Background
Cell-free deoxyribonucleic acid (DNA) is increasingly being used to screen for fetal aneuploidy. The majority of fetal cell-free DNA in the maternal blood results from release from the syncytiotrophoblast as a result of cellular apoptosis and necrosis. Elevated levels of fetal cell-free DNA may be indicative of underlying placental dysfunction, which has been associated with preterm birth. Preliminary studies have demonstrated that fetal cell-free DNA is increased in pregnancies complicated by spontaneous preterm birth. There are limited data on the association between fetal cell-free DNA levels and fetal fraction and preterm birth in asymptomatic women in the first and second trimesters. Preliminary studies have failed to find an association between first-trimester cell-free DNA levels and preterm birth, whereas there is conflicting evidence as to whether elevated second-trimester cell-free DNA is associated with a subsequent spontaneous preterm birth clinical event.
Objective
The objective of the study was to evaluate the association between first- and second-trimester cell-free DNA fetal fraction and preterm birth.
Study Design
This was a retrospective cohort study of women with singleton pregnancies at increased risk for aneuploidy who had cell-free DNA testing at 10–20 weeks’ gestation between October 2011 and May 2014. The cohort was subdivided by gestational age at the time of cell-free DNA testing (10–14 weeks or 14.1–20 weeks). The primary outcome was preterm birth less than 37 weeks’ gestation, and the secondary outcomes were preterm birth at less than 34 weeks’ gestation and spontaneous preterm birth at less than 37 and 34 weeks’ gestation.
Results
Among 1349 pregnancies meeting inclusion criteria 119 (8.8 %) had a preterm birth prior to 37 weeks with 49 cases (3.6 %) delivering prior to 34 weeks. Whereas there was no significant association between fetal fraction and the preterm birth outcomes for those who underwent cell-free DNA testing at 10–14 weeks’ gestation, there were significant associations among those screened at 14.1–20.0 weeks’ gestation. Fetal fraction greater than or equal to the 95th percentile at 14.1–20.0 weeks’ gestation was associated with an increased risk for preterm birth less than 37 and 34 weeks’ gestation (adjusted odds ratio, 4.59; 95% confidence interval, 1.39–15.2; adjusted odds ratio, 22.0; 95% confidence interval, 5.02–96.9).
Conclusion
Elevated fetal fraction levels at 14.1–20.0 weeks’ gestation were significantly associated with an increased incidence of preterm birth. Our findings warrant future exploration including validation in a larger, general population and investigation of the potential mechanisms that may be responsible for the initiation of preterm labor associated with increased fetal cell-free DNA.
Cell-free deoxyribonucleic acid (cfDNA) is increasingly being used to screen for fetal aneuploidy. Fetal cfDNA can be detected in maternal blood as early as 4 weeks’ gestation. The fetal fraction, which is the percentage of cfDNA of fetal origin, exceeds 4% of all of the cfDNA in the majority of pregnant women, beginning at 10 weeks’ gestation and continues to increase with advancing gestational age. Fetal cfDNA is primarily placental in origin. It is likely released from the syncytiotrophoblast layer of the placenta as a result of cellular apoptosis and necrosis. Circulating fetal cfDNA can potentially be utilized as a marker to provide information regarding placental health and disease.
There is preliminary evidence that fetal cfDNA is increased in pregnancies complicated by spontaneous preterm birth, putatively caused by the breakdown of the placental barrier in anticipation of labor. There are limited data on the association between fetal cfDNA levels and fetal fraction and preterm birth in asymptomatic women in the first and second trimesters.
Preliminary studies have failed to find an association between first-trimester cfDNA and the fetal cfDNA levels and preterm birth, whereas there is conflicting evidence as to whether elevated second-trimester cfDNA is associated with a subsequent spontaneous preterm birth clinical event.
The goal of the present study was to further explore the association between cfDNA fetal fraction and preterm birth in the first and second trimesters in our large data set.
Materials and Methods
This is a retrospective cohort study of women with singleton pregnancies who had cfDNA testing at the University of Pennsylvania at 10–20 weeks’ gestation between October 2011 and May 2014. The Institutional Review Board of the University of Pennsylvania approved the study.
