Indications
Congenital
Myelodysplasia
Tethered spinal cord
Exstrophy (classic, cloacal, epispadias)
Sacral agenesis
Caudal regression
Posterior urethral valves
Acquired neurogenic
Spinal cord injury
Spinal tumors
Myelitis
Multiple sclerosis
Idiopathic
Acquired non-neurogenic
Detrusor overactivity
Defunctionalized bladder in patients on dialysis
Infectious
Tuberculosis
Schistosomiasis
Inflammatory
Interstitial cystitis
Radiation cystitis
Chemotherapy-induced cystitis
Iatrogenic
Intraoperative loss of bladder wall (after extirpative surgery for malignancy)
Urinary undiversion
Neurogenic conditions caused by congenital, acquired, or traumatic etiologies, especially myelodysplasia in children and spinal cord injuries in adults, are probably the most frequent indications. Although there is considerable variation in institutional rates, it has been estimated that 5–30 % of patients with spina bifida will undergo AC [13, 14]. Multiple sclerosis, with its inherent progressive neuromuscular deterioration that may make intermittent self-catheterization difficult [15], is preferably managed with medical therapy such as anticholinergics and botulinum toxin but occasional cases may be amenable to AC.
Patients with severe refractory idiopathic overactive bladder (OAB) or rarely interstitial cystitis can also be offered such a procedure, but this should be a last resort option for carefully selected cases, as pointed out by the American Urological Association guidelines [16, 17].
Benign diseases with decrease in compliance from collagen deposition in the bladder wall are other indications. Tuberculosis cystitis, which used to be the number one indication in the past, is now a rarity [15]. Schistosomiasis, an endemic parasitic infection found primarily in the Middle East and Africa, may cause bladder wall fibrosis in 2 % of cases [1]. Reduced bladder capacity may be improved by AC.
Pelvic radiation therapy or surgical resection for colorectal, gynecological, or urological malignancies may also compromise the urinary tract and necessitate AC.
Finally, a defunctionalized bladder in a patient on dialysis awaiting renal transplantation might warrant AC to restore proper lower urinary tract function and protect the allograft, although its timing (before, after, or even concomitantly with the transplantation) is controversial [18], the concern being the risk of severe sepsis in the immunosuppressed patient [1].
Contraindications
Poor baseline renal function may potentially expose patients to severe electrolyte abnormalities and worsening renal function and is a relative contraindication. However, in patients with renal dysfunction that is a direct result of bladder dysfunction with elevated storage pressure, AC may be appropriate and help stabilize renal function [19]. Other relative contraindications include inflammatory bowel disease (Crohn’s disease), irradiated bowel, short gut syndrome, bladder tumors, and liver failure [20].
Preoperative Surgical Considerations
Extensive history and physical exam, serum chemistries (complete blood count, electrolyte and creatinine levels, coagulation factors), urine cytology, urinalysis, and cultures are required. All patients should also undergo anatomic and functional assessment of the urinary tract with cystoscopy (to exclude intravesical abnormalities such as tumors), urodynamics (to characterize bladder capacity, compliance, or uninhibited detrusor contractions, to assess detrusor and Valsalva leak point pressures, and to rule out infravesical obstruction), cystography (to rule out vesicoureteral reflux), and upper tract imaging (ultrasound or computerized tomography; to document the presence of hydronephrosis or stone disease). A history of bowel disease may require preoperative bowel imaging studies or colonoscopy. A voiding diary can help in planning for the final reservoir size by assessing 24-h urine volume [21], although the augmentation usually enlarges with time. The need for latex precautions for at-risk patients with spinal dysraphism should also be kept in mind. Planning for other concomitant procedures such as a continent catheterizable stoma (using the Mitrofanoff or Monti principles), ureteral reimplantation, or bladder neck management is an essential prerequisite before considering the intervention [22]. Finally, since CIC is frequently a necessary adjunct, adequate manual dexterity and cognitive abilities are prerequisites to be considered an adequate surgical candidate and it is crucial, in the preoperative visits, to reinforce the importance of compliance with CIC postoperatively [23].
