Major Criteria
• Pruritus
• Typical Morphology and Distribution
– Flexural lichenification in adults
– Facial and extensor involvement in infancy
• Chronic or chronically relapsing dermatitis
• Personal or family history of atopic disease (asthma, allergic rhinitis, atopic dermatitis)
Minor Criteria
• Xerosis
• Ichthyosis/hyperlinear palms/keratosis pilaris
• Immediate skin test reactivity
• Elevated serum IgE
• Early age of onset
• Tendency for cutaneous infections
• Tendency to nonspecific hand/foot dermatitis
• Nipple eczema
• Cheilitis
• Recurrent conjunctivitis
• Dennie-Morgan infraorbital folds
• Keratoconus
• Anterior subcapsular cataracts
• Orbital darkening
• Facial pallor/facial erythema
• Pytiriasis alba
• Anterior neck folds
• Pruritus when sweating
• Intolerance to wool and lipid solvents
• Perifollicular accentuation
• Food hypersensitivity
• Course influenced by environmental and/or emotional factors
• White dermatographism or delayed blanch to cholinergic agents
Table 29.2
UK working party’s diagnostic criteria for AD
Must have: Evidence of pruritic skin, including parental report of a child rubbing or scratching |
Plus 3 or more of the following: • History of involvement of skin creases, including antecubital fossae, popliteal fossae, neck, areas around eyes, fronts of ankles • History of generally dry skin within the past year • Symptom onset under age 2 (not used if child is under 4 years) • Visible evidence of dermatitis involving flexural surfaces (or dermatitis affecting the cheeks, forehead, and extensor surfaces in children under 4) (modified from Williams [32]) |
Clinical Features
Infantile AD is generally characterized by acute skin lesions with erythematous and edematous papules and plaques, accompanied by oozing, vesicles, and crusts. There may also be subacute lesions, which are erythematous with fine scaling and less oozing and crusting. The lesions typically appear on the cheeks, neck, scalp, trunk, and extensor surfaces of the infant, usually sparing the diaper area (Fig. 29.1).
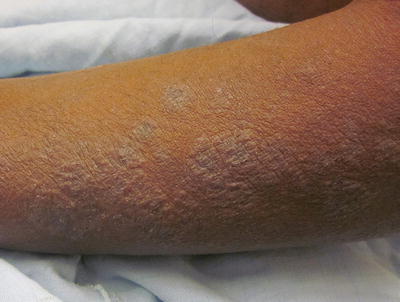

Fig. 29.1
Lichenoid atopic dermatitis on the forearm of a Hispanic male
Childhood AD is usually characterized by subacute to chronic lesions. Chronic rubbing and scratching leads to skin thickening and accentuation of skin lines, known as lichenification. Affected areas classically include flexural areas of the body, such as the antecubital and popliteal fossae, neck, posterior auricular area, wrists and ankles, as well as the face, hands, and feet. Nodular, prurigo-like lesions can also develop in some cases (Fig. 29.2).
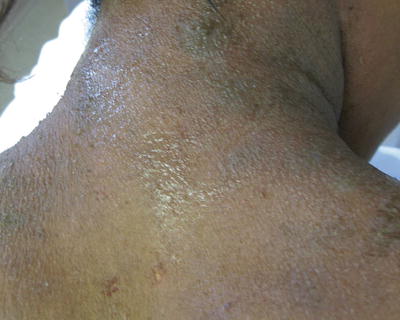

Fig. 29.2
Chronic lichenification on the neck of a teenage African American female with atopic dermatitis
Intense pruritus is the most prominent feature of the disorder and can be severe, causing flares, exacerbations, and chronic excoriations of the skin. For this reason, AD is referred to as the “itch that rashes”. Itching and scratching leads to secondary skin changes, which contribute to disruption of the epidermal barrier, perpetuating the disease and ultimately worsening the “itch-scratch cycle” [30]. In severe cases of the disorder, especially those whose disease persists into adulthood, disease may even progress to generalized erythroderma [29].
Many children have improvement of their AD in the summer and flare during the autumn and winter, possibly due to a combination of the therapeutic effect of UV rays and greater humidity in the former and low humidity and exposure to indoor heating in the latter [30]. On the other hand, many children report itch with sweating [33], which is often problematic in the summer months.
