Therapeutic Hypothermia
INDICATION
Randomized clinical trials performed around the world have shown that therapeutic hypothermia reduces death and major neurodevelopmental disability for infants with moderate-to-severe hypoxic ischemic encephalopathy (HIE).1–7
Hypothermia is initiated within 6 hours after birth and continued for 72 hours, with a target temperature of 33°C–34°C for whole-body hypothermia and 34°C–35°C for selective head cooling. Eligible infants were 35 weeks or greater gestational age in 2 trials and 36 weeks or greater or 37 weeks or greater in the remaining trials. A recent systemic review and meta-analysis that included 7 large randomized clinical trials and 1214 newborns showed a reduction in the risk of death or major neurodevelopmental disability (risk ratio [RR], 0.76; 95% confidence interval [CI], 0.69–0.84) and an increase in the rate of survival with normal neurological function (RR, 1.63; CI, 1.36–1.95) at age 18 months.8 The number needed to treat is 7 to prevent 1 case of neonatal death or major disability. Newborns with moderate HIE had a greater reduction in the risk of death or major neurodevelopmental disability at age 18 months (RR, 0.67; 95% CI, 0.56–0.81) when compared to those with severe HIE (RR, 0.83; CI, 0.74–0.92). There was no difference in head vs whole-body cooling. Follow-up at 6 to 7 years of age of the National Institute of Child Health and Human Development (NICHD) Neonatal Network trial participants found that death or an IQ score below 70 occurred in 47% of the hypothermia group compared to 62% of the control group (p = .06).9 This result was not statistically significant; however, hypothermia was associated with a lower death rate without an increase in the rate of severe disability in survivors.
Clinical Findings
History
The recognition of moderate or severe HIE requires a detailed obstetric history, including any acute perinatal event, such as a placental abruption, cord events such as prolapse or complete knot, maternal hemorrhage, uterine rupture, prolonged fetal bradycardia, or nonreassuring fetal heart tracings. Other essential criteria include Apgar score of 5 or less at 10 minutes or the continued need for ventilator support initiated at birth and continued for more than 10 minutes.
Physical
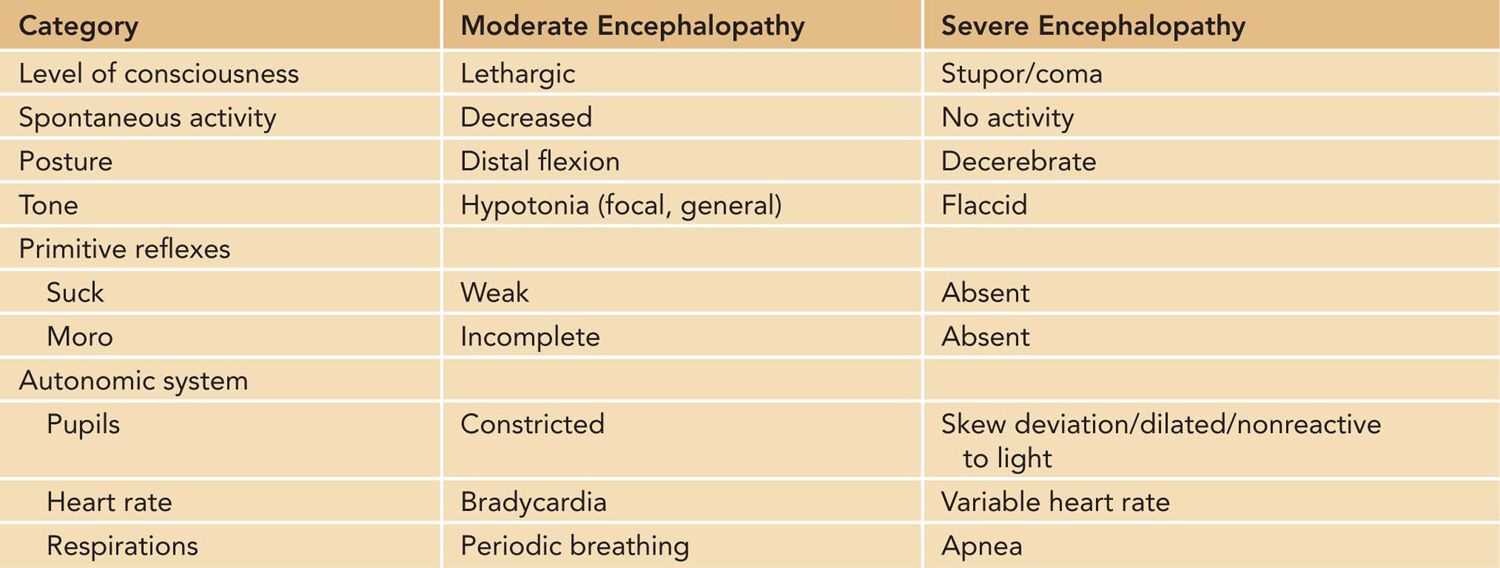
A modified Sarnat neurologic examination is performed to determine eligibility for therapeutic hypothermia unless seizures have been observed. Abnormalities must be identified in at least 3 of the following 6 categories: level of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic nervous system (Table 76-1).