Bowel Preparation and Antibiotic Coverage
Preoperative mechanical bowel preparation continues to be an issue of controversy. In the colorectal surgery literature, it does not lower the rate of postoperative complications (wound infection, intraperitoneal abscess, or anastomotic leak) [24, 25] and some patients have reported adverse events from it [26–28]. There are two pediatric studies that have reported no increase in complications after AC following no preoperative mechanical bowel preparation [29, 30]. However, there are no prospective randomized trials so caution should be exercised especially in patients with ventriculoperitoneal shunts that may be exposed to fecal contamination intraoperatively [31]. Even though there is no consensus among the studies with the duration, the dosage, and the type of bowel preparation to use, we agree with the most recent CDC guidelines (published in 1999) [32] and still routinely prescribe mechanical bowel preparation and clear fluids diet the day prior to the intervention.
The periprocedural systemic administration of an antimicrobial agent to reduce infectious risks in contaminated urology surgery [33] is an evidence-based supported concept, but the literature is not clear about the optimal therapeutic regimen (type of medication, dosage, and route of administration). Available practice guidelines recommend a combination of either second- or third-generation cephalosporin with an aminoglycoside and metronidazole, with special caution for patients with prosthetic devices such as ventriculoperitoneal shunts or orthopedic hardware [32, 33].
Choice of Intestinal Segment
Different parts of the GI tract from stomach to sigmoid have been used for bladder augmentation, each having its pros and cons (Table 12.2), but none being the ideal segment.
Table 12.2
Advantages and disadvantages of different tissues used for augmentation cystoplasty
Tissue | Advantages | Disadvantages |
|---|---|---|
Stomach | Produces less mucus Lower incidence of bacteriuria Rich blood supply More appropriate for patients with renal insufficiency | Risk of bladder ulcers and perforation Hematuria–dysuria syndrome Vit B12 malabsorption |
Jejunum | Severe electrolyte abnormalities Risk of profound dehydration Iron and calcium deficiencies | |
Ileum | Mobile, small diameter, easy to handle Well-defined, reliable blood supply Less severe metabolic complications | Lipid malabsorption (Vit A, D, E, K) Vit B12 deficiency (anemia) Incidence of bowel obstruction more common than colon Lack of bile salt reabsorption (diarrhea) Metabolic acidosis Sometimes short mesentery |
Colon | Transverse colon safer if prior pelvic radiation Fewer nutritional problems Redundant in spina bifida patients Antireflux tunnels easily made | Metabolic acidosis Produces more mucus than ileum (increased risk of UTIs and stones) |
Ureter | Urothelial lined Requires no intestinal resection | Limited availability (need for hydroureter) |
Autoaugmentation | Urothelial lined Requires no intestinal resection | Technically demanding Limited gain in capacity and compliance Risk of perforation |
Tissue engineering | Theoretically unlimited donor tissue | Still experimental |
Ileum is unquestionably the most widely used bowel segment in reconstructive urology. The versatility it provides allows the surgeon to refashion it in a multitude of different techniques that have been elaborated over the years [34]. With overall fewer complications than other bowel segments, it is mobile, easy to handle, with a constant blood supply, and available in large quantity [35]. However, ileum might not be ideally suited in situations where the mesentery is short, or for those with significant adhesions or prior small bowel resections [23]. Caution should be also exercised when considering ileocystoplasty for patients who underwent prior pelvic radiation therapy [36].
If functional or anatomic factors preclude the use of ileum, colon is often the second choice. Advantages of colocystoplasty include a thick muscular wall, large lumen, and abundant mesentery guaranteeing adequate bladder capacity and maneuverability [37], while disadvantages include more mucus production with increased risk of urinary tract infections (UTI) and stone formation [38]. More specifically, the sigmoid is sometimes redundant in patients with neurogenic bowel dysfunction which makes it an attractive alternative for this population [22]. Finally, the cecum, in conjunction with the terminal ileum, can be used as a continent catheterizable channel [39]. The ileocecal valve provides the continence mechanism, but its resection renders the patient vulnerable to diarrhea [40], which occasionally may be severe and difficult to manage. This may render the patient at risk for malabsorption and fecal incontinence [35].
Gastrocystoplasty as an alternative has been mostly reported in the pediatric literature [41, 42]. Despite clear advantages to the use of the stomach such as less mucus production, a lower incidence of bacteriuria, and a rich blood supply, the technique has declined in popularity due to significant limitations, mostly from debilitating hematuria–dysuria syndrome [43].
The use of jejunum results in severe metabolic disorders in 25–40 % of cases and therefore should not ordinarily be utilized [44, 45]. Although not formerly contraindicated, its use should be limited to those extremely rare cases where any other segment of the GI tract is not available for the augmentation, a situation we have not yet encountered.
Alternative tissues have also been described. Several series have reported satisfactory outcomes with ureterocystoplasty [38, 46, 47]. This necessitates a hydroureter from a nonfunctioning renal unit and is practically rarely applicable [47, 48]. In autoaugmentation (or detrusor myectomy) of the bladder, the creation of a urothelial pseudodiverticulum is technically demanding and usually only provides a slight gain in capacity and compliance [49]. With such disappointing efficacy, the technique is rarely reported.
Finally, none of the bowel segments has been shown to be clearly superior to another in all circumstances so the reconstructive urologist needs to be proficient in several techniques making use of various segments in order to individualize the decision and optimize results.
Technique
With the ultimate goals of lowering urinary storage pressures, preserving renal function, maximizing continence, and hopefully maintaining volitional voiding, the clam ileocystoplasty is the most commonly reported technique.
With the patient supine or in the low lithotomy position, a midline infraumbilical incision is usually adequate to gain intraperitoneal access, but a short Pfannenstiel incision has also been described [50]. With a Foley catheter in the surgical field, the native bladder is distended. The loose areolar tissue is bluntly dissected to expose the anterior and perivesical spaces [51]. With stay sutures on either side, the bladder is bivalved in the coronal or sagittal plane [11]. A “smile” incision has also been described [52]. The bladder incision should be a broad opening to create a large anastomosis and prevent an hourglass deformity [35]. Occasionally, supratrigonal cystectomy can be done for conditions such as severe interstitial cystitis [53].
A 15–40 cm segment of ileum is then harvested approximately 20 cm proximal to the ileocecal valve on a broad well-vascularized pedicle that may be identified by transillumination. A side-to-side stapled or hand-sewn ileoileostomy reestablishes bowel continuity cephalad to the isolated segment and the mesenteric defect may be closed to prevent internal hernias, or alternatively left open if judged to be wide enough. The isolated segment is irrigated thoroughly until return is clear to prevent intraperitoneal soiling as much as possible. It is then detubularized on its antimesenteric side to prevent intraluminal pressure rises from peristaltic contractions. The segment is reconfigured into a U, S, or W shape [36, 54] (Figs. 12.1 and 12.2). The surgeon should make sure that the segment reach down into the pelvis without tension or twist on the mesentery. The ileal patch can then be reapproximated to the edges of the cystotomy, starting posteriorly, with one to two layers of 2-0 continuous absorbable sutures. The vesico-intestinal anastomosis is tested for watertightness and drains are inserted prior to closing the abdominal wall in a standard fashion. The goal is to create a new reservoir in as spherical a configuration as possible to maximize the surface area as per Laplace’s law.