Associated Features
Filaggrin is a structural protein which plays an important role in maintaining the integrity of the epidermal barrier. An intact epidermal barrier limits transepidermal water loss as well as the inflow of irritants and pathogens. Filaggrin is one of the last components to be incorporated into the formation of the cornified envelope of the epidermis, which is chiefly made up of the outer membranes of terminally differentiated keratinocytes [34]. Subsequent to its integration into the stratum corneum, filaggrin is also broken down into a number of hydrophilic amino acids and other components which together make up natural moisturizing factor (NMF) [35], an important substance in preserving skin hydration. As such, filaggrin (FLG) loss-of-function gene mutations lead to a deficiency of NMF [36], which likely contributes to xerosis in AD [37]. A number of FLG mutations have now been identified and have been shown to be a risk factor for AD [38, 39]. These mutations are rather common, detected in up to 10 % of western European and North American populations [40] and AD affects 42 % of mutation carriers [41]. A few studies have attempted to determine the prevalence of mutation in different racial and ethnic groups [42–44]. One pediatric cohort study found that among all patients with at least one variant of FLG mutation, 27.5 % were white and only 5.8 % were African-Americans [45].
Xerosis, or dry skin, is present in most cases of AD and is often accompanied by fine “dry” scale. One case series found that xerosis was present in 100 % of children with AD at 2 years of age [33]. Xerosis is usually worse in the winter, likely secondary to low indoor humidity. The etiology of xerosis in AD is multifactorial, including decreased epidermal hydration in conjunction with increased transepidermal water loss through a defective epidermal barrier [46] and lower levels of natural moisturizing factor secondary to filaggrin mutations [37].
Ichthyosis vulgaris is a condition characterized by extreme white-brown scale of the skin, especially on the lower extremities. Ichthyosis vulgaris is also caused by similar mutations of the filaggrin gene as those which cause AD [37].
AD is often accompanied by one or more atopic disease. In the USA, 25.1 % of children with AD reportedly had a history of asthma, 34.4 % had hay fever, and 15.1 % had food allergy [4]. Children with severe AD are more likely to have atopic disease than those with mild AD [4]. There are several physical signs that may serve as clinical clues of atopic disease, such as Dennie-Morgan lines that are horizontal skin folds under the lower eyelids; transverse nasal crease or “allergic salute” from rubbing of an itchy nose in hay fever, and “allergic shiners” that result from darkening of the periorbital areas secondary to edema in hay fever are common [47].
Several other dermatologic findings are associated with AD. In order of frequency, from highest to lowest, hyperlinear palms, keratosis pilaris, pytiriasis alba (hypopigmented, scaly patches, commonly occurring on the cheeks but can occur at virtually any body site), and white dermatographism also frequently affect patients with AD [33] (Fig. 29.3).
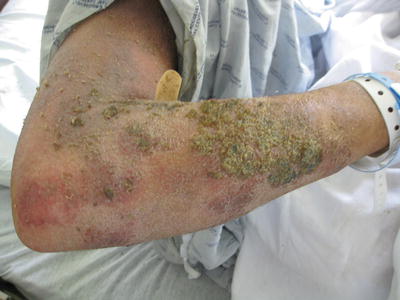

Fig. 29.3
Superinfected atopic dermatitis with oozing and crusting
A lower irritant threshold is also associated with AD and it is hypothesized that this is due to intrinsically hyperreactive inflammatory cells [48]. A filaggrin deficient animal model provides further support for the notion that a defective epidermal barrier reduces the inflammatory threshold to common topical irritants [49]. Irritant reaction to various consumer items is also common in AD; many patients, especially women, have reported adverse skin reactions to scented products in several studies [50, 51].
Complications
According to Hanifin and Rajka’s list of minor diagnostic criteria, AD patients have increased risk of skin infections in general. Staphylococcal (S.) aureus has been shown to colonize the skin of most patients with AD [52]. It is thought that super-antigens released by S. aureus contribute to AD flares and perpetuate active lesions [53]. Alternatively, inflammation in AD may predispose toward greater colonization by S. aureus. Impaired epidermal barrier and decreased naturally occurring antimicrobial peptides may predispose AD patients to more infections [54]. The rate of bacterial or viral infections found in an inpatient study was approximately 30 % for AD patients, in contrast to 6.7 % of those with psoriasis [55]. Interestingly, children with AD were also found to have increased rates of extra-cutaneous infections, including Streptococcal pharyngitis, colds and sinus infections, urinary tract infections, and recurrent otitis media, among others. These findings suggest that an element of immune dysfunction may contribute to the pathogenesis of AD and an overall increased susceptibility to infection [56]. There is some controversy as to whether AD patients have an increased risk of infection with methicillin-resistant S. aureus (MRSA) versus methicillin-sensitive S. aureus (MSSA) strains. It has been suggested that AD patients may be more susceptible to MRSA infection since anti-Staphylococcal antibiotics are often a part of their long-term treatment regimen [57]. Several recent studies found a low rate of MRSA in AD patients [58] and determined that children with AD were actually less likely to be infected with community acquired MRSA than the general outpatient pediatric population [59]. On the other hand, the prevalence of MRSA in AD children colonized with Staphylococcal species was determined to be between 18.3 and 30.8 % in other studies [60, 61] and it was concluded that this could present a significant reservoir of MRSA in the population [60].