Table 76-1 Criteria for Defining Moderate and Severe Encephalopathy
Baseline Tests
The laboratory eligibility criteria for therapeutic hypothermia include a pH of 7.0 or less or base deficit of less than 16 mmol/L in an umbilical cord gas or any gas during the first hour after birth. If the pH is between 7.01 and 7.15, base deficit is between 10 and 15.9 mmol/L, or no blood gas is available, additional criteria are required, including an acute perinatal event and 10-minute Apgar score of 5 or less or assisted ventilation for 10 or more minutes.
Several of the randomized clinical trials required amplitude-integrated electroencephalography (aEEG) with abnormal background activity for study entry; many centers continue to use this eligibility criterion for therapeutic hypothermia.1,4,5
Other baseline laboratory studies performed in this population include a complete blood cell count (CBC), blood culture, C-reactive protein value, coagulation panel, complete metabolic panel, and creatinine kinase, troponin, and lactate values. Conventional EEG, when performed with video recording, helps with identification of infants with clinical and subclinical seizures.
Differential Diagnosis
The following diagnostic categories must be considered when evaluating an encephalopathic infant, particularly in the absence of an acute perinatal event:
• Metabolic disorders
• Sepsis
• Neuromuscular disorders
• Chromosomal disorders and genetic syndromes
• Central nervous system malformations or injury (eg, subdural or subgaleal hemorrhage)
Exclusions
The following are exclusions for treatment:
1. Infants with lethal chromosomal or congenital anomalies are usually excluded.
2. Infants presenting after 6 hours of age are often not cooled as there are few data suggesting benefit. The window for therapeutic hypothermia is not specifically known; however, it is known that neuroprotection is influenced by the timing of therapy.10,11 The NICHD Neonatal Research Network is currently conducting a trial to evaluate the effect of cooling initiated between 6 and 24 hours of age (http://ClinicalTrials.gov, identifier: NCT00614744).
3. Infants with profound asphyxia are not likely to benefit; however, early identification of these infants is challenging. At this time, no reliable early clinical markers of nonresponse have been identified, and in the absence of this information, therapeutic hypothermia is initiated and later discontinued if other information, such as neurologic examination, EEG, and magnetic resonance imaging (MRI), confirm that the prognosis is poor.
4. The use of therapeutic hypothermia to treat mild HIE is controversial. Two trials unintentionally enrolled newborns with mild HIE.6,7 Zhou et al found no death or severe disability in the newborns with mild HIE; Jacobs et al reported a sizable rate of death and disability associated with mild HIE.6,7 The latter study did not use a standardized neurologic assessment tool or formally certify the transport personnel who assessed newborns for eligibility. Misclassification of the level of encephalopathy is potentially responsible for the higher-than-anticipated rates of death and disability.
5. Parental refusal is a cause for exclusion.
PREPARATION
Ethical Considerations and Consent
Neonatal therapeutic hypothermia has been studied in a specific patient population: near-term infants who are less than 6 hours of age and meet strict criteria for moderate-to-severe HIE. Multiple prospective, randomized, controlled trials have shown improved neurodevelopmental outcomes in infants with HIE who are cooled. Based on the results of these studies, it would be unethical to withhold this treatment from near-term infants with moderate-to-severe HIE.12 Parents must be informed of the availability of this therapy in a timely fashion and offered the option of transfer to another facility if hypothermia is not available at the birth hospital.
