Bladder Diseases in Childhood
Craig A. Peters
Harvard Medical School, Boston Children’s Hospital, Boston, Massachusetts 02115.
INTRODUCTION
Normal bladder function is essential to all children in their physical and psychosocial development. Abnormalities of bladder function may have profound effects on the well-being of any child, ranging from simple family tension as a result of persistent enuresis, to permanent loss of renal function due to hypertonic bladder dysfunction. The work of the bladder rests on two basic functions: (1) storage and (2) emptying. Storage of urine for a socially acceptable period of time requires the bladder to accommodate by enlarging its capacity without increasing its pressure. Bladder compliance is the critical parameter involved in normal storage. Normal emptying of the bladder permits continence of urine between emptying and efficient evacuation of the stored urine in a timely fashion, under voluntary control. This action requires the coordination of the detrusor muscle of the bladder itself with the bladder neck or internal sphincter and the skeletal or voluntary sphincter. The contraction must be sustained for a sufficiently long period to permit complete emptying. Discoordination of the activities of the detrusor and sphincter produce significant derangements in bladder function and may produce permanent damage.
A basic understanding of the activity of the bladder is essential to permit clinical assessment and management of various bladder disorders. Bladder dysfunction has become an active area of investigation as the clinical, social, and economic significance of bladder diseases has become evident. Understanding of childhood bladder dysfunction on a clinical basis has developed rapidly, and the basic scientific knowledge is beginning to catch up and offer the potential for novel therapeutic interventions.
This chapter briefly reviews normal bladder anatomy and function, patterns of bladder development, and the manifestations of bladder diseases. The assessment and management of the major categories of bladder dysfunction are presented. Areas of developing understanding that will influence therapy are also be reviewed.
Normal Bladder Anatomy and Function
Although the basic structure and function of the bladder may appear simple, it is an organ dependent on complex interactions of autonomic and voluntary neural regulation, as well as smooth and skeletal muscle groups, to store and expel a hypertonic solution without significant absorption or inadvertent leakage. Clinically significant bladder disorders are the result of various failures in these processes. The functional anatomy of the urinary bladder has been the more recent subject of vigorous investigation in the adult, principally aimed at understanding bladder outlet obstruction in the male, incontinence in the female, and neurologic bladder disease in the spinal cord injury patient. Many of the principles of bladder function in the adult are applicable in the child, yet this assumption should not be too broadly applied.
Anatomy
The bladder in the young child is an intraabdominal organ that becomes progressively intrapelvic with age. It is extraperitoneal, which permits most surgical exposures to be performed without peritoneal entry. The ureters enter posterolaterally from the deep posterior pelvis and are associated with the obliterated umbilical arteries, the vas deferens in the male, and the uterine ligaments and fallopian tubes in the female. The base of the bladder rests on the pelvic floor, or the urogenital diaphragm, an important structure in the maintenance of continence. The support of the bladder neck provided by the pelvic floor and skeletal pelvis is particularly important for continence.
The bladder is usually divided into two anatomic parts, the body or detrusor, and the base, including the trigone and bladder neck. These are of distinct embryologic origin,
with functional and neurophysiologic differences. The detrusor is composed of an inner epithelial layer with three cell layers, including a basal cell layer and a relatively impermeable lumenal epithelial layer. Beneath the uroepithelium is the lamina propria, consisting of connective tissue elements, which may have significant functional importance as a mediator of bladder compliance (1). The muscular layers of the bladder are not as distinctly organized into longitudinal and circumferential layers as in the intestine, but are more of an interdigitating meshwork of smooth muscle bundles and connective tissue elements, including fibroblasts, collagen fibers, and elastin, along with several other extracellular matrix components.
with functional and neurophysiologic differences. The detrusor is composed of an inner epithelial layer with three cell layers, including a basal cell layer and a relatively impermeable lumenal epithelial layer. Beneath the uroepithelium is the lamina propria, consisting of connective tissue elements, which may have significant functional importance as a mediator of bladder compliance (1). The muscular layers of the bladder are not as distinctly organized into longitudinal and circumferential layers as in the intestine, but are more of an interdigitating meshwork of smooth muscle bundles and connective tissue elements, including fibroblasts, collagen fibers, and elastin, along with several other extracellular matrix components.
UROEPITHELIUM
The biologic properties of the uroepithelium are only recently coming to be recognized. Previously considered impermeable, there is evidence to indicate selective permeability with ion transport that may be influenced by mechanical factors. A protective layer of glycosaminoglycans probably acts as a protective coat and disruptions in this layer are believed to be related to several inflammatory conditions, including interstitial cystitis (2). They are likely to play a role in resistance to infection as well. The uroepithelium appears to be important in the developmental regulation of bladder formation (3,4,5), and it may continue to play a regulatory role in the function of the detrusor muscle. This relationship is likely to be a two-way communication network.
