per cell at any particular time. These mRNAs correspond to approximately 20,000 different genes, which are simultaneously expressed within a cell at any given state. The majority of these mRNAs are constantly engaged in translation. Pre-mRNA is further processed by a chemical modification at the 5′ end of the transcript, resulting in the addition of a 7-methyl guanosine group, which is known as capping. This modification occurs cotranscriptionally, and it is important in mRNA transport, stability, and translation. Furthermore, a stretch of 50 to 300 adenosine nucleotides is added to the 3′ end of the transcript, which is known as the poly A tail. The poly A tail also plays a role in mRNA transport, stability, and translation. The same mRNA could present poly A tails of different sizes within the same cell. This difference in poly A length seems to be an indicator of the mRNA age. Thus, older mRNA has apparently shorter poly A tails than newly transcribed ones. The presence of the poly A tail within mRNAs has been exploited to isolate mRNA pools from the total cellular RNA population by affinity chromatography on immobilized oligo dT. Moreover, oligo dT is commonly used in the synthesis of complementary DNA (cDNA). Thus, it is important to differentiate an mRNA from an RNA precursor or transcript. An mRNA is the fully processed transcript, including capping and the poly A tail. When the mRNA is fully processed, it is transported to the cytosol with the help of several proteins via pores on the nuclear envelope.
STABILITY OF MRNAS ALSO REGULATES GENE EXPRESSION
The rate of polypeptide biosynthesis is dependent on the concentration of the particular mRNA within the cytosol. The cytosolic concentration of a particular mRNA is determined by the relationship between the rate of mRNA synthesis (transcription and processing), transport outside the nucleus, and degradation. A cell is constantly synthesizing and degrading mRNA, which represents a steady-state level. This continuous production and destruction of molecules appears as a waste of resources. However, it plays a critical role in gene expression regulation within the cell, which is the product of a constant adaptation to a new physiological condition. Cells are neither isolated nor in a still environment. The surroundings of cells are continuously changing due to the arrival of new chemicals that can be used as fuel, changes in pH or oxygen tension, or the interaction with neighboring cells. For each change, cells respond by activating a particular metabolic pathway or mounting a mechanism of defense, etc. These changes
require a reprioritization of gene expression. How does this reprioritization occur? The cell needs to synthesize new molecules (e.g., necessary enzymes), which require transcription and translation. Subsequently, preexisting molecules that are no longer necessary must be discarded. In addition, the synthesis of these unnecessary molecules should be stopped by discontinuing transcription and/or degrading preexisting mRNAs. Every mRNA has a particular half-life in normal conditions and contains the information necessary for its own persistence or degradation. The kinetics of this process may be modified by the environment, resulting in the stabilization or accelerated degradation of the respective mRNA. In addition, mRNAs are apparently degraded after a fixed number of translation cycles. Messages are degraded by RNAses present within the cell, which are always ready to act. The presence of regulatory proteins associated with the mRNA molecule (mRNA binding proteins) is responsible for protecting or destabilizing the message. Moreover, mRNAs and other RNA molecules are single-stranded nucleic acids that can form hydrogen bonds within two regions of the same molecules creating a secondary structure. Thus, hairpin and clove structures are frequently observed in RNA molecules. These secondary structures also play a major role in mRNA stability and translation. There are different mechanisms for mRNA degradation, which may be related to the nature of a particular message. The first step in the degradation of many mRNAs is the loss of the 5′ cap structure. This step is followed by the removal of the poly A tail, which occurs in a stepwise process. The majority of mRNAs are degraded by an exo RNAse in a 3′ to 5′ direction. For other mRNAs, degradation occurs in a 5′ to 3′ direction without poly A tail removal. It is also possible that mRNAs are degraded by the action of endo RNAses. In addition, systems to avoid the production of abnormal proteins are present. For example, if a message contains a nonsense (stop) codon, the message is degraded by the nonsense-mediated decay system. The mechanism of this process is not known, but there is increasing evidence that it is related to the presence of a nuclear protein that accompanies the mRNA and may occur within the nucleus. Another system is RNA interference (RNAi), in which the appearance of a double-stranded RNA is cleaved into 21 nucleotide fragments triggering the degradation of the complementary mRNA. RNAi is currently being used by many investigators to silence the expression of a particular gene after artificially introducing a duplex RNA molecule within the cell.
require a reprioritization of gene expression. How does this reprioritization occur? The cell needs to synthesize new molecules (e.g., necessary enzymes), which require transcription and translation. Subsequently, preexisting molecules that are no longer necessary must be discarded. In addition, the synthesis of these unnecessary molecules should be stopped by discontinuing transcription and/or degrading preexisting mRNAs. Every mRNA has a particular half-life in normal conditions and contains the information necessary for its own persistence or degradation. The kinetics of this process may be modified by the environment, resulting in the stabilization or accelerated degradation of the respective mRNA. In addition, mRNAs are apparently degraded after a fixed number of translation cycles. Messages are degraded by RNAses present within the cell, which are always ready to act. The presence of regulatory proteins associated with the mRNA molecule (mRNA binding proteins) is responsible for protecting or destabilizing the message. Moreover, mRNAs and other RNA molecules are single-stranded nucleic acids that can form hydrogen bonds within two regions of the same molecules creating a secondary structure. Thus, hairpin and clove structures are frequently observed in RNA molecules. These secondary structures also play a major role in mRNA stability and translation. There are different mechanisms for mRNA degradation, which may be related to the nature of a particular message. The first step in the degradation of many mRNAs is the loss of the 5′ cap structure. This step is followed by the removal of the poly A tail, which occurs in a stepwise process. The majority of mRNAs are degraded by an exo RNAse in a 3′ to 5′ direction. For other mRNAs, degradation occurs in a 5′ to 3′ direction without poly A tail removal. It is also possible that mRNAs are degraded by the action of endo RNAses. In addition, systems to avoid the production of abnormal proteins are present. For example, if a message contains a nonsense (stop) codon, the message is degraded by the nonsense-mediated decay system. The mechanism of this process is not known, but there is increasing evidence that it is related to the presence of a nuclear protein that accompanies the mRNA and may occur within the nucleus. Another system is RNA interference (RNAi), in which the appearance of a double-stranded RNA is cleaved into 21 nucleotide fragments triggering the degradation of the complementary mRNA. RNAi is currently being used by many investigators to silence the expression of a particular gene after artificially introducing a duplex RNA molecule within the cell.
CYTOSOL: MAJOR SITE OF PROTEIN SYNTHESIS
The cytosol is the major center of metabolic activity within the cell. This compartment is also the major site of protein biosynthesis. The cytosol is an aqueous environment bordered by lipid membrane structures. Although water is the major component of the cytosol, the density of macromolecules within this compartment is very large. The concentration of macromolecules within the cytosol has been calculated on the order of 60%. In spite of this high concentration, the diffusion of substances within the cytosol is optimal. This high density of macromolecules within the cytosol results in a potential problem for the proper folding of newly synthesized polypeptides. Therefore, the presence of molecular chaperones is required to assist the folding process. Although the synthesis of most cellular proteins occurs in the cytosol, a few proteins are synthesized within the mitochondria. The center of protein biosynthesis is the ribosome. Ribosomes are made up of two subunits (40S and 60S in eukaryotes). Each subunit is composed of proteins and RNAs. The major RNA component is named 18S and 28S rRNA, with sizes of 1.9 and 4.6 kb, respectively. There are 27 different polypeptides in the 60S and 20 in the 40S subunits. Their organization results in a very compact structure, which is necessary for proper function. Ribosomal subunits are synthesized independently in the nucleolus in equal molar quantities, approximately 2 × 107 subunits per cell. The 40S subunit is involved in the recognition of the mRNA to be translated, which is activated upon binding to methionine-charged tRNA. This subunit interacts with the cap structure and scans the message for the detection of the translation initiation site, which corresponds to an AUG triplet of nucleotides, usually in the context of ACCAUGG. This triplet (AUG) encodes the amino acid methionine, which is therefore the first amino acid in all eukaryotic proteins. When the AUG is found, the 60S subunit is recruited forming a functional ribosome, the 80S particle, or monosome, and translation is initiated. Amino acids are covalently bound to the nascent polypeptide by a peptide bond (elongation). The nascent polypeptide is pushed out of the ribosome via a channel in the 60S subunit. When a stop codon is found (UAA, UAG, UGA), translation is terminated and the nascent polypeptide is released. This process is governed by a series of proteins (translation initiation, elongation, and release factors) that work in an orderly manner to obtain the final product, the protein. The absence of any translational factor is sufficient to stop the whole process. To increase the efficiency of the translation, several ribosomes are sequentially added to the same mRNA. Thus, several copies of the protein can be made simultaneously from a single mRNA template. When a single ribosome is bound to a message, the complex is named a monosome. Subsequent addition of ribosomes results in disomes, trisomes, etc., and polysomes when it is impossible to count the number of ribosome particles within the mRNA. The different association of ribosomes with the mRNA, as well as the two respective subunits, can be separated by sucrose gradient centrifugation providing
a particular pattern, which is known as polysome profile (Fig. 1-2). The biosynthesis of proteins could be visualized by pulse chase experiments in which cells are fed with radiolabeled amino acids. The synthesis of a particular protein is detected by a technique known as immunoprecipitation, using antibodies specific for the protein of interest. Proteins that are part of the cytosol, nucleus, mitochondria, etc., are synthesized in soluble ribosomes. The released polypeptides are free to diffuse within the cytosol to reach their final destination. There are some proteins that need to be secreted outside the cell or that are components of cellular membranes (membrane proteins). They are synthesized in membrane-bound ribosomes within the endoplasmic reticulum (ER).
a particular pattern, which is known as polysome profile (Fig. 1-2). The biosynthesis of proteins could be visualized by pulse chase experiments in which cells are fed with radiolabeled amino acids. The synthesis of a particular protein is detected by a technique known as immunoprecipitation, using antibodies specific for the protein of interest. Proteins that are part of the cytosol, nucleus, mitochondria, etc., are synthesized in soluble ribosomes. The released polypeptides are free to diffuse within the cytosol to reach their final destination. There are some proteins that need to be secreted outside the cell or that are components of cellular membranes (membrane proteins). They are synthesized in membrane-bound ribosomes within the endoplasmic reticulum (ER).
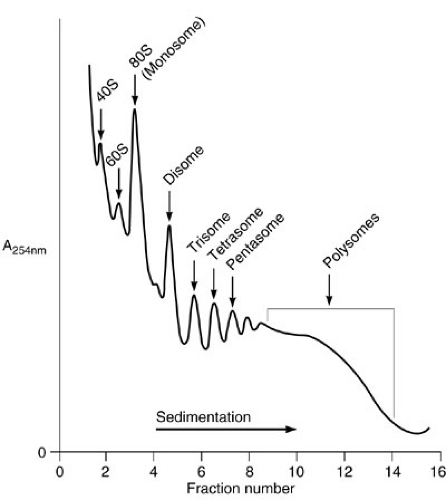 FIGURE 1-2. Polysome profile after density centrifugation.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|