Herpes simplex virus may trigger eczema herpeticum, widely disseminated viral super-infection of eczematous skin. Classically, monomorphic, umbilicated vesicles form especially on the head, neck, and trunk. These vesicles later evolve into “punched out” crusted erosions. It is usually accompanied by fever and lymphadenopathy and may spread to various organs including the eyes and brain if not treated appropriately with systemic antiviral therapy such as acyclovir [62]. Clinicians from one German hospital found a dramatic increase in incidence of this complication between 1969 and 1981 [63]. Infection with molluscum contagiosum can also be widespread with numerous umbilicated papules, though without systemic manifestations [62].
AD has a major impact on patient quality of life. Patients with AD were found to have significantly lower scores measuring quality of life compared to healthy control subjects and the general population [64]. A significantly smaller percentage of pediatric patients with AD in the USA identify as being in overall excellent health compared to those not affected with AD (55.2 % versus 63.4 %) [4]. Pruritus often poses a significant challenge and may be so severe as to cause sleep disturbances, with 10.8 % of US pediatric AD patients reporting at least 4 nights per week of impaired sleep [4]. Repeated sleep deprivation results in daytime fatigue, decreased capacity to complete daily tasks, reduced ability to function at school or work and may impair overall behavioral and psychological development in children. Patients may also suffer from psychological distress and a feeling of social stigma and may experience both functional and social limitations. In a community survey of teenagers and adults with AD, many patients reported limitations in activities of daily living, the most common of which was clothing choice, reported in 35 % of respondents. Limitations in social functioning and self-perception were also reported, with 20–25 % of participants declaring feeling embarrassed or angry about their appearance. In those with severe disease, 18 % often or always felt uncomfortable in group settings and 16 % were less satisfied with personal relationships [65].
Daily treatment regimens require a significant time commitment and may place a burden on home and family life, both financial and emotional. The financial implications of AD encompass direct costs such as out-of-pocket payment for various skin care products, medications, visit copays, and purchase of special clothing, as well as indirect costs, including absences from school or work [66]. A total of approximately $213 is spent per patient every year on emollients and over-the-counter medications [65].
There may also be an association between childhood AD and mental health disorders, including attention deficit (hyperactivity) disorder, depression, anxiety, conduct disorder, and autism [67–72]. Further, more severe AD appears to be associated with even higher prevalences of these disorders.
When the lesions of AD resolve, they can leave pigmentation alterations in their wake. Both hyper and hypopigmentation changes occur. This is especially true in the case of patients of different ethnic and racial groups with naturally darker skin and can be a source of much emotional distress [29]. Pityriasis alba is another pigmentation disorder that may be associated with AD. It is characterized by asymptomatic, hypopigmented, poorly demarcated, patches, usually on the face. Its etiology is somewhat controversial, though studies have linked atopy and xerosis with its pathogenesis [73]. In a clinical survey of 56 Korean patients with pityriasis alba, 18 % were found to have a history of AD [74]. In a histopathological analysis of samples from these patients, a reduced number of melanosomes within keratinocytes and reduced pigment in the epidermis were found. Daily frequency of bathing and water temperature were found to correlate positively with the presence of pityriais alba in a retrospective study, which provided support for the notion that xerosis, as in AD, may be an important aspect of its pathogenesis [75].
With longstanding disease, ocular complications can also arise, including keratoconus, keratoconjunctivitis, uveitis, and anterior and posterior subcapsular cataracts [29]. These complications have been well described in adults, with a frequency in AD between 25 and 50 % [76–78]. The severity of AD positively correlates with the development of ocular complications [79]. Posterior subcapsular cataracts are slightly more common; however, anterior subcapsular cataracts are more specific to AD [80]. Less is known about the prevalence of ocular complications in childhood AD. In a prospective study of 59 children with AD, 23 % of participants had a form of ocular disease associated with AD [81]. The majority of these cases were asymptomatic benign papillofollicular conjunctivitis, but findings also included one case each of bilateral nuclear cataract, purulent bacterial conjunctivitis, and chronic atopic blepharoconjunctivitis. These findings suggest that the occurrence of severe ocular complications of AD is rare in children with moderately severe disease.
Clinical Presentation and Special Considerations for Skin of Color
The classical presentations of AD are essentially the same across various racial and ethnic groups. However, there are a number of distinguishing features that are more characteristic of skin of color and must be considered. First, erythema is not as pronounced on more darkly pigmented skin. Erythema in skin of color often appears violaceous. Second, many patients of Black, African, and/ or Afro-Caribbean descent have papular/follicular eczema and/or lichenoid lesions. Indeed, 54.1 % of patients with AD in a region of southeastern Nigeria were found to have papular lichenoid lesions [82]. This can present an obstacle to a physician making the diagnosis and/ or attempting to assess the severity of AD, as erythema is a characteristic that is included in a number of the popular scoring tools, including SCORAD (Scoring Atopic Dermatitis) and EASI (Eczema Area and Severity Index). This phenomenon could lead to a delay in diagnosis and treatment and, ultimately, more severe disease at presentation [19, 23]. When the lesions of AD resolve, they can cause persistent dyschromia or pigmentary alterations. These are almost always more pronounced in patients of racial and ethnic groups with darker skin [23].
Papular eczema presents with a pattern of small, distinct papules, especially on the trunk, as opposed to the more conventional patches and plaques and can present with severe pruritus. This particular morphology has been observed to be more common in skin of color [82, 83], as is a pattern of perifollicular accentuation [84]. In the Nigerian study, 70.3 % of patients had a scattered, often perifollicular, micropapular rash, on the extensor aspects of the joints. In a series of case reports, investigators identified three children with AD, two of which were African American, who developed prominent, pruritic, follicular papules in the periumbilical area as well as on the flexural areas of the arms and legs [85]. Such a pattern should alert the physician to the diagnosis even if other signs have not yet made their appearance.
Asian patients may present with “sandpaper-like lesions” on their extensor surfaces, wrist dermatitis, and hyperkeratotic papules as accompanying features [86]. African-American children and adolescents often present with lesions on the extensor surfaces of the body as opposed to the more traditional presentation of flexural involvement in this age group [18, 23]. Dennie Morgan lines are more commonly noted in patients of color, even in those without AD [87]. Ichthyosis vulgaris has also been reported to be more common in patients of color [82].
Treatment
The cornerstones of AD therapy are protecting the integrity of the epidermal barrier by using emollients, avoiding triggering factors and implementing gentle skin care
Topical anti-inflammatory agents, including corticosteroids and calcineurin inhibitors, are first-line therapy for AD.
Many cases can be managed with emollients and topical therapy, but systemic anti-inflammatory therapy and other adjunctive treatments may be necessary.
It is important not to undertreat and to initiate appropriately aggressive treatment plans to gain control and maintain remission of disease.
The management of AD requires a multi-tiered approach, which includes managing acute flares, as well as maintenance therapy to prevent the occurrence of future exacerbations. First and foremost, an extremely important aspect of therapy is education. Helping patients and families understand the nature of the disease, explaining the importance of good skin care, and avoidance of irritants and instruction on proper application of medications are all critical in creating a successful partnership, which optimizes the treatment and control of this disorder. Offering emotional support and directing families to helpful resources and support groups can help provide them with the tools they need to deal with the challenges of this condition.
Current treatment places an emphasis on avoiding potential trigger factors, skin hydration, and the repair and protection of the epidermal barrier, the disruption of which seems to play a large role in the pathogenesis of the disease. Mild cases can usually be treated with continuous application of emollients and low potency topical corticosteroids during flares. Moderate to severe cases require higher potency corticosteroids and other anti-inflammatory medications and, possibly, other adjunctive therapies. Usage of twice-weekly corticosteroids can be helpful in the maintenance of clear skin for children with frequent flares [88]. A summary of available treatments is outlined in Table 29.3.