MUSCULAR COMPONENTS
The muscular layer of the trigone merges inferiorly into the proximal urethra and taken together this muscular complex functions as a sphincter. Although there is no anatomically distinct internal sphincter, its functional existence is supported by urethral pressure studies, videourodynamic observations, and by the fact that continence may be maintained with its sole presence or lost with injury. Its function as a sphincter is augmented by soft-tissue coaptation and elasticity, loss of which will impair continence. These smooth muscle fibers may be seen to interdigitate with skeletal muscle of the external sphincter (6,7). The skeletal muscle sphincter is more anatomically distinct, and acts to maintain continence with activity and permit voluntary termination of urination. Discoordination of the activity of the sphincters with that of the detrusor may lead to significant bladder dysfunction.
Innervation and Neural Control of Bladder Function
The bladder is richly innervated by sacral parasympathetic fibers from S2 to S4, thoracolumbar sympathetic fibers, and sacral somatic fibers traveling through the pudendal nerves (8). Bladder function is regulated through the interactions of both autonomic and voluntary neural systems, with both spinal and brainstem reflex arcs, as well as cerebral control over their activity. A variety of neural effectors are active in normal bladder function, and many neurotransmitters have been shown to influence bladder function. The basic bladder functions and their neural control include filling and storage, which requires relaxation of the detrusor and concomitant sphincter contraction, and emptying, which is dependent on a sustained bladder contraction with coordinated sphincteric relaxation.
Filling and storage of the bladder must be at low pressure. Two primary factors contribute to these properties of the bladder. The smooth muscle of the bladder must relax against a passive stretch induced by increasing bladder volume (9). Smooth muscle relaxation has been shown to be an active process, mediated at a molecular level by dephosphorylation of myosin light chains, as well as changes in a Ca++-dependent force maintenance system referred to as a latch state (10,11). Centrally, relaxation is mediated through storage reflexes that increase the inhibitory impulses to the bladder as intravesical pressure rises. These impulses are mostly sympathetic via the β-adrenergic receptor system. This occurs with increased sphincter activity and is believed to be controlled at the level of the lateral pontine reticular formation (8). This relationship underscores the conceptual notion that bladder function is often determined by balances between excitatory and inhibitory influences. Although bladder function is biphasic (storage and emptying), switching between these phases appears to be the result of alterations in thresholds of activity that are influenced by a dynamic balance of excitatory and inhibitory influences (12).
The extracellular matrix is also important in bladder storage characteristics, possibly more than the muscular components, and is described by the viscoelastic properties of the connective tissue matrix (13). The importance of this element of bladder compliance is particularly relevant in the child with a noncompliant bladder from prior obstruction because developmental alterations in matrix remodeling have been shown to occur with fetal obstruction (14). The folding and unfolding of connective tissue bundles, and the properties of individual fibers such as elastin, contribute to the overall properties of the bladder as it fills with urine (15,16).
Bladder emptying requires initiation and maintenance of a detrusor contraction, coordinated with relaxation of the sphincters under voluntary control. The infant demonstrates involuntary or reflex voiding. More recent studies suggest a level of cortical control and involvement with infantile voiding (17). Central coordination is through the pontine micturition center, which acts as a switch,
triggered by a level of afferent or sensory impulses from the bladder as it fills. Activation of the sacral parasympathetic nerve fibers inhibit the somatic pathways to the urethral sphincter and permit relaxation. Sympathetic activity, which permits bladder filling, is inhibited and parasympathetic activation of the bladder occurs. Cortical inhibition of these reflexes can prevent onset of micturition.
triggered by a level of afferent or sensory impulses from the bladder as it fills. Activation of the sacral parasympathetic nerve fibers inhibit the somatic pathways to the urethral sphincter and permit relaxation. Sympathetic activity, which permits bladder filling, is inhibited and parasympathetic activation of the bladder occurs. Cortical inhibition of these reflexes can prevent onset of micturition.
The mediators of bladder neural control have been widely studied. The principal neuropharmacologic mechanisms include the cholinergic and adrenergic systems, as well as purinergic and peptidergic mechanisms, are likely to play an important role in function and dysfunction. Cholinergic receptors in the bladder body are largely muscarinic (M2) and act to stimulate muscle contraction. They are balanced by adrenergic β2 receptor mechanisms. Purinergic receptors responsive to adenosine triphosphate or adenosine contribute to the contractile response, and may serve to initiate a contraction, which is then sustained by cholinergic activity. Adrenergic activity in the bladder neck and urethra is mostly alpha-receptor mediated and stimulatory. Selective agonists and antagonists have permitted demonstration of site-specific receptor subtype distribution, and therefore, more specific pharmacologic control over function. Peptidergic neurotransmission has been documented in the bladder, including activity of vasoactive intestinal peptide, neuropeptide-Y, substance P, somatostatin, calcitonin gene-related peptide, cholecystokinin, and enkephalin immunoreactive fibers (18,19). The precise functional role(s) of these neurotransmitters remains to be defined.