The situation becomes less clear if the patient is identified as a candidate for cooling outside the 6-hour window postbirth, if the infant is less than 36 weeks’ gestational age, or if the neurologic status does not meet the specific criteria used in randomized clinical trials. Because the complications of hypothermia are few, it could be argued that ethically any patient who might benefit from the therapy should be offered treatment, for instance, the infant who qualifies for treatment but does not arrive at a tertiary facility until 7 hours of age or the infant who qualifies for treatment but is 35 weeks’ gestation. Is it ethical to withhold treatment from such patients because they do not meet criteria used in studies? Conversely, is it ethical to treat patients who may not benefit from the therapy? Some centers have chosen only to treat infants who meet the strict criteria followed in the clinical trials; others decide whether to treat on an individual basis. If treatment is offered to infants who do not meet the criteria used in randomized clinical trials, informed parental consent is essential.12
Another ethical dilemma encountered by centers that offer hypothermia treatment is the potential for delay in outcome prediction. The early sequelae of HIE present during the 72-hour period in which cooling has been shown to be helpful. Cooling can suppress seizures and alter the EEG pattern, confounding the prediction of poor neurologic outcome based on electrographic evidence. Imaging studies such as computed tomography (CT) or MRI are difficult, if not impossible, to obtain in the cooled infant. Cooled patients are often treated with sedatives or narcotics that alter neurologic status. Discussions about prognosis and decisions about continuation of intensive care are often delayed until after the 72-hour treatment window, by which time the cardiorespiratory status of these patients may have improved to such an extent that discontinuation of support is no longer an option. The result can be prolongation of life in a severely damaged infant, greatly altering the options available to parents.13 If during the 72-hour treatment period the prognosis for a severely ill infant becomes hopeless, parents should be informed that they may choose to end hypothermia treatment and consider withdrawal of intensive support.12
Multiple studies have shown that cooling should begin as soon as possible following a neurologic insult, hence the 6-hour postbirth window used in the clinical trials completed to date. Informed parental consent is mandatory when offering a treatment within the confines of a clinical trial. Obtaining consent within the 6-hour window can be difficult for many reasons and may result in nontreatment because of lack of consent. Because hypothermia is considered by many to be standard of care for moderate-to-severe HIE in the first 6 hours of life, consent is not routinely required. In fact, failure to treat based on lack of consent could be viewed as unethical. Outside a study protocol, treatment should be initiated within the 6-hour window, with the intent of informing the parents and reaching agreement about treatment as soon as possible.12
Monitoring for Treatment
Intensive Care Monitoring
Infants with neonatal encephalopathy are at risk for multiorgan failure. These infants should be cared for in a level III neonatal intensive care unit (NICU) that can provide intensive monitoring and cardiorespiratory support, correction of metabolic disturbances, treatment of seizures, access to neuroimaging, and consultation with pediatric neurology.
Laboratory Workup
The CBC with platelet count; coagulation panel; chemistries, including liver enzymes; and lactate should be monitored.
Although no increased risk for bleeding has been seen in the randomized controlled trials of hypothermia therapy, changes in hematologic parameters are evident with cooling. Lower platelet counts, abnormalities of platelet activation and aggregation, and delayed activation of the fibrinolytic system have been reported.
Blood Gas Monitoring
Temperature Correction
Blood gas equipment is calibrated to analyze a sample at a “typical” patient body temperature, generally 37°C. Two of the early trials of hypothermia recommended that blood gases run on cooled patients should be performed on an instrument calibrated to the core temperature.1,3 In an evaluation of the iStat® instrument (Abbott Point of Care, Princeton, NJ), blood gases from patients cooled to 33.5°C were 15% lower on the corrected instrument than on the instrument calibrated to 37°C, and pH was 15% higher (Sheehan, personal communication). Failure to monitor ventilation with an instrument calibrated to the appropriate temperature in hypothermic patients can result in nontreatment of hypocarbia and alterations in cerebral blood flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