Table 29.3
Treatments for atopic dermatitis
Lifestyle modification: Limit bathing time to 15 min Do not use very hot water for bathing Use fragrance-free, gentle cleansers Avoid known allergens Avoid overheating Use of humidifiers Use mild clothes detergents Wear soft, itch-free fabrics Manage stress |
Skin hydration and epidermal barrier repair: Regular application of emollients, especially ointments or thick creams Application immediately after bathing |
Anti-inflammatory therapy: • Topical Anti-inflammatory Therapy – Topical Corticosteroids Low potency for mild cases, face, and intertriginous areas Mid-high potency for moderate to severe/refractory cases Application immediately after bathing, before emollients Wet wrap technique for enhanced effect – Topical Calcineurin Inhibitors For topical corticosteroid failure and for face and intertriginous areas Pimecrolimus 1 % cream for mild-moderate disease, ages 2 and over Tacrolimus 0.03 % ointment for moderate-severe disease in children over 2 Tacrolimus 0.1 % ointment for moderate-severe disease in adults • Systemic Anti-inflammatory Therapy – Systemic Corticosteroids Short course for severe case refractory to topical therapy |
Pruritis control: Oral antihistamines at bedtime Ice Moisturizers with menthol, phenol, or topical anesthetic |
Antimicrobial therapy : • Decolonization – Bleach baths – Intranasal mupirocin • Treatment of Superinfection – Topical/oral antibiotic treatment |
Other therapies: For severe, refractory cases • Phototherapy – Narrowband-UVB for chronic, moderate disease – UVA1 for acute exacerbations and severe disease • Systemic Immunomodulatory Therapy – Cyclosporine – Azathioprine – Mycophenolate Mofetil – Methotrexate • Targeted Molecular Therapy – Omalizumab – Rituximab – Mepolizumab |
Food allergy testing Food allergy workup in a selective subset of patients Food allergen avoidance if workup is positive |
Alternative therapy: Chinese herbal therapy Dietary lipid supplements |
Avoidance of Trigger Factors
There are numerous factors that can potentially exacerbate AD and these should be avoided in patients as much as possible. Excessive bathing with very hot water, low indoor humidity, and perspiration can all cause decreased skin hydration and contribute to disruption of the epidermal barrier [89]. Indoor humidity drops precipitously with prolonged use of indoor heating. Thus, patients can be counseled to lower the thermostat by a few degrees and open the window to allow naturally humid outdoor airflow. Some patients may benefit from using a cool-mist humidifier; however, evidence is lacking for their effectiveness [90]. Individuals with AD have a lower threshold for itch and may be sensitive to overheating, wool or other rough fabrics, and harsh soaps and detergents; other known allergens should be avoided as well [91]. The role of aeroallergens such as dust mites have not been clearly defined as yet [91]. Emotional distress has also been shown to play a role in disease exacerbation [89]. Racial and ethnic differences in relation to various trigger factors have not been described.
Maintaining Skin Hydration
Continuous skin hydration with emollients is a crucial component of therapy for AD as it helps restore essential lipids in the skin and contributes to the integrity of the epidermal barrier. Hydration is best accomplished via ointments, which have high lipid content, applied immediately after a lukewarm bath or shower to seal in moisture (“soak and seal” method). However, patient preference and compliance must be taken into account. Some patients may dislike the greasy feel of ointments and prefer thick creams with a low water content. This can be a viable option, especially in those with milder disease. Lotions have a high water content and can exacerbate xerosis by evaporation from the surface of the skin and are generally discouraged. Products with added ceramides or natural moisturizing factor ingredients may replete deficient barrier elements for some children of color. Emollients should be applied twice daily, covering the entire surface area of the body. Scented varieties or those containing potentially irritating ingredients should be avoided [91]. Topical medications should be applied before emollients.
Anti-inflammatory Therapy
Topical Anti-inflammatory Therapy
Topical corticosteroids are the mainstay of AD therapy, especially for acute flares. These agents reduce inflammation by suppressing the expression of certain transcription factors and pro-inflammatory cytokines and act on a number of immune cells [92]. The goal of therapy is daily use of an agent potent enough to bring about resolution of the flare and, possibly, subsequent tapering of the medication to every other day use until maintenance therapy with intermittent topical corticosteroids is initiated. Low potency corticosteroids such as hydrocortisone 1–2.5 % cream or ointment are appropriate for infants and children with mild disease. Medium potency ointments, such as fluocinolone 0.025 %, fluticasone proprionate 0.005 %, or triamcinolone 0.1 % are commonly used for more moderate to severe disease. For refractory cases, higher potency ointments may be used for a brief period of time with a transition to lower potency options when the worst of the flare has calmed. High potency ointments are particularly useful in areas with thick, lichenified plaques and for lesions on the palms and soles. Use of these agents, especially long-term use, is generally avoided in treating the face and skin folds, because of the potential side effects, which includes skin atrophy [91]. Maintenance therapy for up to approximately 16 weeks after a flare, with intermittent use of topical corticosteroids, may help to reduce relapses [93]. The ointment vehicle of these agents acts as its own emollient, and, like other emollients used for basic, daily skin care, should be applied immediately after bathing to enhance skin hydration.
Wet wrap therapy may be a useful technique to incorporate for acute flares, especially in cases of severe or refractory AD [91]. With this approach, topical medication and emollients are applied to the patient’s skin. A layer of damp material (gauze, cotton pajamas, etc. soaked in warm water) is then wrapped around the affected area, immediately followed by another layer of dry material. The wraps are left on overnight and may be used with once-daily topical treatment for several days. This form of treatment is beneficial in that it enhances skin hydration and acts as a physical barrier to scratching. It also serves as an occlusive dressing which allows more effective penetration of topical medications into affected skin [91].
Two topical calcineurin inhibitors are currently available as effective second-line treatment options for AD in patients over age 2 [94]. Tacrolimus 0.03 % and 0.1 % ointment are FDA approved for moderate to severe disease. Pimecrolimus 1 % cream is often used for mild to moderate disease. These agents block the pro-inflammatory downstream effects of calcineurin, which activates T-cells by upregulating expression of interleukin-2. Calcineurin inhibitors do not result in cutaneous atrophy as may occur with topical corticosteroids, and thus, are ideal for use on the face and intertriginous areas. They may also be used in individuals with frequent exacerbations who would otherwise need constant steroid treatment [91]. There is data in the literature to indicate that pimecrolimus is equally efficacious in all races and ethnicities for mild to moderate disease [95]; however, tacrolimus may be more effective at the 0.1 % concentration for children of color [96]. Their major side effect is a burning sensation at the application site, which is more pronounced with tacrolimus and may subside with ongoing use. The burning sensation is often worse when tacrolimus is applied on wet skin, which precludes it being used in a “soak and smear” approach. Another, theoretical side effect is increased risk of malignancy for which the FDA has placed a black-box warning, although this matter is highly controversial and has not been well elucidated.
Systemic Anti-inflammatory Therapy
Occasionally, systemic corticosteroids may be used in a case of severe disease which is unresponsive to appropriate treatment with topical therapy. A short course may be implemented with careful monitoring for potential side effects. The patient should be switched to topical therapy or other systemic therapies that are more suitable for long-term use as soon as possible to avoid these side effects as well as rebound flares [97]. In our experience, it is common for patients with chronic moderate to severe AD to rapidly flare upon tapering and discontinuation of prednisone and other oral corticosteroids. Oral corticosteroid therapy, therefore, may become addictive and should be used with caution.
Pruritus Control
Adjunctive pharmacotherapy to treat pruritus may be important in controlling the itch-scratch cycle of AD. This is usually accomplished with sedating antihistamines, given at bedtime, which are particularly useful in patients who suffer from difficulty falling asleep or have poor sleep efficiency due to itchiness [98]. Applying ice or topical agents that contain menthol, phenol, antihistamines, pramoxine, or topical anesthetics may provide some relief. However, some of these topical agents work via the creation of distracting sensations, which ultimately may enhance irritation [30]. There is currently only modest objective evidence demonstrating efficacy of these agents [99]. Currently, the most effective treatments for itch in AD are topical or systemic anti-inflammatory agents [100].
Antimicrobial Therapy
The preponderance of individuals with AD are colonized with Staphylococcus aureus and bacterial counts increase with disease flares [101–103]. Bleach baths have been developed to treat prophylactically with the goal of eliminating Staph colonization. One cup of standard household bleach is diluted in a lukewarm bath with 40 gallons of water twice weekly. This adjunctive therapy resulted in improved symptoms on the trunk and extremities [104, 105]. However, the bleach may provoke itching or burning of the skin, especially in those with open cuts, excoriations, or fissures. Thus, bleach baths may be better suited for maintenance therapy rather than for acute AD flares. Topical mupirocin, applied intranasally, is widely used to decolonize the nose, particularly in those with a propensity for impetigenization. However, combination topical mupirocin and corticosteroids offered no advantage over topical corticosteroid monotherapy [106]. Therefore, use of mupirocin for the treatment of AD per se is not recommended. In cases of bacterial superinfection, appropriate topical or oral antibiotics are warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