The effect of bladder pathology on neural regulation of function is critical to recognize, particularly in the developing child. Abnormal function, as in obstruction, alters development, which then further affects function. Although there is little alpha-adrenergic activity in the normal bladder body, increases in alpha-sensitivity has been shown to follow obstruction. Changes in the level of existing receptors occur with both prenatal and postnatal obstruction, and with abnormal innervation (20,21,22). The functional changes induced by obstruction also affect central neural activity and patterns of innervation (23). This may have long-lasting effects, even after the inducing abnormality has been corrected. The concept of neural plasticity in the regulation of bladder function, particularly in the developing bladder, is critical (24).
Normal bladder function may be seen as a dynamic balance between the two basic phases of bladder activity, storage, and emptying. There are neural, muscle, and matrix components contributing to that balance. Abnormalities in these components are the basis for many bladder disorders. Improved understanding of the normal and abnormal integration of those factors and their clinical manifestations will permit more specific therapeutic intervention for children with bladder disorders.
BLADDER DEVELOPMENT
Embryonic Formation and Associations
The bladder forms from the embryonic endoderm and the trigone from the mesoderm (25). The bladder epithelial surface derives from the endodermal layer and may have a significant role in the induction and maturation of the mesodermal layer as it becomes the detrusor muscle and connective tissues of the bladder wall. As the ventral plate of the embryo infolds, the combined bladder and gut structures take on the form of a tube and become the cloaca (Latin for “sewer”) as a common channel. There is as yet no opening at what will become the perineum, but the allantois is patent and runs parallel to the umbilical vessels. Separation of the ventral bladder and dorsal gut structures occurs between 5 and 8 weeks, concurrent with separation of the ureter and mesonephric duct with ingrowth of the urorectal septum. The trigone (triangular area bounded by the ureteral orifices and the bladder neck) is initially mesodermal, but is ultimately surfaced by endodermal epithelium. The cloacal membrane remains closed at this time, but is hypothesized to rupture early in the production of cloacal exstrophy. When fully separate from the hind gut, the bladder is beginning to receive urine from the metanephric kidneys (8 weeks). The ureteral structures and associated mesonephric structures are still developing. It is clear that epithelial and mesenchymal interactions similar to the induction of the bladder mesoderm continue to influence maturation of these structures.
Development of Musculature
An important element in the bladder that develops in the late embryonic and early fetal period is the bladder musculature. Muscle cells are first seen about 7 weeks, and recognizable bundles are present by 12 weeks (26). Bladder myocytes either migrate from surrounding mesenchyme, or the undifferentiated mesodermal cells surrounding the bladder epithelium are induced to differentiate in a muscular pattern. Urothelial regulation of bladder myogenesis and differentiation has been demonstrated in rodent recombination models (3,5). The mediators of those interactions remain to be identified, but hold promise to understand aberrations of development, as well as potential for therapeutic intervention.
Bladder filling and emptying also plays a role in modulating this process; bladders that have never functioned (i.e., exstrophy of the bladder) have a variety of developmental differences from normal bladders. The onset of this interaction may not be active until closure of the urachus, which is about 16 weeks in the human. Innervation of the bladder has begun by this time because receptors for various neurotransmitters have been shown in the early fetal bladder (27). Muscularization of the bladder
continues with further growth and presumed integration of muscles and nerves. Little is known as to the mediators of bladder innervation in the fetus. The functional characteristics of bladder smooth muscle cells in the fetus have been investigated, with focus on the ontogeny of calcium regulation of contractility (28) as well as the presumed role of nitric oxide (29). Sphincter function can be demonstrated at 8 weeks, and is followed by development of the ureterovesical junction. Development of the external urethral sphincter is an important element in the functional patterns of the developing bladder and may contribute to abnormal functional maturation (30,31).
continues with further growth and presumed integration of muscles and nerves. Little is known as to the mediators of bladder innervation in the fetus. The functional characteristics of bladder smooth muscle cells in the fetus have been investigated, with focus on the ontogeny of calcium regulation of contractility (28) as well as the presumed role of nitric oxide (29). Sphincter function can be demonstrated at 8 weeks, and is followed by development of the ureterovesical junction. Development of the external urethral sphincter is an important element in the functional patterns of the developing bladder and may contribute to abnormal functional maturation (30,31).
The functional development of the extracellular matrix of the fetal bladder has been examined in a variety of systems. Developmental relative increases of collagen type I have been reported in the bovine fetus (32,33) and associated with developmental increases in bladder compliance (28). These observations differ from those of Swaiman (34) in the human, in which the developmental balance between type I and III collagen decreases, which is the reverse of that seen in the fetal calf. Other components of the matrix are likely be equally important, particularly elastin. An important component of extracellular matrix (ECM) homeostasis is the degradation arm of collagens and elastins. Connective tissue breakdown is mediated through a family of proteins, the matrix metalloproteinases (MMPs), which selectively degrade particular components of the ECM. Regulation of the activity of degradation will therefore have a major role in the matrix composition in the bladder, and this degrading activity is directly inhibited by the tissue inhibitors of metalloproteinases (TIMPs), endogenous and semispecific inhibitors of the MMPs (35). In fetal obstruction, the expression and activity of the TIMPs is increased, producing a decrease in the degradation of the matrix, which ultimately leads to increased amounts of ECM.