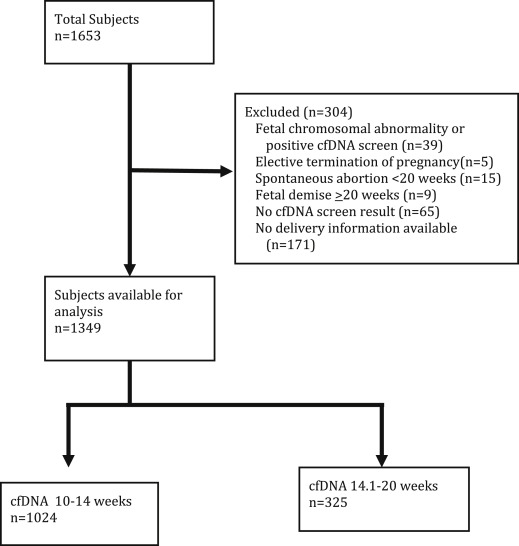
The primary outcome was preterm birth at less than 37 weeks’ gestation and the secondary outcomes were preterm birth at less than 34 weeks’ gestation and spontaneous preterm birth at less than 37 and 34 weeks’ gestation. Patients with singleton pregnancies that delivered as of Oct. 1, 2014, were included in the analysis. Women carrying a fetus with a chromosomal abnormality or with a screen-positive cfDNA result, those with an elective termination of pregnancy, a spontaneous loss less than 20 weeks, or a fetal demise at greater than or equal to 20 weeks, no cfDNA test result available, and those for whom pregnancy outcome data were unavailable were excluded. All included patients delivered at our institution.
The demographic and pregnancy outcome data were obtained from our Reproductive Genetics and Obstetrics databases and medical record review. The medical records for all preterm birth deliveries were reviewed by a maternal-fetal medicine specialist (L.D.), who was blinded to the fetal fraction data to confirm the diagnosis of preterm birth and spontaneous preterm birth. Spontaneous preterm birth included those with spontaneous onset of labor and those with preterm premature rupture of membranes. It is the protocol at our institution to induce labor in the setting of preterm premature rupture of membranes at 34 weeks’ gestation unless there is a maternal or fetal indication for earlier delivery.
During the study time period, it was the practice policy at our institution to offer cfDNA screening to women at increased risk for fetal aneuploidy including women aged 35 years or older, women with a positive aneuploidy screening test (most commonly a sequential screen or quad screen), fetuses with sonographic findings associated with aneuploidy, women with a history of a child affected with a trisomy, or a parent with a balanced Robertsonian translocation with increased risk of trisomy 13 or trisomy 21.
Diagnostic testing with chromosomal microarray analysis was recommended in cases with fetal structural anomalies. The cfDNA screening was offered to patients who declined diagnostic testing. Maternal blood samples were obtained for cfDNA testing for fetal aneuploidy. All of the samples were sent to the same laboratory (Sequenom, Inc, San Diego, CA). For samples drawn prior to Oct. 24, 2013 (n = 1047), fetal fraction was determined using methylation techniques as previously described.
Fetal fraction estimation for samples drawn beginning on Oct. 24, 2013 (n = 302), was determined using a multivariable model derived by machine learning approaches using regional read depth counts from autosomes generated by whole-genome low coverage massively parallel single-end sequencing. Automatic data normalization procedures ensured that sequencing fetal fraction estimates showed an identical distribution and no significant bias compared with methylation fetal fraction estimates.
Fetal fraction multiples of the median (MoM) were determined by using the median for each gestational age week based on the technology used to determine fetal fraction. The fetal fraction data were provided by the laboratory for the purpose of this study because this information is not routinely included in the laboratory reports.
Statistical analysis
The cohort was subdivided by gestational age based on the trimester, first (10–14 weeks) vs second (14.1–20 weeks), at the time of cfDNA testing. We used statistical software (SPSS Inc, Chicago, IL) to perform descriptive analyses and logistic regression. Continuous data were found to be nonparametric; therefore, Mann Whitney U tests and χ 2 analyses (for categorical data) were performed to determine the association between maternal demographic variables and pregnancy outcomes.