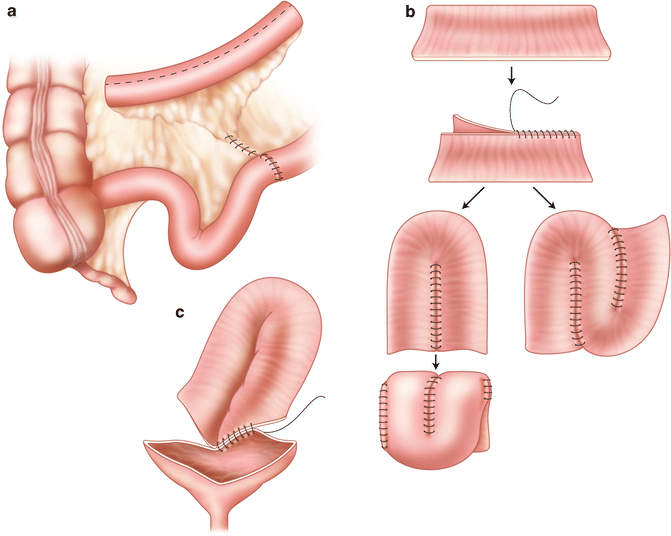
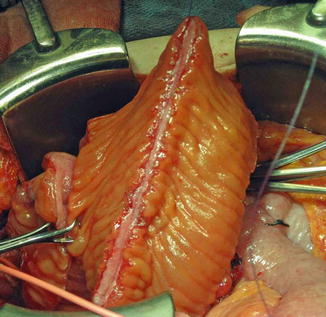

Fig. 12.1
(a–c) (a) Ileocystoplasty. A 20- to 40-cm segment of ileum at least 15 cm from the ileocecal valve is removed and opened on its antimesenteric border. Ileoileostomy reconstitutes the bowel. (b) The opened ileal segment should be reconfigured. This can be done in a U, S, or W configuration. It can be further folded as a cup patch. (c) The reconfigured ileal segment is anastomosed widely to the native bladder

Fig. 12.2
A 40 cm length of ileum is shown. The segment has been isolated from the GI tract and reconfigured. The antimesenteric border was incised and the bowel segment was detubularized into an inverted U shape. It will be anastomosed to the bladder
More recently, minimally invasive (laparoscopic and robot-assisted) approaches have become more frequently reported from a number of institutions. Depending on the surgeon’s experience and expertise, most of the suturing can be performed intra- or extracorporeally [55]. More technically demanding, these minimally invasive techniques have the advantages of reduced perioperative pain and morbidity, shorter hospital stay, and cosmetic superiority, with outcomes similar to the open surgery [20, 56–58].
Postoperative Care
As for any major abdominal surgery, intravenous fluids and bowel rest are maintained for several days with close monitoring of inputs and outputs. Routine nasogastric tube drainage is not required as it has not been shown to lead to faster bowel function recovery [59]. Antibiotic prophylaxis for 24 h [60], thromboprophylaxis, and early ambulation are also recommended. A closed-suction pelvic drain is kept until output tapers off or once peritoneal fluid is confirmed with fluid chemistries [21].
Proper drainage and irrigation of the augment, initially several times a day with 50–100 mL of sterile saline and then gradually reduced to an as-needed basis, are necessary to prevent mucus from accumulating, plugging the catheters and disrupting the fresh anastomosis. We usually favor a Foley urethral catheter (16–18 Fr) and a suprapubic (SP) Malecot catheter (20–22 Fr). Both tubes are kept for approximately 4 weeks, after which a cystogram is performed. The Foley is then removed if there is no extravasation and patients resume spontaneous voiding. Post-void residuals are closely monitored initially as well as on a long-term basis, and CIC is initiated when needed. The SP tube may be kept for 1–2 more weeks longer and used for daily bladder irrigation. Although there is no evidence to support it, antimicrobial prophylaxis can be administered at the time of catheter removal since bacterial colonization has likely occurred [33].
Complications
Flood and colleagues reported an overall 28 % early and 44 % late complication rate in this difficult group of patients, with a chance of requiring a reintervention ranging from 15 % to 40 % [61–63]. Table 12.3 lists the possible consequences of such procedure.