Fetal Bladder Function
It has long been recognized that bladder function is ongoing in the fetus, and it is likely that structural and functional development is dependent on bladder activity. Defunctionalized bladders in the fetal sheep have altered patterns of contractile protein isoforms and altered concentrations of muscarinic cholinergic receptors (20). Connective tissue elements are influenced by this activity, in that defunctionalized bladders show reduced procollagen type III gene expression. Although many of the mechanisms of interaction are unclear, it has been shown that fetal bladder function is a critical aspect of development (36).
Ultrasonographic images of the human and sheep fetal bladders show continued filling and emptying cycles in later gestation, with contractions every 10 to 15 minutes (37,38). The response to pharmacologic agents has also been described, including agents commonly used in obstetric practice. Magnesium sulfate, for example, will suppress almost all bladder contractions in the fetus, and postnatal voiding may be impaired until this effect has cleared.
Perinatal Bladder Function
Postnatally, there is a maturational process in development of normal bladder function (17). Infant voiding is characterized by small infrequent and incomplete voiding. Interrupted voiding may be seen in 60% of preterm infants and 30% of full-term children. Infant bladders tend to be more hyperactive with voiding at low volume and with high pressure. The most likely cause of this pattern is immature coordination between the detrusor and the sphincter mechanisms. There seems to be a relationship with voiding and sleep, whereby voiding is usually associated with arousal from sleep in the full-term child and less so in the preterm infant. The association with arousal suggests a role for higher cortical control, rather than a completely autonomic pattern as previously believed.
Ultrasonographic Appearances
Maternal-fetal ultrasound can detect many bladder abnormalities, both directly and indirectly. Identification of the bladder on maternal fetal ultrasound should be possible by 16 to 18 weeks in the normal fetus. The inability to demonstrate the bladder despite two or more attempts is abnormal and should prompt consideration of severe renal dysfunction, bladder exstrophy, or a severe abnormality of the bladder neck or ureteral positioning. Protrusion of tissue at the level of the lower umbilicus without a visible bladder lumen should raise the possibility of bladder or cloacal exstrophy, and this may be associated with a low set umbilical cord. When visible, the bladder should be filled with echo-transmitting fluid without debris or internal echogenic structures. A ureterocele may be demonstrated prenatally when present (usually associated with upper pole hydronephrosis). Massive dilation of the fetal bladder may be seen with bladder outlet obstruction as from valves, with massive vesicoureteral reflux (39), and with the prune belly syndrome (PBS). Bladder wall thickness, the condition of the renal parenchyma, and the status of the amniotic fluid are useful in distinguishing between these (40). The bladder affected by posterior urethral valves may not be massively dilated, but may have a very thickened wall and be only moderately full. The kidneys and ureters are dilated and the renal parenchyma may be echogenic, indicating some degree of dysplasia (41). Male gender should be identified in such cases and oligohydramnios may be present (42). Caution needs to be exercised in the clinical distinction between obstructive and nonobstructive conditions of the fetal bladder.
MANIFESTATIONS OF BLADDER DISEASE
The principal manifestations of bladder disorders include incontinence of urine, infection, hydronephrosis, hematuria and dysuria, or an alteration in voiding pattern. Each may be present in a spectrum of severity, with specific characteristics indicative of the underlying condition. Recognition of these signs and symptoms is not always immediate and in certain situations they should be specifically sought.
Incontinence
Inadvertent urinary emptying, incontinence, may be one of the most troubling symptoms to afflict a child after the age of toilet training. Causes range from behavioral patterns to major structural anomalies of the urinary tract or nervous system. The patterns of incontinence serve as the most useful initial tool to define the cause. Nighttime incontinence, nocturnal enuresis, is most often a maturational, self-limited entity, but should prompt a thorough history and physical examination to rule out subtle manifestations of more serious disease. Daytime wetting is primary if it has always been present or secondary if it develops after normal training. Constant dampness in a girl would suggest an ectopic ureter to the distal urethra, introitus, or vagina. Total incontinence, wetness without a dry interval, suggests a significant structural defect of the bladder neck (epispadias, bilateral ureteral ectopia) or a neurologic defect. Lesser degrees of these conditions may be manifest by episodic incontinence, as with stress or movement. Urgency incontinence, in which the child senses the need but cannot inhibit voiding, may be behavioral or neurologic; associated signs or symptoms should be sought. Overflow incontinence may be suspected in someone with small to moderate amounts of leakage, usually without urgency to void, and a full bladder will be identified with examination or catheterization. A structural, neurologic, or behavioral basis may be present and require careful further evaluation.
Infection
Urinary tract infections (UTIs) are one of the most common health problems in children (43) and may be a manifestation of bladder dysfunction. Inadequate bladder emptying, with or without obstruction, is the most frequent underlying cause. The association with upper tract infection of the kidney, pyelonephritis (44), is largely dependent on the presence or absence of reflux, upper tract drainage, and the nature of the infecting organism. Diagnosis of infection is a critical guide to further evaluation and should be based on a properly collected specimen. In the sick child, in whom antibiotics are to be started empirically, a definitive culture must be obtained at the outset, using catheterization or a suprapubic aspirate. Clean voided specimens in toilet-trained children with minimal symptoms are acceptable. A bag-collected specimen is of value in a negative culture or in the unusual pure growth of high colony count (even so, the false-positive rate is about 50%); no major therapeutic decision should be based on such a specimen.
The most appropriate evaluation of a child with a UTI remains controversial (45,46). The clinical distinction between cystitis and upper tract infection remains challenging and unreliable. Infants with a culture-proven infection should undergo ultrasonographic imaging and cystography. Older children may be equally served with ultrasonography alone if normal, although the transition age is not defined. The nature of the infection should guide evaluation. Most major structural or functional abnormalities will present with febrile infections, but this is not universal.
Hydronephrosis
Bladder dysfunction may be associated with hydronephrosis as a causative agent or a concomitant factor. Bladder outlet obstruction due to either structural or functional reasons may produce upper tract dilation. Usually this is bilateral and symmetric (Fig. 97-1), but may be unilateral.
Posterior urethral valves may cause unilateral high-grade dilation with renal parenchymal disruption [vesicoureteral reflux and dysplasia (VURD) association] (47), in which one renal unit acts as the pressure release of the high-pressure bladder. Very often, obstructed bladders will be thick-walled and hypertrophic. As a result, there may be an element of obstruction at the ureterovesical junction contributing in part or whole to the upper tract dilation (48). This factor may be difficult to assess, but may be demonstrated by reducing bladder pressures with temporary diversion.
Posterior urethral valves may cause unilateral high-grade dilation with renal parenchymal disruption [vesicoureteral reflux and dysplasia (VURD) association] (47), in which one renal unit acts as the pressure release of the high-pressure bladder. Very often, obstructed bladders will be thick-walled and hypertrophic. As a result, there may be an element of obstruction at the ureterovesical junction contributing in part or whole to the upper tract dilation (48). This factor may be difficult to assess, but may be demonstrated by reducing bladder pressures with temporary diversion.
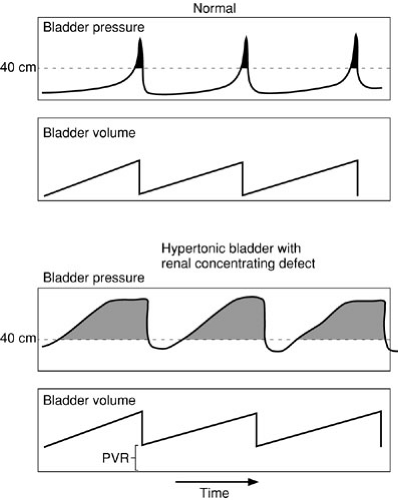
The effect of bladder pressure on upper tract dilation is due to the imbalance of ureteral peristaltic pressure moving urine into the bladder and the level of pressure within the bladder cavity. Forty centimeters of water pressure is the generally accepted maximal level of pressure generated by the ureter to transmit urine; vesical pressures exceeding this will cause cessation of flow into the bladder and if pressures exceed this, as during a bladder contraction, increased pressure may be transmitted to the kidneys (49). The effect of prolonged elevated pressures on the upper urinary tracts and renal parenchyma has been demonstrated clinically and may produce progressive renal functional deterioration and renal failure. The maximal pressures reached by the bladder with contraction are not the most important factor, however. The total work over time done against the kidney by bladder pressure is most critical and should be assessed to guide management. The product of pressure over the “safe” level of 35 or 40 cm H2O and the time during which this occurs is the work done against the kidney. Normal voiding pressures exceed 35 cm H2O, yet occur only six to eight times per day for 1 to 2 minutes; bladder pressures in the normal then fall to 5 cm H2O for the rest of the time. If bladder pressures reach near 35 cm H2O quickly as the bladder refills and stay at that level for most of the bladder filling cycle, the total work against the kidney over a 24-hour period is far greater than in the normal (Fig. 97-2). This concept of a “safe” period of bladder filling is critical to determining appropriate bladder management. Hydronephrosis associated with bladder dysfunction should be sought in patients in whom high-pressure bladder patterns may be seen, including those with neuropathic bladders or previously obstructed bladders (so-called “valve” bladders) (50,51). When present, hydronephrosis must be dealt with aggressively and monitored diligently, by addressing the bladder dysfunction.
Hematuria
Blood in the urine is a dramatic symptom and must be dealt with thoroughly. In children, however, it does not have the same sinister implications as in adults. The pattern of hematuria will often provide sufficient clues to permit a diagnosis and guide further evaluation. Total gross hematuria, bloody urine from beginning of voiding to the end, may be associated with flank pain or dysuria, or may be painless. Flank pain would focus attention on the ureters and kidneys; dysuria on the bladder and urethra and painless hematuria requires a full evaluation. Ultrasonographic examination of the entire urinary tract is a useful first step and will serve to identify upper tract lesions and most bladder neck lesions such as tumors or polyps. Viral cystitis in children will often be associated with severe voiding pain and urgency, and may have such marked edema of the entire bladder wall as to mimic a neoplastic process. Eosinophilic cystitis may present in a similar
manner with a discrete mass noted on imaging. This process is of unclear origin and may represent an allergic process. It is variably responsive to corticosteroids and will eventually resolve spontaneously. Biopsy may be needed to rule out a true neoplasm. Cystoscopy in children is seldom revealing and may serve more to reassure anxious parents. Often a watchful waiting period is useful because there are few lesions that must be immediately identified that would be missed by a careful physical examination (including rectal examination) and ultrasonography. The nature of the bleeding may serve to guide this decision. Terminal hematuria, occurring at the end of voiding is indicative of a process at the bladder base or more commonly of the urethra. In peripubertal boys, this is often due to a process termed benign urethrorrhagia, (52) a self-limited condition of unknown etiology, without long-term sequelae. Cystoscopy is not needed in most cases, which should resolve within 6 to 8 weeks. Meatal bleeding is most often due to urethral meatal stenosis. This is readily confirmed on examination and by observation of the voiding stream, which typically deviates upward and is thin and jetlike.
manner with a discrete mass noted on imaging. This process is of unclear origin and may represent an allergic process. It is variably responsive to corticosteroids and will eventually resolve spontaneously. Biopsy may be needed to rule out a true neoplasm. Cystoscopy in children is seldom revealing and may serve more to reassure anxious parents. Often a watchful waiting period is useful because there are few lesions that must be immediately identified that would be missed by a careful physical examination (including rectal examination) and ultrasonography. The nature of the bleeding may serve to guide this decision. Terminal hematuria, occurring at the end of voiding is indicative of a process at the bladder base or more commonly of the urethra. In peripubertal boys, this is often due to a process termed benign urethrorrhagia, (52) a self-limited condition of unknown etiology, without long-term sequelae. Cystoscopy is not needed in most cases, which should resolve within 6 to 8 weeks. Meatal bleeding is most often due to urethral meatal stenosis. This is readily confirmed on examination and by observation of the voiding stream, which typically deviates upward and is thin and jetlike.
Dysuria
Painful urination is a common symptom in children and may be relatively mild or extreme. On occasion, this will also be described as suprapubic pain after voiding. Severe abrupt pain during voiding is termed strangury and may indicate episodic obstruction as from a stone or bladder neck polyp, particularly if urine flow stops. Dysuria is most often the result of UTI, but may also be due to simple perineal irritation, obstruction, or abnormal voiding dynamics. The extreme manifestation of the latter is bladder sphincter discoordination (dyssynergy) and may produce pain in the neurologically intact child. This may be structural or behavioral. In boys with dysuria, it is prudent to rule out a bladder neck obstructive process such as a tumor with a rectal examination, ultrasound, or both. This is an unusual, but not infrequently missed diagnosis. Unusual causes of dysuria in children that should be considered include interstitial cystitis, eosinophilic cystitis, and granulomatous disease of the bladder.
Altered Voiding Pattern (Frequency, Decreased Flow, Retention)
Changes in voiding pattern may indicate a variety of problems, most of which may be identified with a careful history and examination. Frequency of urination is most likely due to infection, but may represent bladder instability due to a neurologic or obstructive process. In kindergarten age boys, however, this is most often a behavioral, self-limited process termed poikilouria (53). Diminished flow may be the result of outlet resistance such as a urethral stricture or valves, but may also be due to impaired contractility of the bladder. Urinary retention may result from a semiacute obstructive process, such as tumor of the bladder base or prostate, or from bladder decompensation in a chronic setting of partial obstruction or massive reflux. Neurologic causes must also be considered. Unusually, this is due to viral infection (herpes) and is rarely idiopathic or behavioral (54).