A multivariable logistic regression was performed to determine the association between fetal fraction and pregnancy outcomes of interest, controlling for potential confounders. Collinearity (to identify highly related variables) and correlations of continuous fetal fraction (MoM) with demographics were assessed using χ 2 analyses, Spearman, or Pearson’s correlation as appropriate. Test characteristics (sensitivity, specificity, and area under the receiver-operator curve) of fetal fraction to predict each preterm birth outcome were performed.
Results
One thousand six hundred fifty-three women with singleton pregnancies who had cfDNA testing at our institution between October 2011 and May 2014 delivered as of Oct. 1, 2014. One thousand three hundred forty-nine cases were included in the analysis. Details regarding the excluded cases are shown in Figure 1 . The characteristics of the study population are presented in Tables 1 and 2 .
| Characteristic | Gestational age at testing | ||
|---|---|---|---|
| 10–14.0 wks (n = 1024) | 14.1–20.0 wks (n = 325) | P value | |
| Maternal age, y | 36.9 (35.4, 38.9) | 34.8 (30.3, 37.3) | <.001 |
| Race | <.001 | ||
| African American | 142 (14.0) | 145 (44.84) | |
| Asian | 108 (10.6) | 24 (7.4) | |
| Caucasian | 709 (69.9) | 127 (39.2) | |
| Hispanic | 28 (2.8) | 13 (4.9) | |
| Other/missing | 37 (3.6) | 16 (4.8) | |
| BMI at enrollment | 24.2 (21.6, 27.7) | 25.7 (23.0, 32.5) | <.001 |
| Nulliparity | 419 (40.9) | 126 (38.8) | .517 |
| Indication for NIPT | <.001 | ||
| AMA | 945 (92.2) | 157 (48.3) | |
| Abnormal ultrasound | 5 (0.5) | 14 (4.3) | |
| Positive first-trimester, sequential or quad screen | 30 (2.9) | 132 (40.6) | |
| Other/missing | 44 (4.3) | 8 (2.5) | |
| History of PTB | 95 (9.3) | 41 (12.6) | .091 |
| Gestational age | 12.0 (11.1, 12.7) | 17.0 (15.7, 18.1) | n/a |
| Fetal fraction (MoM) | 1.00 (0.73, 1.37) | 1.01 (0.75, 1.36) | .249 |
| Characteristic | 10–14.0 wks | 14.1–20.0 wks | ||||
|---|---|---|---|---|---|---|
| PTB < 37 wks (n = 87) | No PTB (n = 937) | P value | PTB < 37 wks (n = 32) | No PTB (n = 293) | P value | |
| Maternal age, y | 37.8 (35.6, 40.4) | 36.9 (35.4, 38.9) | .031 | 36.0 (33.9, 39.2) | 34.7 (30.2, 37.1) | .037 |
| Race | .001 | .339 | ||||
| African American | 26 (29.9) | 116 (12.4) | 19 (59.4) | 126 (43.0) | ||
| Asian | 12 (13.8) | 96 (10.2) | 2 (6.3) | 22 (7.5 ) | ||
| White | 47 (54.0) | 662 (70.7) | 10 (31.3) | 117 (39.9) | ||
| Hispanic | 1 (1.1) | 27 (2.9) | 0 | 13 (4.4) | ||
| Other/missing | 1 (1.1) | 36 (3.8) | 1 (3.1) | 15 (5.1) | ||
| BMI at enrollment | 25.6 (22.0, 32.1) | 24.2 (21.6, 27.5) | .013 | 26.2 (23.0, 34.8) | 25.7 (23.0, 32.3) | .567 |
| Nulliparity | 37 (42.5) | 394 (40.5) | .745 | 8 (25.0) | 118 (40.3) | .092 |
| Indication for cfDNA | .048 | .091 | ||||
| AMA | 85 (97.7) | 860 (87.1) | 20 (62.5) | 137 (46.8) | ||
| Abnormal ultrasound | 0 | 5 (0.5) | 1 (3.1) | 13 (4.4) | ||
| Positive serum screen | 1 (1.1) | 29 (3.1) | 9 (28.1) | 123 (42.0) | ||
| Other/missing | 1 (1.1) | 43 (4.6) | 2 (6.3) | 20 (6.8 ) | ||
| History of PTB | 30 (34.5) | 65 (6.9) | <.001 | 12 (37.5) | 29 (9.9) | <.001 |
There were a total of 119 preterm births prior to 37 weeks (8.8%) including 49 cases delivering prior to 34 weeks (3.6%). Eighty-seven of the 119 subjects who experienced preterm birth prior to 37 weeks had cfDNA testing at 10–14.0 weeks and 32 had cfDNA testing at 14.1–20 weeks. Of the 49 subjects with preterm births at less then 34 weeks’ gestation, 34 had cfDNA testing performed between 10 and 14.0 weeks’ gestation and 15 had cfDNA testing between 14.1 and 20 weeks. Sixty-eight of the preterm births were spontaneous preterm births including 40 cases of preterm premature rupture of the membranes and 1 case of cervical insufficiency.
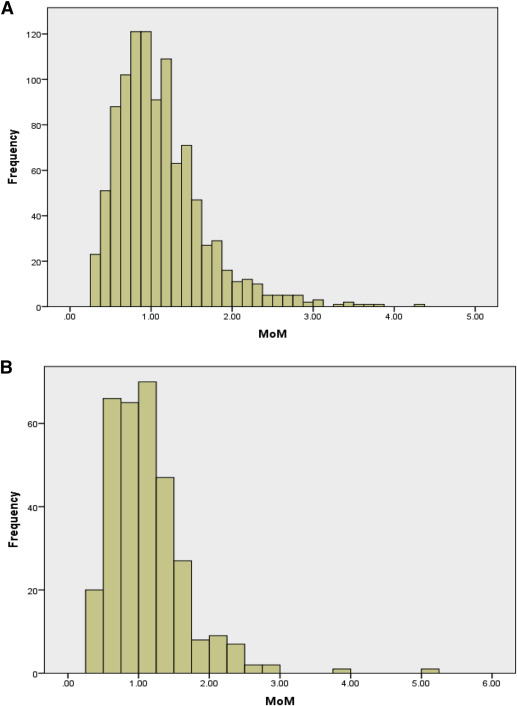
The fetal fraction distribution for the women with cfDNA testing performed between 10 and 14.0 and between 14.1 and 20.0 weeks’ gestation is shown in Figure 2 . Although collected as a continuous variable, the fetal fraction was also dichotomized into < 95th percentile and ≥ 95th percentile based on the precedent established by the existing literature. The 95th percentiles for fetal fraction for the 10–14.0 and the 14.1–20 week groups were 2.15 MoM and 2.18 MoM, respectively.
The adjusted odds of each preterm birth outcome by continuous fetal fraction and dichotomous fetal fraction (< 95th percentile and ≥ 95th percentile) are presented in Table 3 . Although there was no significant association between fetal fraction and the preterm birth outcomes for those who underwent cfDNA testing between 10 and 14 weeks’ gestation, there were significant associations among those screened at 14.1–20.0 weeks’ gestation.
| Outcome | aOR (95% CI) continuous fetal fraction (MoM) | P value | aOR (95% CI) dichotomous fetal fraction | P value |
|---|---|---|---|---|
| Fetal fraction drawn 10–14 wks | ||||
| PTB < 34 wks (n = 34) | 0.91 (0.42–1.95) a | .80 | 0.71 (0.09–5.62) a | .75 |
| sPTB < 34 wks (n = 24) | 0.93 (0.39–2.22) a | .87 | 0.93 (0.12–7.52) a | .95 |
| PTB < 37 wks (n = 87) | 0.97 (0.60–1.56) a | .88 | 1.53 (0.56–4.15) a | .40 |
| sPTB < 37 wks (n = 52) | 0.63 (0.32–1.23) a | .17 | 0.81 (0.19–3.56) a | .78 |
| Fetal fraction drawn 14.1–20 wks | ||||
| PTB < 34 wks (n = 15) | 6.69 (2.50–17.9) b | .001 | 22.0 (5.02–96.9) b | .001 |
| sPTB < 34 wks (n = 8) | 3.77 (1.21–11.8) b | .022 | 18.6 (2.88–119) b | .002 |
| PTB < 37 wks (n = 32) | 2.06 (1.07–3.96) b | .031 | 4.59 (1.39–15.2) b | .013 |
| sPTB < 37 wks (n = 16) | 1.95 (0.91–4.15) b | .086 | 4.43 (0.98–19.8) b | .052 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