Table 12.3
Complications of augmentation cystoplasty
Early | Late |
|---|---|
Infection Wound infection Intraperitoneal abscess UTI Ventriculoperitoneal shunt sepsis | Metabolic disturbances Metabolic acidosis Hypokalemia Hypocalcemia Hypomagnesemia Hyperammonemia (encephalopathy) |
Wound dehiscence | Mucus accumulation |
Prolonged ileus | Urolithiasis |
Peroneal nerve palsy | Bacteriuria/Pyelonephritis |
Anastomotic leak Urinary Intestinal | Bowel dysfunction Diarrhea Fecal incontinence Bowel obstruction |
Fistula | Neoplasia |
Hemorrhage | Bladder perforation |
Death | Upper tract deterioration/Renal dysfunction |
Malabsorption Vitamins A, D, E, K deficiencies Bile salts (gallbladder stones) Vitamin B12 deficiency (megaloblastic anemia) | |
Hematuria–dysuria syndrome (gastric) | |
Drug absorption toxicities | |
Bone demineralization/Impaired growth | |
Urinary retention/Diverticularization | |
Incontinence |
Early Complications
Cardiovascular, respiratory, thromboembolic, and gastrointestinal complications can occur in the early postoperative course, as for any major abdominal surgery. The most common ones include wound infection or dehiscence (5–6 %), prolonged ileus (5 %), anastomotic leakage (2–4 %), catheter obstruction from mucus, ventriculoperitoneal shunt sepsis (0–20 %) [64], and peroneal nerve palsy [19]. Contemporary publications in the literature report a mortality rate of 0–3.2 % [19].
Late Complications
Bacteriuria
Asymptomatic bacteriuria in patients on CIC is nearly universal regardless of the segment considered and should not be treated except for infection with urease-splitting organisms [65]. Recurrent episodes of symptomatic cystitis do require treatment, but symptoms may be nonspecific. Risk factors predisposing to UTI include mucus accumulation, stasis, and CIC [66]. Symptomatic urinary tract infection which occurs in 5–40 % of patients [19, 67, 68] requires antibiotic treatment. With regard to prophylaxis, as recommended by the European Association of Urology, low-dose, long-term, antibacterial prophylaxis may be an option for patients with recurrent UTI, but there is a risk of emergence of multiresistant organisms [69].
Urolithiasis
The incidence of calculi in augmented bladders ranges from 10 % to 50 % [70, 71]. Stone composition is more commonly struvite or calcium oxalate [72]. Stasis, incomplete emptying, excessive mucus production, and chronic bacteriuria, especially if urease-splitting organisms, predispose to stone formation [73, 74]. Patients with a continent catheterizable channel (which may not drain the bladder completely), those using urethral CIC (compared to those voiding spontaneously), and patients with urease-splitting bacteriuria are at increased risk [19, 75]. Permanent sutures can serve as a nidus and should be avoided as much as possible [35]. Prevention strategies include increased fluid intake, routine bladder irrigation, prompt treatment of UTI, and staple exclusion at the time of surgery [74].
Perforation
Bladder perforation is a potentially life-threatening complication and is due to either overdistension or trauma from catheterization. It has been reported up to 13 % [76]. Patients with neurogenic bladders, those with competent bladder necks, those without a catheterizable channel, and those who abuse alcohol appear to be at an increased risk [19, 77–79]. Patients can present with an acute abdomen, but symptoms can be more subtle in neurologically impaired patients. The diagnosis can be made with a CT cystogram but requires a high index of suspicion [76] and low threshold for exploratory laparotomy [79]. The area of perforation is usually at the vesico-intestinal anastomosis or within the weaker bowel wall [80]. Emphasizing the importance of compliance with regular CIC can help obviate the risk of early perforation [76].
Mucus Production
The average daily mucus production is about 40 g and does not taper off with time despite villous atrophy [1, 81]. With time, accumulated proteinaceous material can become a nidus for infection and stone or impair adequate bladder emptying [19]. Colonic segments produce more mucus than ileum [82]. Daily irrigation can help reduce mucus retention [83]. These can be augmented with acetylcysteine or urea irrigations which help dissolve mucus [82] or oral ranitidine which may help to reduce mucus production [84].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