ASSESSMENT OF BLADDER FUNCTION
History
The history of the child’s signs and symptoms must be carefully elicited and may serve to specifically focus attention on the appropriate diagnostic possibilities. This serves to guide further evaluation. Important elements of the history have been noted previously in association with the specific signs and symptoms. Other features to consider include pregnancy and birth history of the child, developmental history, and medication exposure. In those children with identified diseases or conditions, their surgical history is essential and may be complex; their response to therapy and prior imaging and urodynamic studies are an essential part of the history. Associated symptoms including abdominal pain, constipation, and fevers need to be elicited. Parental views of “normal” are important because they are often divergent from true “normal.” To assess voiding patterns it may be very helpful to have the family complete a voiding diary to document actual voiding frequency and volumes. Many times this reveals a pattern unrecognized by the parent.
Imaging
Pediatric urologic imaging is highly specialized and critically important in evaluation and management planning (55). Ultrasonography has become a mainstay of bladder imaging due to its high resolution and absence of radiation (Fig. 97-3). Functional inferences may be made, particularly with regard to the bladder, if attention is paid to the state of bladder filling and efficiency of emptying [postvoid residual (PVR) volume], and the bladder wall thickness (40). Ultrasound is particularly useful in long-term follow-up studies.
Cystography is an important means of bladder evaluation and provides information regarding configuration, emptying, and the presence of reflux. The urethra should be well visualized in an adequate study (56). If voiding cannot be induced, the study should not be considered adequate and should be repeated, if clinically indicated. Radiographic cystography [voiding cystourethrography (VCUG) or micturating cystourethrogram] is usually the best first study to define anatomy and function, whereas
radionuclide cystography is a useful follow-up or screening tool due to its lower radiation exposure.
radionuclide cystography is a useful follow-up or screening tool due to its lower radiation exposure.
Computed tomography and magnetic resonance imaging (MRI) are important means of defining structural relationships of the bladder within the pelvis, particularly in the setting of tumor or trauma.
Urodynamic Evaluation
Functional bladder evaluation is most accurately based on urodynamic studies (UDS), which provide objective information regarding the parameters of bladder activity that are the basis for the consequences of bladder dysfunction (57). These include the ability of the bladder to store adequate amounts of urine at low pressures and to empty urine efficiently at appropriate pressures, and the ability of the sphincter mechanisms to retain urine for socially acceptable periods of time and relax adequately during voiding. Cystometry is a record of bladder pressure with filling and normally demonstrates low pressures within the bladder as volume increases (Fig. 97-4A). As capacity is approached, pressures increase slightly until a voiding contraction is initiated. Pressures then rise rapidly, and if accompanied by sphincter relaxation, voiding occurs. Bladder contractions occurring during filling that cannot be suppressed by the patient are termed uninhibited contractions and may be a manifestation of neurogenic, structural, and functional abnormalities. A poorly compliant bladder demonstrates early increases in pressure with filling, and this becomes important when those pressures approach the clinically important threshold of 35 to 40 cm H2O (Fig. 97-4B). Resting pressure, as correlated with volume, can also be a useful measure of compliance and may reflect more realistic patterns than filling cystometry (58). Absence of a voiding contraction may be seen with neuropathic bladder dysfunction or postobstructive dysfunction. In those patients, abdominal straining may be demonstrated as they attempt to empty a noncontracting bladder.
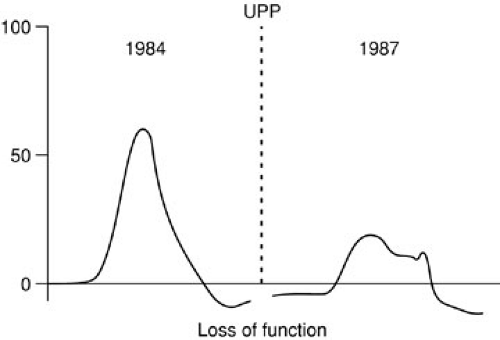
The activity of the bladder sphincter mechanism may be examined using pressure measurements within the urethra, estimating both the level of the pressure, as well as the length over which that pressure is exerted (functional urethral length) (Fig. 97-5). There is not universal agreement as to the interpretation or utility of urethral pressure measurements. Uroflowmetry can add information to the clinical assessment of incontinence in some situations (59). An alternative measure of sphincter function is the leak point pressure either with filling or with stress such as increased abdominal pressure (valsalva) (60). Electromyography of the urethral sphincter is a specialized study that may permit identification and characterization of neurologic bladder dysfunction, and bladder sphincter incoordination (dyssynergy) in particular (Figs. 97-6, 97-7A, and 97-7B).
NEUROGENIC BLADDER DISEASES
Table 97-1 provides a list of congenital and acquired neurogenic bladder diseases.
Spinal Dysraphisms: Meningomyelocele
Presentation
The child born with meningomyelocele is readily apparent and must be assessed expeditiously. The appearance of a membrane-covered sac along the back is unmistakable (Fig. 97-8). This sac contains neural elements and will usually become infected if not surgically closed in the first few days of life. Fetal coverage of the defect has been introduced with apparent reduction in the need for postnatal ventricular shunting, but the effect on bladder function seems minimal (61,62). Studies of aborted fetuses with myelomeningocele have shown early changes of bladder wall fibrosis, suggesting an early insult to bladder development. Whether these changes are due to abnormal innervation affecting bladder development or are the result of abnormal function altering development is unclear. These findings are strong indicators of the profound effect of these lesions on normal bladder development and ultimately function (63).
Neurosurgical closure of the spinal cord defect is usually performed in the first 2 days of life. A basic urologic assessment prior to closure is optimal, including renal and bladder ultrasound and neurologic examination of the perineum to assess innervation of the sphincter muscles (57). The presence of hydronephrosis in the newborn indicates abnormal bladder dynamics, usually due to bladder-sphincter dyssynergy. These babies require the most meticulous follow-up to minimize upper tract damage and to preserve bladder compliance. In those with normal upper urinary tracts, an assessment of bladder emptying is important to determine whether intermittent catheterization is needed (64). PRV measurements by catheter or ultrasound are appropriate.
Following neurosurgical closure, some babies will experience a period of spinal shock, with bladder flaccidity and poor emptying. Catheterization or credé emptying for this limited period is appropriate. Urodynamic assessment in this period is seldom useful. After the child has stabilized from the closure, baseline urodynamic assessment should be performed; this may be from 2 to 6 months of life. The principal aim is to identify those babies with risk of developing upper urinary tract damage from high bladder pressures. Detrusor-sphincter dyssynergy is the usual cause of this, and when demonstrated on UDS, our institution has recommended a program of anticholinergic medication to reduce bladder contractile tone (Fig. 97-9), and intermittent catheterization to permit emptying (64). It has been shown that this prophylactic treatment will avoid upper tract deterioration and may well maintain bladder compliance (64,65,66). Fewer of these children require augmentation cystoplasty than their counterparts managed expectantly (Fig. 97-10).
Medical Management
The two aims of management of children with myelodysplasia are (1) preservation of renal function and (2) social continence. The first requires normal storage function of
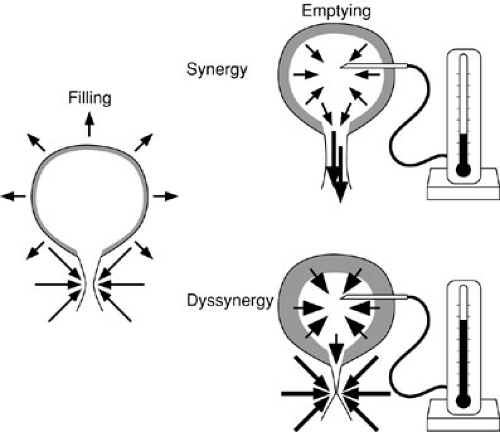
the bladder by maintaining low pressures during bladder filling. With neural abnormalities, the bladder may become noncompliant through two mechanisms. The first is denervation supersensitivity in which reduction in nerve input into the bladder muscle leads to an increase in the density of neurotransmitter receptors (67). The functional consequence of this is bladder instability and hypercontractility. The resting tone of the bladder may be higher than normal. Reduced inhibitory innervation that mediates relaxation may also be active in this context. The second mechanism is from functional bladder outlet obstruction in the setting of detrusor sphincter dyssynergy. Every time the detrusor contracts, the sphincter contracts with it (Fig. 97-6). Voiding pressures are elevated, and the bladder behaves as if obstructed. Smooth muscle hypertrophy begins and increased connective tissue deposition occurs (68,69). Alterations in elastin concentration and distribution are also seen, reducing the compliance of the bladder wall. Some groups have advocated sphincterotomy by dilation to eliminate this obstructive effect until the child is old enough to be definitively treated (70). Intermittent catheterization can
be taught to parents and may be a more acceptable and lasting solution in these children, without the risk of permanent sphincteric injury.
the bladder by maintaining low pressures during bladder filling. With neural abnormalities, the bladder may become noncompliant through two mechanisms. The first is denervation supersensitivity in which reduction in nerve input into the bladder muscle leads to an increase in the density of neurotransmitter receptors (67). The functional consequence of this is bladder instability and hypercontractility. The resting tone of the bladder may be higher than normal. Reduced inhibitory innervation that mediates relaxation may also be active in this context. The second mechanism is from functional bladder outlet obstruction in the setting of detrusor sphincter dyssynergy. Every time the detrusor contracts, the sphincter contracts with it (Fig. 97-6). Voiding pressures are elevated, and the bladder behaves as if obstructed. Smooth muscle hypertrophy begins and increased connective tissue deposition occurs (68,69). Alterations in elastin concentration and distribution are also seen, reducing the compliance of the bladder wall. Some groups have advocated sphincterotomy by dilation to eliminate this obstructive effect until the child is old enough to be definitively treated (70). Intermittent catheterization can
be taught to parents and may be a more acceptable and lasting solution in these children, without the risk of permanent sphincteric injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree