Cardioversion and Defibrillation
Richard J. Scarfone
Christine S. Cho
Introduction
Synchronized cardioversion is the application of direct current electricity to terminate dysrhythmias. Current is timed (synchronized) so that it is delivered outside the vulnerable phase of the cardiac cycle to minimize the risk of precipitating ventricular fibrillation (VF). Types of dysrhythmias seen in children that may require synchronized cardioversion include supraventricular tachycardia (SVT), atrial fibrillation, atrial flutter, and stable ventricular tachycardia (VT). Defibrillation is the application of a high initial dose of direct current electricity that is not timed to the cardiac cycle (asynchronous). A large segment of the myocardium is depolarized, rendering it refractory to further disorganized cardiac conduction. Defibrillation is used in the treatment of VF or pulseless VT (1). The development of the automatic external defibrillator (AED) is an important recent advancement in defibrillator technology. AED use for pediatric patients is discussed later in this chapter, after standard cardioversion and defibrillation techniques.
Because the most common cause of cardiopulmonary arrest in children is respiratory failure leading to hypoxemia and asystole rather than primary cardiac dysfunction with dysrhythmias, cardioversion and defibrillation are not frequently performed in this patient population. However, recent advances in pediatric cardiothoracic surgery have led to substantially higher survival rates among children with congenital heart disease, and these patients are at increased risk for dysrhythmias. In addition, children who accidentally or intentionally ingest excessive amounts of medications such as tricyclic antidepressants or sympathomimetics may also develop atrial and ventricular dysrhythmias. These drugs have become more prevalent in society as ever-increasing numbers of adolescents and adults are treated with antidepressant medications. For those children who present with cardiac dysrhythmias and hemodynamic compromise, cardioversion and defibrillation represent life-saving interventions that must be performed in a timely and appropriate manner.
Anatomy and Physiology
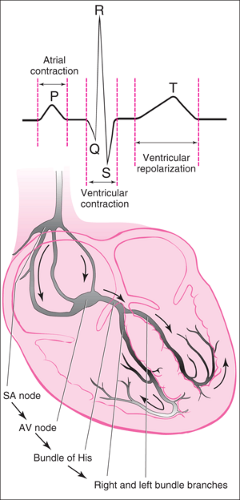
Under normal circumstances, the sinoatrial (SA) node, located in the right atrium, serves as the primary source of cardiac impulses (Fig. 23.1). A depolarization wave travels through the atria to the atrioventricular (AV) node at the lower portion of the right atrium. Here conduction of the current is slowed, allowing sufficient time for completion of atrial contraction. The impulse is then conducted along the bundle of His through the right and left bundle branches to the Purkinje fibers, resulting in an organized ventricular depolarization and contraction.
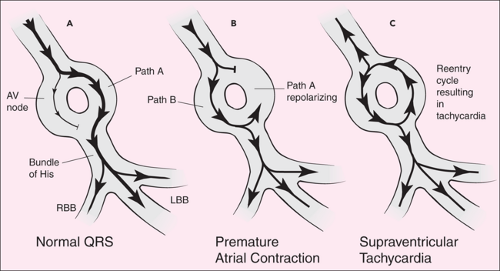
Tachyarrhythmias commonly occur as a result of re-entrant conduction (2). As shown in Figure 23.2, during re-entry the usual conduction route for an electrical impulse (path A) is in a state of depressed excitability. While the current travels a more slowly conducted secondary path (path B), path A repolarizes. The current is then conducted along path A, but in a retrograde direction. The resulting pattern of rapidly cycling current (down path B and up path A) produces repetitive depolarizations leading to tachyarrhythmias. Re-entry may result in a rapid atrial and/or ventricular rate, depending on the extent of conduction from the atria to the ventricles and the location of the re-entrant circuit within the myocardium.
With cardioversion, an externally applied electrical current depolarizes a segment of the myocardium, rendering it refractory to continued depolarization by the re-entry impulse. This often allows the SA node to resume its role as pacemaker, because it normally has the greatest intrinsic automaticity. A synchronized cardioversion impulse is timed so that it is not given during the vulnerable period of the cardiac cycle in the
phase of early repolarization, represented on the ECG tracing by the beginning of the T wave. An electric shock delivered at this time (the so-called R on T phenomenon) may produce VF (3).
phase of early repolarization, represented on the ECG tracing by the beginning of the T wave. An electric shock delivered at this time (the so-called R on T phenomenon) may produce VF (3).
The physiology of fibrillation is not well understood. Factors such as ischemia and acidosis are believed to decrease the refractory period that myocardial cells normally enter following depolarization (4). Numerous depolarization wavefronts that arise outside of the sinus node may then be conducted, beginning a series of re-entry circuits leading to asynchronous activity. Fibrillation may occur in both the atria and the ventricles. In most instances, atrial fibrillation is not a serious dysrhythmia, as long as the resulting ventricular rate maintains a stable blood pressure. Therefore, emergent therapy is usually unnecessary. In contrast, VF is extremely dangerous if untreated. The ventricles contract in an ineffective and disorganized manner, leading to a rapid progression of minimal to nonexistent cardiac output, inadequate tissue perfusion, hypoxemia, and death. The heart is said to resemble a “bag of worms” during the chaotic depolarizations of VF. Electrical defibrillation is often the only intervention that will reverse this lethal cascade of events. Defibrillation involves an externally applied asynchronous electrical impulse that results in the depolarization of a large segment of ventricular tissue. After depolarization, these cells are refractory to ectopic impulses. As with direct cardioversion, the SA node can then take over the function of generating cardiac depolarizations, ideally resulting in a resumption of normal sinus rhythm.
Indications
The decision to apply electrical current to the heart depends on the specific dysrhythmia present and the extent of hemodynamic instability. Signs of hemodynamic compromise among children include hypotension, agitation or lethargy, weak pulses, mottling, and delayed capillary refill. With children, proper assessment of blood pressure must be based on the normal range for each age group in question. A listing of normal blood pressure ranges for pediatric patients is presented in Table 4.3.
Supraventricular Tachycardia
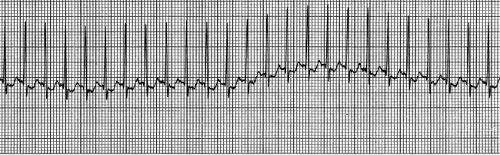
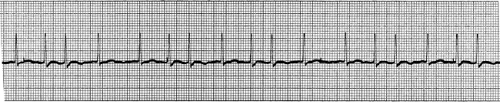
SVT is the most common significant dysrhythmia in children (5). It most often occurs as a result of re-entry as described previously, although a non–re-entrant ectopic atrial focus may be the cause. Distinguishing SVT from sinus tachycardia can be difficult in children, although the degree of tachycardia with SVT is typically more extreme. In infants, the rate of SVT ranges from 220 to 320 bpm, and in older children from 150 to 250 bpm. Fever, anxiety, pain, and respiratory distress are a few conditions that may contribute to sinus tachycardia, but the heart rate rarely exceeds 210 bpm, even in young infants. In addition to extreme tachycardia, ECG findings consistent with SVT include a regular RR interval that does not vary with respiratory pattern, narrow QRS complexes, and dysmorphic or absent P waves (Fig. 23.3). Causes of SVT in children include congenital heart disease, Wolff-Parkinson-White syndrome, fever, and medications such as sympathomimetics. One case report described a child with albuterol-induced SVT, which was terminated with adenosine (6). Among infants, 50% of cases are classified as idiopathic (7).
Severe tachycardia associated with this condition results in decreased diastolic filling time, eventually leading to congestive heart failure. Older children with SVT typically come to
medical attention relatively early in their clinical course when they complain of palpitations or chest pain. Consequently, they may not have significant hemodynamic compromise at presentation. In contrast, infants and young children with SVT will often have signs of significant cardiac decompensation (congestive heart failure, inadequate peripheral perfusion) when diagnosed. Thus, children with SVT will have a variable clinical presentation ranging from tachycardia alone to cardiogenic shock, depending on the duration of the dysrhythmia.
medical attention relatively early in their clinical course when they complain of palpitations or chest pain. Consequently, they may not have significant hemodynamic compromise at presentation. In contrast, infants and young children with SVT will often have signs of significant cardiac decompensation (congestive heart failure, inadequate peripheral perfusion) when diagnosed. Thus, children with SVT will have a variable clinical presentation ranging from tachycardia alone to cardiogenic shock, depending on the duration of the dysrhythmia.
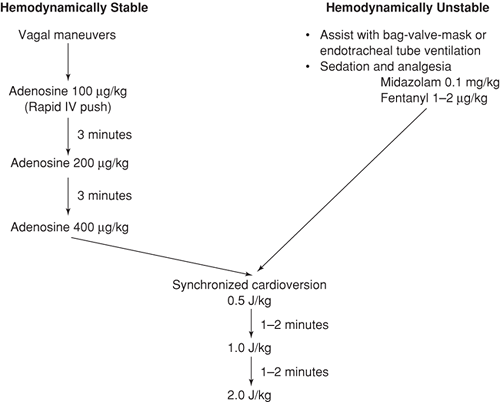
For children with stable hemodynamics, maneuvers designed to increase vagal stimulation to the heart may be both diagnostic and therapeutic in the management of SVT (see Chapter 70). For those with SVT, vagal maneuvers either will have no effect on the heart rate or will result in an abrupt termination of the dysrhythmia. For those with sinus tachycardia, vagal stimulation may result in a gradual decrease in the heart rate, followed by a steady return to the initial rate. If vagal maneuvers are unsuccessful, the next step is to administer intravenous medication. For the past 15 years, adenosine has been the drug of choice in treating the hemodynamically stable child with SVT (8,9,10,11,12,13,14,15,16,17). Synchronized cardioversion is indicated for patients who fail to respond to vagal maneuvers and pharmacologic agents or who manifest signs of hemodynamic instability. One cautionary note regarding the use of synchronized cardioversion is that low energies should be used for the child with SVT who is taking digoxin, because ventricular dysrhythmias may be precipitated in this setting
(3). The recommended management approach is shown in Figure 23.4.
(3). The recommended management approach is shown in Figure 23.4.
Atrial Fibrillation and Atrial Flutter
Although relatively uncommon in children, atrial fibrillation and atrial flutter should be recognized and treated appropriately. Atrial fibrillation appears on the ECG tracing as fine oscillating waves between QRS complexes without definable P waves. The hallmark of atrial fibrillation is an irregular ventricular response (Fig. 23.5). Atrial flutter is characterized by a regular ventricular rhythm with “sawtooth” waves between each QRS complex (Fig. 23.6). Atrial rates may exceed 400 bpm with either atrial fibrillation or atrial flutter, but since typically not all of the impulses are conducted to the ventricles, the ventricular rate will be considerably less. The clinical state of the patient depends on the rate of the ventricular response, the effects of any underlying heart disease, and the duration of the dysrhythmia. With a very rapid ventricular rate, diastolic filling time is diminished, resulting in decreased cardiac output.
With atrial fibrillation, ample time is usually available to initiate drug therapy in an attempt to terminate the dysrhythmia. If the patient fails to respond to drug therapy or becomes hemodynamically unstable, however, synchronized cardioversion is indicated. Success rates as high as 90% have been reported for adults with no underlying heart disease who were treated with direct cardioversion for atrial fibrillation (3). For atrial flutter, on the other hand, synchronized cardioversion is now considered the treatment of choice, even when the patient is stable (3). This is true because atrial flutter is notoriously difficult to convert with drug therapy, and successful treatment with cardioversion has been reported in 72% to 100% of cases (3).
Ventricular Tachycardia
VT is also an uncommon dysrhythmia in the pediatric population. Conditions that may predispose to VT in children include underlying heart disease, a tricyclic antidepressant overdose, and disease processes leading to hyperkalemia such as renal failure or congenital adrenal hyperplasia.
As with SVT, VT may occur either as the result of re-entry or an ectopic focus, although here the abnormality is obviously ventricular in origin. On the ECG, the characteristic feature of VT is wide QRS complexes (greater than 0.12 seconds) with a bundle branch morphology (Fig. 23.7). The rate may vary from 120 to 400 bpm but is typically 200 to 300 bpm. P waves may be absent, retrograde (i.e., inverted and appearing after the QRS complex), or unrelated to the QRS complex (AV dissociation). SVT with aberrant conduction can be difficult or impossible to distinguish from VT based on ECG alone. However, because fewer than 10% of children with SVT have aberrant conduction, the clinician must assume the presence of VT when treating a child with a wide complex tachycardia (1).
Children with VT decompensate far more rapidly than those with SVT. As with SVT, tachycardia can result in a decreased ventricular filling time, which in turn leads to diminished stroke volume and cardiac output. Prolonged VT may also degenerate into VF. For patients who are hemodynamically stable, intravenous amiodarone is the treatment of choice (1). If the patient fails amiodarone therapy or shows signs of hemodynamic decompensation while still having a palpable pulse, synchronized cardioversion is indicated. For patients with pulseless VT, the most recent American Heart Association (AHA) guidelines recommend immediate asynchronous defibrillation (1). Studies with adults show that cardioversion is successful in converting VT in over 95% of cases (3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree