Assisted Reproductive Technologies
 |
Assisted reproductive technologies (ART) encompass all techniques involving direct manipulation of oocytes outside of the body. The first and still most common form of ART is in vitro fertilization (IVF), but other related techniques also reside within the realm of ART. The success of modern ART has completely revolutionized both the evaluation and treatment of infertility. Some traditional diagnostic methods and treatments have been rendered obsolete and others have only limited applications because ART is simply more effective. The trend is clear and certain to continue.
IVF involves a sequence of highly coordinated steps beginning with controlled ovarian hyperstimulation with exogenous gonadotropins, followed by retrieval of oocytes from the ovaries under the guidance of transvaginal ultrasonography, fertilization in the laboratory, and transcervical transfer of embryos into the uterus. The first pregnancy resulting from IVF was reported in 1976, and was ectopic.1 The first child resulting from IVF was born in 1978.2 Over the more than 30 years since, ART has been greatly refined and expanded, resulted in millions of births worldwide, and now accounts for 1-3% of all births in the U.S. and Europe. ART includes methods for assisted fertilization by intracytoplasmic sperm injection (ICSI) using sperm isolated from the ejaculate or obtained by microsurgical epididymal sperm aspiration (MESA) or testicular sperm extraction (TESE), assisted embryo hatching, and preimplantation genetic diagnosis (PGD). In most cases, IVF is used to help an infertile couple conceive their own biological child, but donor sperm, donor oocytes, and gestational surrogates also play an important role in modern ART.
Other forms of ART include tubal transfer of oocytes and sperm (gamete intrafallopian transfer; GIFT), zygotes (zygote intrafallopian transfer; ZIFT), or embryos (tubal embryo transfer; TET) via laparoscopy. Whereas these more invasive techniques once had certain advantages over traditional IVF for some infertile couples, they now have only very limited indications.
A truly comprehensive discussion of ART is well beyond the scope of any single book chapter. The objective here is to provide an overview of the indications for ART, the most common methods for ovarian stimulation, oocyte retrieval, sperm recovery, fertilization, and gamete/embryo transfer, and the results and complications of ART, with emphasis on newly developing technologies and areas of controversy.
Indications for IVF
IVF was first developed as a method to overcome infertility resulting from irreparable tubal disease, but now is applied much more broadly for the treatment of almost all causes of infertility. IVF is most clearly indicated when infertility results from one or more causes having no other effective treatment; severe tubal disease relating to previous infection or advanced endometriosis and severe male factor infertility are the most obvious examples. IVF also is often the best treatment for couples with multifactor infertility because it can address or overcome all contributing causes at the same time. IVF is a legitimate treatment option for women with age-related or otherwise unexplained infertility and represents the treatment of last resort when other treatments fail.
In women with premature ovarian failure or reproductive aging and healthy women beyond normal reproductive age, IVF using oocytes from a young donor is highly successful. For women with normal ovaries but no functional uterus (müllerian agenesis, severe intrauterine adhesions, previous hysterectomy) and those with medical disorders that preclude pregnancy due to serious health risks, IVF with embryo transfer to a gestational surrogate still offers the possibility of genetic offspring. In couples who carry autosomal or sex-linked genetic disorders or balanced chromosomal translocations, IVF with preimplantation genetic diagnosis can avoid the risk of delivering an affected child.
Tubal Factor Infertility
Before the advent of IVF, women with irreparable bilateral tubal obstruction were essentially sterile, and the prognosis for those with less severe distal disease was only fair. In the modern era of ART, surgical treatments are declining in importance and the prognosis for women with tubal factor infertility has improved dramatically. Approximately 9% of patients using ART have a primary diagnosis of tubal factor infertility.3 The relative advantages and disadvantages of surgery and IVF for the treatment of tubal factor infertility and the factors bearing on a choice between the two are discussed in depth in Chapter 27 and summarized here.
Reconstructive surgery remains a viable option for young women with mild distal tubal obstruction or peritubular adhesions (because postoperative live birth rates can exceed 50%),4,5 and 6 but IVF is the treatment of choice for women with severe distal disease. Results achieved with surgery have varied, but success rates (10-35%) are generally lower than with IVF and the risk of ectopic pregnancy is higher (5-20%).3,7,8,9 and 10 In 2007, the overall IVF live birth rate (per cycle start) for U.S. women with tubal factor infertility (all ages) was 30.7%.3 IVF is also the best treatment for women who remain infertile for more than a year after tubal surgery (the likelihood for success diminishes progressively with time after operation), for older women with significant distal tubal disease (cycle fecundity is low after distal tubal surgery and time is limited), and for women with recurrent distal tubal obstruction (repeated attempts to correct distal tubal occlusive disease are rarely successful).
Although not candidates for reconstructive surgery, women with severe distal tubal disease still can benefit from surgery before IVF. A substantial body of evidence indicates that communicating hydrosalpinges (proximal patency and distal occlusion) decrease the probability of both pregnancy and live birth after IVF by approximately one-half. The mechanism for the adverse effect of hydrosalpinges on IVF outcomes could involve mechanical interference with implantation or toxic effects on the embryo or endometrium.11,12,13,14 and 15 A 2010 systematic review including five randomized trials involving 646 women observed that the odds of achieving an ongoing pregnancy were twice as great after laparoscopic salpingectomy for hydrosalpinges before IVF (OR=2.14, CI=1.23-3.73).16 Laparoscopic proximal occlusion of the tubes also increased the odds of clinical pregnancy, compared to no intervention (OR=4.66, CI=2.47-10.01), and neither surgical procedure was superior.16 Other treatments have been suggested, such as ultrasound-guided aspiration of hydrosalpinges after oocyte retrieval,17 but are unproven, and evidence suggests the fluid re-accumulates rapidly.18
Proximal tubal occlusion observed during hysterosalpingography (HSG) often is not real and results from “cornual spasm” or other technical pitfalls of the procedure (Chapter 27). Efforts to confirm the diagnosis are justified; otherwise many women may needlessly undergo IVF. Common methods include repeated HSG19 and laparoscopic “chromotubation.”20,21 and 22 Fluoroscopic or hysteroscopic selective tubal cannulation both establish the diagnosis and provide the means for successful treatment.19,20,23,24,25,26 and 27 Micro-surgical segmental resection and anastomosis is another proven treatment for true proximal tubal obstruction,28,29,30 and 31 but requires uncommon technical expertise. IVF is the obvious alternative when cannulation is contraindicated (salpingitis isthmica nodosa) or technically unsuccessful, and when infertility persists for more than 6-12 months after the procedure.
Approximately 1 million U.S. women have an elective tubal sterilization procedure each year; up to 7% regret the decision and about 1% later request its reversal.32,33 The most commonly cited reasons for regret include new relationships, changes in family planning goals, and death of a child. Regrets are more common in younger women, those who were unaware of the spectrum of contraceptive options, women whose decision for sterilization was influenced by a third-party (partner, other family member, friend, or physician), and in those sterilized postpartum or after an abortion.34,35 Women 30 years old or younger are twice as likely as older women to express regret, 3.5 to 18 times more likely to request information about reversal of the procedure, and approximately eight times more likely to have a sterilization reversal or IVF.36
Young women sterilized using rings or clips and women having no other infertility factors have the best surgical prognosis; success rates are lower for older women, those sterilized by cautery (particularly multiple-burn techniques), and women with other infertility factors.37,38,39,40,41,42,43 and 44 Although conception rates are quite good (45-82%) after microsurgical tubal anastomosis in properly selected candidates, IVF is a legitimate alternative to surgery, particularly for older women, those with a poor surgical prognosis or preferring to avoid surgery, and women who desire only one additional pregnancy.
Endometriosis
The association between endometriosis and infertility and the pathogenic mechanisms involved are considered at length in Chapter 29. In brief summary, 20-40% of infertile women have endometriosis and accumulated evidence indicates that fertility decreases with the severity of the disease. Endometriosis may cause infertility by distorting adnexal anatomy and interfering with ovum capture,45 or possibly by impairing oocyte development, early embryogenesis, or endometrial receptivity.46,47,48,49 and 50 IVF should be expected to
overcome any anatomical obstacles, and although it would seem less likely to conquer functional disorders of oocyte, embryo, or endometrial development, outcomes in women with endometriosis suggest it can. Endometriosis is the primary diagnosis in approximately 5% of patients using ART.3
overcome any anatomical obstacles, and although it would seem less likely to conquer functional disorders of oocyte, embryo, or endometrial development, outcomes in women with endometriosis suggest it can. Endometriosis is the primary diagnosis in approximately 5% of patients using ART.3
Treatment options for infertile women with advanced stages of endometriosis include conservative surgical treatment and IVF. For those with severe symptoms, surgery is the most logical treatment. Data from case series suggest that cumulative pregnancy rates 1-3 years after surgical treatment are approximately 50% for women with endometriomas,51,52,53 and 54 and about 30% for women with complete cul-de-sac obliteration.51,55 Careful surgical technique is important because ovarian function can be compromised by excision of excessive tissue or damage to hilar vessels56; the risk of ovarian failure after excision of bilateral ovarian endometriomas is approximately 2.5%.57 After surgical treatment, the choice between expectant management, empirical treatment, and IVF should be based on age, the surgical results, and the severity of any other coexisting infertility factors.
Asymptomatic infertile women with advanced endometriosis, including those with ovarian endometriomas, can be treated surgically or proceed directly to IVF. There is no evidence to indicate that endometriomas have any important adverse effect on the response to ovarian stimulation or IVF outcomes.58,59,60,61,62,63,64 and 65 Consequently, endometriomas can be left untreated before IVF. Aspiration of endometriomas before ovarian stimulation or at time of oocyte retrieval has been associated with an increased risk for developing an ovarian abscess,66,67 and 68 although the risk appears quite low.69
Treatment options for asymptomatic women with known or suspected minimal or mild endometriosis and no other infertility factors include expectant management, surgical treatment, empiric treatment with clomiphene or exogenous gonadotropins and IUI, and IVF. In older women, those with other coexisting infertility factors, and women who have failed other forms of treatment, IVF is often the best overall choice.
Results of a 2006 systematic review including three randomized trials involving 165 infertile women with endometriosis of varying severity suggest that treatment with a GnRH agonist for 3-6 months before IVF can increase the odds of clinical pregnancy (OR=4.28, CI=2.0-9.15).70 However, because prolonged treatment with a GnRH agonist also can decrease response to ovarian stimulation, most clinicians do not favor suppressive treatment before IVF.
Male Factor Infertility
Poor semen quality is the sole cause of infertility in approximately 20% of infertile couples and an important contributing factor in another 20-40% of couples with reproductive failure.71,72 Many infertile men have disorders than can be corrected medically or surgically if properly diagnosed and treated, allowing them to achieve natural conception with their partners.72 In others, mild but important semen abnormalities can be overcome by IUI.
When treatment is not possible or fails, and insemination with donor sperm is not an acceptable option, IVF and ICSI, using sperm isolated from the ejaculate or extracted from the epididymis or testis, offers realistic hope for success. The evaluation and treatment of male factor infertility are the focus of Chapter 30. Discussion here is limited to the indications for ART.72
The likelihood of male factor infertility is increased in men whose ejaculates consistently exhibit a sperm concentration under 15 million sperm/mL, less than 32% progressive motility, or fewer than 4% morphologically normal sperm (strict criteria, WHO III standard).73
The overall odds of male infertility increase with the number of abnormal parameters in the subfertile range; the probability is two to three times higher when one is abnormal, five to seven times higher when two are abnormal, and approximately 16 times greater when all three parameters are abnormal.74 Additional genetic evaluation is indicated for men with severe oligospermia (sperm concentration <5 million/mL) whose sperm may be used for ICSI (Chapter 30).
The overall odds of male infertility increase with the number of abnormal parameters in the subfertile range; the probability is two to three times higher when one is abnormal, five to seven times higher when two are abnormal, and approximately 16 times greater when all three parameters are abnormal.74 Additional genetic evaluation is indicated for men with severe oligospermia (sperm concentration <5 million/mL) whose sperm may be used for ICSI (Chapter 30).
Medical or surgical treatment to improve or normalize poor semen quality is always the first and best option, when that is possible. When treatment is not feasible or proves unsuccessful, timely IUI can help to improve cycle fecundity in some couples with male factor infertility.
Best results with IUI are achieved when the number of total motile sperm in the insemination specimen exceeds a threshold of approximately 10 million75,76 and 77 and 14% or more of sperm have normal morphology (strict criteria; WHO III standard).78 Higher counts do not further increase the likelihood for success75,79 and IUI is seldom successful when fewer than 1 million total motile sperm are inseminated.80,81 Success rates with IUI are best when 14% or more of sperm have normal morphology (strict criteria), intermediate with values between 4% and 14%, and generally quite poor when fewer than 4% of sperm are normal.78 The likelihood of success with IUI also decreases with increasing female partner age and with coexisting infertility factors (ovulatory dysfunction, uterine and tubal factors).
When IUI is not possible, the prognosis for success with IUI is poor, or IUI proves unsuccessful and therapeutic donor insemination is rejected, IVF is the logical alternative. Approximately 18% of patients using ART have a primary diagnosis of male factor infertility.3
Conventional fertilization rates in IVF cycles are decreased when the total motile sperm count is less than 2-3 million (post-wash).82 Numerous studies have observed that conventional fertilization rates also are decreased when less than 4% of sperm are morphologically normal.83,84,85,86 and 87 Although severe teratospermia is widely accepted as an indication for assisted fertilization by ICSI, some observing no differences in fertilization, pregnancy, and live birth rates achieved with ICSI, compared with conventional fertilization, do not regard isolated teratospermia as an indication for ICSI.88,89,90 and 91
Ovulatory Dysfunction
For women with ovulatory disorders (hypogonadotropic hypogonadism, polycystic ovary syndrome, thyroid disorders, hyperprolactinemia), ovulation induction alone generally restores fertility (Chapter 31), but for some who require exogenous gonadotropins, ovulation induction proves difficult to achieve or consistently results in excessive ovarian stimulation and cycle cancellation for undue risk of ovarian hyperstimulation syndrome (OHSS) and high-order multiple gestation. For these difficult patients, IVF is an obvious treatment alternative, making their high sensitivity to gonadotropin stimulation an asset instead of a liability. Ovulatory dysfunction is the primary diagnosis in approximately 7% of patients using ART.3
Unexplained Infertility
The incidence of unexplained infertility ranges from 10% to as high as 30% among infertile populations, depending on diagnostic criteria.92,93 and 94 For women with unexplained infertility, treatment options (cycle fecundability in parentheses) include expectant management (2-4%),95 IUI (2-4%),96,97 empiric treatment with clomiphene (2-4%)96,98 or exogenous
gonadotropins (5-7%),99 combined treatment with IUI and clomiphene (5-10%)100,101 and 102 or gonadotropins (7-10%),99,100,103 and IVF (25-45%).3,104 As might be expected, success rates with all forms of treatment decline progressively with increasing age of the female partner.
gonadotropins (5-7%),99 combined treatment with IUI and clomiphene (5-10%)100,101 and 102 or gonadotropins (7-10%),99,100,103 and IVF (25-45%).3,104 As might be expected, success rates with all forms of treatment decline progressively with increasing age of the female partner.
Among couples with unexplained infertility, IVF is the preferred treatment for some and the treatment of last resort for others. In either case, there is no question that IVF is the most effective treatment for couples with unexplained infertility. A higher incidence of fertilization failure has been observed in several, but not all, studies of IVF outcomes in couples with unexplained infertility,105,106,107 and 108 prompting many to recommend ICSI when IVF is planned. Approximately 12% of patients using ART have a diagnosis of unexplained infertility.3
Ovarian Failure and Diminished Ovarian Reserve
IVF using oocytes from a known or anonymous young donor was first developed for women with premature ovarian failure or menopause.109 Now, oocyte donation is most commonly performed in women over age 42, those with grossly abnormal ovarian reserve test results, and women whose IVF cycles consistently yield poor quality embryos (Chapter 27). Approximately 10% of patients using ART have a primary diagnosis of diminished ovarian reserve.3
 |
Other Indications for IVF and Related Technologies
Although less commonly encountered, there are a number of other legitimate indications for IVF and related ART procedures.
Fertility preservation is fast becoming a more common indication for ART. Women with cancer or other illnesses requiring treatments (chemotherapy, radiation therapy) that pose a serious threat to future fertility may be candidates for urgent IVF and cryopreservation of
embryos before treatment begins, if time and health allow.110 Oocyte cryopreservation is a viable option for women in similar circumstances having no male partner,111 and is rapidly emerging as an option for young women at risk for premature ovarian failure,112,113 healthy aging women, and others who anticipate delayed childbearing.111,112,114,115
embryos before treatment begins, if time and health allow.110 Oocyte cryopreservation is a viable option for women in similar circumstances having no male partner,111 and is rapidly emerging as an option for young women at risk for premature ovarian failure,112,113 healthy aging women, and others who anticipate delayed childbearing.111,112,114,115
For women with normal ovaries but no functional uterus, due to a developmental anomaly (müllerian agenesis), advanced disease (multiple myomas, severe intrauterine adhesions), or a previous hysterectomy, and for women with medical conditions that preclude pregnancy due to serious health risks, gestational surrogacy offers the opportunity to have their own genetic offspring.116,117
For couples at risk for transmitting a specific genetic disease or abnormality to their offspring, IVF with preimplantation genetic diagnosis (PGD) provides the means to identify and exclude affected embryos and thereby avoid that risk. PGD is applied most commonly in couples who carry autosomal recessive and sex-linked disorders or harbor a balanced chromosomal translocation.118 Women who carry a genetic disorder not amenable to diagnosis by PGD or who decline PGD may be candidates for oocyte donation. Preimplantation genetic screening (PGS) applies the same technology in couples having no known chromosomal or genetic abnormality in efforts to identify and exclude aneuploid embryos where the risk is increased, as in older women, those with a history of recurrent miscarriage, and in women with repeated unexplained IVF failure.118 Although the technical limitations of current methods for PGS have so far prevented the technology from improving live birth rates in at-risk couples, more sophisticated and reliable methods now emerging hold promise.119
Prognostic Factors
The probability for success with IVF relates to several factors, many of which are unfortunately not known until the treatment cycle is well underway (response to stimulation) or even nearing completion (number and quality of embryos). Before an IVF cycle begins, the primary prognostic indicators are maternal age, ovarian reserve, diagnosis, and past reproductive performance.
Maternal Age
The relationship between maternal age and fertility and the physiology of reproductive aging are discussed in detail in Chapters 27 and 28 and only briefly summarized again here, where the focus is on the relationship between maternal age and IVF outcomes.
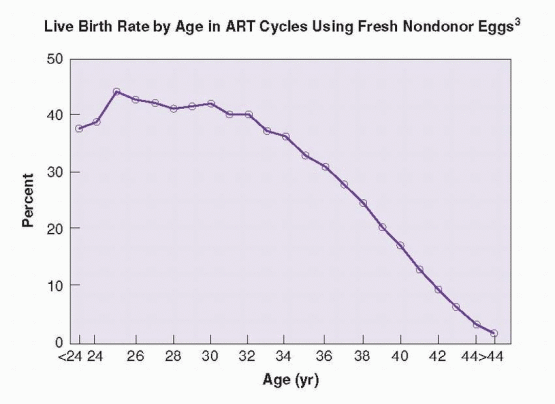
The average age of women using ART in the U.S. is 36 years.3 Maternal age is the one most important factor in determining the likelihood for success with IVF. Although IVF can overcome most causes of infertility in younger women, it cannot negate or reverse the age-related decrease in biologic fertility in older women, particularly those over the age of 40.120 The success rates achieved with IVF, like natural fertility rates, decline as maternal age increases. The pattern reflects a progressive decline in response to ovarian stimulation, resulting in fewer oocytes and embryos, and a decreased embryo implantation rate, due to declining oocyte quality.121,122,123 and 124 In 2007, the percentage of cycles that resulted in a
live birth from fresh nondonor oocytes, by maternal age, was 39.6% for women under age 35, 30.5% for ages 35-37, 20.9% for ages 38-40 yr, 11.5% for ages 41-42, and 5.4% for ages 43-44 years.3 The pattern of decreasing success rates achieved with IVF parallels that associated with other, less complex, forms of treatment for infertility.125
live birth from fresh nondonor oocytes, by maternal age, was 39.6% for women under age 35, 30.5% for ages 35-37, 20.9% for ages 38-40 yr, 11.5% for ages 41-42, and 5.4% for ages 43-44 years.3 The pattern of decreasing success rates achieved with IVF parallels that associated with other, less complex, forms of treatment for infertility.125
 |
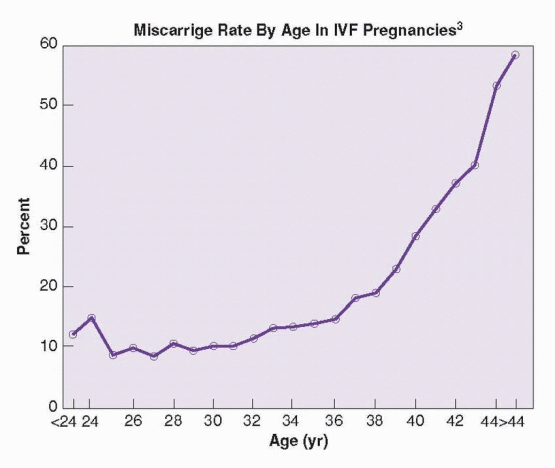
Evidence from numerous lines of investigation indicates that the age-dependent decrease in success rates achieved with IVF relates primarily to an increasing prevalence of aneuploidy in aging oocytes,126,127 and 128 which is reflected in the incidence of miscarriage in pregnancies achieved with ART: less than 14% for women under age 35, 19% at age 38, 28% at age 40, and nearly 60% for women over age 44.3 In a case series of IVF cycles involving women ages 45-49, 70/231 cycles (30%) were cancelled before oocyte retrieval and 34/161 retrievals (21%) resulted in a pregnancy, but only 5/34 pregnancies (15%) and 5/231 cycles (2%) resulted in a live birth.129
 |
Ovarian Reserve
The concept of ovarian reserve, generally defined as the size and quality of the remaining ovarian follicular pool, and the various methods for its measurement are discussed in detail in Chapter 27. In brief summary, the total number of oocytes in any given women is genetically determined and inexorably declines throughout life, from approximately 1-2 million at birth, to about 300,000 at puberty, 25,000 at age 40, and fewer than 1,000 at menopause.128,130,131 and 132 The rate of follicular depletion is not constant, but increases gradually as the number of follicles remaining decreases.133,134,135 and 136 As the size of the remaining follicular pool decreases, circulating inhibin B levels (derived primarily from smaller antral follicles) decrease, resulting in lower levels of feedback inhibition and a progressive increase in serum follicle-stimulating hormone (FSH) levels, most noticeably during the early follicular phase.137,138,139,140,141,142,143,144 and 145 Increasing inter-cycle FSH concentrations stimulate earlier follicular recruitment, resulting in advanced follicular development early in the cycle, an earlier rise in serum estradiol levels, a shorter follicular phase, and decreasing overall cycle length.146,147 and 148
The physiology of reproductive aging provides the foundation for all contemporary tests of ovarian reserve. In clinical practice, the basal early follicular phase (cycle day 2-4) FSH level is the most common test, but antimüllerian hormone (AMH) and antral follicle count are alternatives having significant potential advantages.
As basal FSH levels increase, peak estradiol levels during stimulation, the number of oocytes retrieved, and the probability for pregnancy or live birth decline steadily.149,150,151,152,153,154 and 155
With current assays (using IRP 78/549), FSH levels greater than 10 IU/L (10-20 IU/L) have high specificity (80-100%) for predicting poor response to stimulation, but their sensitivity for identifying such women is generally low (10-30%) and decreases with the threshold value.156 Although most women who are tested have a normal result, including those with a diminished ovarian reserve (DOR), the test is still useful because those with abnormal results are very likely to have DOR. In a 2008 study, an FSH concentration above 18 IU/L had 100% specificity for failure to achieve a live birth.157
The basal serum estradiol concentration, by itself, has little value as an ovarian reserve test,158,159,160 and 161 but can provide additional information that helps in the interpretation of the basal FSH level. An early elevation in serum estradiol reflects advanced follicular development and early selection of a dominant follicle (as classically observed in women with advanced reproductive aging), and will suppress FSH concentrations, thereby possibly masking an otherwise obviously high FSH level indicating DOR. When the basal FSH is normal and the estradiol concentration is elevated (>60-80 pg/mL), the likelihood of poor response to stimulation is increased and the chance for pregnancy is decreased.162,163,164 and 165 When both FSH and estradiol are elevated, ovarian response to stimulation is likely to be very poor.
Antimüllerian hormone (AMH) derives from preantral and small antral follicles. Levels are gonadotropin-independent and vary little within and between cycles.166,167 and 168 The number of small antral follicles correlates with the size of the residual follicular pool and AMH levels decline progressively with age, becoming undetectable near the menopause.169,170,171 and 172
Overall, lower AMH levels have been associated with poor response to ovarian stimulation and low oocyte yield, embryo quality, and pregnancy rates,173,174,175,176 and 177 but studies correlating mean AMH levels with IVF outcomes have not yielded threshold values that can be applied confidently in clinical care.158,174,175,178 In the general IVF population, low AMH threshold values (0.2-0.7 ng/mL) have had 40-97% sensitivity, 78-92% speci-ficity, 22-88% positive predictive value (PPV) and 97-100% negative predictive value (NPV) for predicting poor response to stimulation (<3 follicles, or <2-4 oocytes), but
have proven neither sensitive nor specific for predicting pregnancy.173,179,180 and 181 In women at low risk for DOR, values of 2.5-2.7 ng/mL have had 83% sensitivity, 82% specificity, 67-77% PPV, and 61-87% NPV for clinical pregnancy.159,182 A study in women at high risk for DOR (involving older women, those with an elevated FSH, or history of poor response to stimulation) observed that an undetectable AMH had 76% sensitivity, 88% specificity, 68% PPV, and 92% NPV for three or fewer follicles.174 A higher threshold value (1.25 ng/mL) had 85% sensitivity, 63% specificity, 41% PPV, and 57% NPV for cycle cancellation.160
have proven neither sensitive nor specific for predicting pregnancy.173,179,180 and 181 In women at low risk for DOR, values of 2.5-2.7 ng/mL have had 83% sensitivity, 82% specificity, 67-77% PPV, and 61-87% NPV for clinical pregnancy.159,182 A study in women at high risk for DOR (involving older women, those with an elevated FSH, or history of poor response to stimulation) observed that an undetectable AMH had 76% sensitivity, 88% specificity, 68% PPV, and 92% NPV for three or fewer follicles.174 A higher threshold value (1.25 ng/mL) had 85% sensitivity, 63% specificity, 41% PPV, and 57% NPV for cycle cancellation.160
The antral follicle count (AFC) is the total number of antral follicles measuring 2-10 mm in both ovaries during the early follicular phase and is a useful measure of ovarian reserve because it quantifies the number of follicles at the stage of development that responds to FSH stimulation.183,184,185,186 and 187
Several studies have observed a relationship between the AFC and response to ovarian stimulation in IVF cycles. In the general IVF population, including women at low and high risk for DOR, an AFC threshold value of three to four follicles has high specificity (73-100%) for predicting poor response to ovarian stimulation and failure to conceive (64-100%), but relatively low sensitivity for both endpoints (9-73% for poor response, 8-33% for failure to conceive).160,188,189,190,191,192,193 and 194 The PPV and NPV of AFC have varied widely in studies. A low AFC has high specificity for predicting poor response to ovarian stimulation and treatment failure, making it a useful test, but low sensitivity limits its overall clinical utility.
In summary, none of the ovarian reserve tests currently in use is an accurate predictor of pregnancy in IVF cycles, unless extreme abnormal threshold values are applied, which results in very low sensitivity for identifying women having a poor prognosis.157 The tests are adequate for predicting poor response, which does have prognostic value, although not as much in young women as in older women.195,196 and 197 Although ovarian reserve tests have become a routine element of pre-treatment evaluation for couples planning IVF, it can be argued that routine testing has limited clinical utility in the large majority of patients and can be misleading, especially in women at low risk for having a diminished ovarian reserve.198
Ovarian reserve tests always should be interpreted with caution. Rigid application of test results risks inappropriate recommendations for treatment, or for no treatment, and both must be avoided. An abnormal test result does not preclude the possibility of pregnancy. Except perhaps when grossly abnormal, test results should not be used to deny treatment, but only to obtain prognostic information that may help to guide the choice of treatment and best use of available resources. Although the probability of pregnancy may be low, many with abnormal test results will achieve pregnancy if afforded the chance. Ultimately, regardless of the prognosis, the success rate for any individual woman will be zero or 100%.
Diagnosis and Past Reproductive Performance
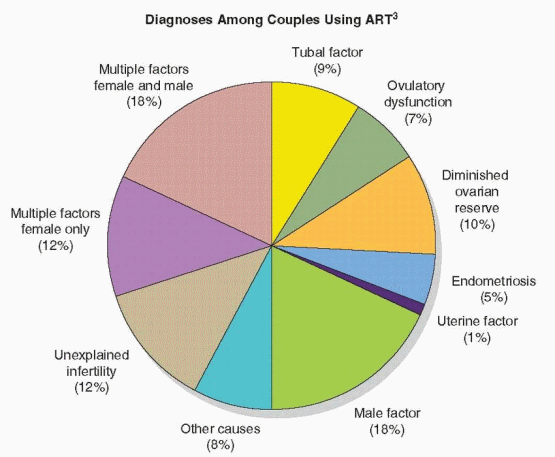
Although the average overall IVF live birth rate per cycle is approximately 29% for all women in the U.S., success rates vary, to some extent, with the cause of infertility. In 2007, the success rates for women with tubal factor infertility, ovulatory dysfunction, endometriosis, male factor, and unexplained infertility were above average, and those for women with multiple infertility factors, a uterine factor, and diminished ovarian reserve were below average. Whereas these data are useful, it is important to note that criteria for the different diagnoses are not standardized and likely vary among treatment centers.
Live Birth Rates in IVF Cycles, By Diagnosis, 20073 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Women with a previous live birth are more likely to succeed with IVF than nulliparous women. In all age categories, success rates for women having one or more previous live births are modestly higher (2-4%) than for women with no previous live births.3 A previous unsuccessful IVF cycle does not decrease the likelihood for success in subsequent cycles until approximately the fourth IVF cycle.199 A history of an earlier unsuccessful pregnancy also has no effect on success rates.3
Other Prognostic Factors
As discussed earlier in the section focused on indications for ART, there is substantial evidence indicating that hydrosalpinges adversely affect IVF outcomes, and that salpingectomy or proximal tubal occlusion before IVF increases the likelihood for achieving a live birth by 2-fold.16 A study evaluating the cost-effectiveness of preliminary salpingectomy concluded the procedure decreases the average cost per live birth, compared to no treatment.200 Laparoscopic salpingectomy before IVF is generally recommended for women with hydrosalpinges.
The effect of uterine myomas on IVF outcomes depends on their location. Submucosal myomas significantly decrease the likelihood for success, subserosal myomas have no significant impact, and the effect of intramural myomas is unclear. Overall, studies examining the effect of submucosal myomas on IVF outcomes indicate they decrease clinical pregnancy rates and delivery rates by approximately 70%,201,202,203,204,205,206 and 207 and increase risk for miscarriage by more than 3-fold.206,207 A 2009 systematic review of studies examining outcomes after submucosal myomectomy concluded that clinical pregnancy rates achieved with IVF were 2-fold higher after surgery than in women with submucous myomas in situ, and comparable to those observed in women without myomas.207 Results of individual studies examining the effects of intramural myomas on IVF outcome are inconsistent, with some observing an adverse effect,208,209,210,211 and 212 and others not.206,213,214,215,216,217 and 218 Although systematic reviews have concluded that intramural myomas have significant negative impact on implantation rates and live birth rates,204,205,219 there is no compelling evidence that their removal improves outcomes.220
Evaluation Before IVF
Individuals and couples planning IVF require additional specific evaluation before a treatment cycle begins. At a minimum, evaluation generally includes a test of ovarian reserve, a current assessment of semen quality, infectious disease screening, a trial transfer, and imaging of the uterine cavity.
Ovarian reserve tests (basal FSH, AMH, antral follicle count) have value for predicting response to gonadotropin stimulation and therefore can be helpful in planning treatment. If a threshold value with high specificity for detecting diminished ovarian reserve is applied, the test can accurately identify women at high risk for poor response and treatment failure.
Semen quality should be assessed not long before the treatment cycle is scheduled to start, even when earlier diagnostic evaluation revealed normal semen parameters, to ensure there has been no appreciable change that might affect the choice between conventional fertilization and intracytoplasmic sperm injection (ICSI). Evaluation of sperm morphology, as judged by “strict” criteria (WHO III standard), also may help to determine whether ICSI should be planned (Chapter 30).83,84,85,86 and 87 Sperm cryopreservation is prudent when semen quality is severely abnormal or there is reason to anticipate difficulty with obtaining a fresh specimen on the day of oocyte retrieval. Although fertilization rates achieved with frozen thawed sperm may be somewhat lower than when fresh sperm are used, pregnancy rates are comparable.224,225
Infectious disease screening is recommended for both partners for human immunodeficiency virus (HIV), hepatitis B (hepatitis B surface antigen), hepatitis C (hepatitis C antibody), and syphilis (rapid plasma reagin), for the protection of medical and laboratory staff, the protection of any fetus that may result from IVF, and protection against the risk for cross-contamination of cryopreserved embryos in storage. Whereas some advocate routine testing for chlamydia and gonorrhea in the female partner, others choose to limit evaluation to women with tubal factor infertility or other risk factors.
A trial transfer helps to determine the technique required to achieve an atraumatic embryo transfer and to identify women whose transfer may be difficult to accomplish, although the orientation of the uterus can change when the ovaries are enlarged after stimulation.226,227
Imaging of the uterine cavity a short time before a cycle of treatment identifies submucosal myomas or endometrial polyps that may interfere with implantation or have an adverse effect on pregnancy outcome. An HSG performed earlier during the diagnostic evaluation may suf-fice if entirely normal and relatively recent (within approximately 6 months), but sonohysterography and hysteroscopy are the more sensitive and preferred methods. Routine office hysteroscopy before IVF can be expected to identify potentially significant abnormalities such as polyps, myomas, adhesions, or septa in 10-20% of patients without symptoms.228,229 and 230 Many prefer sonohysterography to hysteroscopy because it is easier to perform, highly sensitive, and also can detect hydrosalpinges and unsuspected ovarian pathology.231,232 and 233
Ovarian Stimulation Regimens
The ideal ovarian stimulation regimen for IVF should have a low cancellation rate, minimize drug costs, risks and side effects, require limited monitoring for practical convenience, and maximize singleton pregnancy rates. Numerous regimens have been described, ranging
from no stimulation (natural cycles), to minimal stimulation (clomiphene citrate) or mild stimulation (sequential treatment with clomiphene citrate and low dose exogenous gonadotropins), to aggressive stimulation (high dose exogenous gonadotropins, alone or in combination with a gonadotropin-releasing hormone agonist or antagonist). Ovarian stimulation has been a basic element of IVF for more than 25 years, but concerns about multiple pregnancies and the costs of IVF have sparked renewed interest in natural cycle IVF and mild stimulation regimens.
from no stimulation (natural cycles), to minimal stimulation (clomiphene citrate) or mild stimulation (sequential treatment with clomiphene citrate and low dose exogenous gonadotropins), to aggressive stimulation (high dose exogenous gonadotropins, alone or in combination with a gonadotropin-releasing hormone agonist or antagonist). Ovarian stimulation has been a basic element of IVF for more than 25 years, but concerns about multiple pregnancies and the costs of IVF have sparked renewed interest in natural cycle IVF and mild stimulation regimens.
Natural Cycle
The first birth resulting from IVF derived from a single oocyte collected in a natural ovulatory cycle.2 Compared to stimulated IVF cycles, natural cycle IVF offers a number of attractive advantages. Natural cycle IVF involves only monitoring the spontaneous cycle and retrieving a single oocyte before the midcycle LH surge occurs. It is physically less demanding, requires little or no medication, decreases costs by 75-80%,234,235 and all but eliminates risks for multiple pregnancy and ovarian hyperstimulation syndrome (OHSS). The chief disadvantages of natural cycle IVF are high cancellation rates due to premature LH surges and ovulation, and the comparatively low success rate, which is approximately 7%.236
When oocyte retrieval is based on detection of the midcycle rise in LH, careful and frequent monitoring is required and procedures are difficult to schedule efficiently. Alternatively, exogenous human chorionic gonadotropin (hCG) can be administered when the lead follicle reaches a size consistent with maturity, thereby better defining the optimum time for oocyte retrieval.235 Adjuvant treatment with a GnRH antagonist also can be used to prevent a premature LH surge, but requires “add-back” treatment with exogenous FSH, and success rates are still quite low, ranging up to 14% per cycle in non-randomized trials.237,238,239 and 240 In one large cohort study involving 844 treatment cycles in 350 good prognosis patients, the cancellation rate was 13%, the pregnancy rate was 8% per cycle and the cumulative pregnancy rate after three “modified natural IVF cycles” was 21%.241 In a cohort of infertile couples with male factor infertility, success rates in modified natural cycles have reached as high as 13% per cycle, with a cumulative pregnancy rate of 44% after six treatment cycles.242
Clomiphene Citrate
Clomiphene citrate was the first method of ovarian stimulation used in IVF,243,244 but now has been almost entirely replaced by more effective stimulation regimens using human menopausal gonadotropins (hMG) or FSH, in combination with a GnRH agonist or antagonist.245
Clomiphene (100 mg daily) usually is administered for 5-8 days, beginning on cycle day 3, and induces development of two or more follicles in most normally ovulating women,246,247 and 248 although egg yields (1-3) are only slightly greater than in unstimulated cycles and substantially lower than in cycles stimulated with exogenous gonadotropins.248,249 and 250 Cycle cancellation rates are somewhat lower than in natural cycles and the numbers of oocytes retrieved, embryos transferred, and pregnancy rates are greater. As in natural cycles, exogenous hCG is administered when the lead follicle reaches mature size and a GnRH antagonist can be used to prevent a premature endogenous LH surge.
Sequential treatment with clomiphene (100 mg daily for 5 days) and modest doses of exogenous gonadotropins (150-225 IU daily beginning on the last day of clomiphene treatment or the day after) stimulates multifollicular development more effectively than treatment with
clomiphene alone.251,252 and 253 Drug costs and monitoring requirements are moderately higher, but still substantially less than in standard stimulation regimens involving higher dose gonadotropin treatment after down-regulation with a long-acting GnRH agonist (described below).254,255 In one comparative trial, higher cancellation rates and lower pregnancy rates were observed in sequential clomiphene/gonadotropin cycles.255 In another, the sequential stimulation regimen yielded fewer oocytes and embryos, but pregnancy rates were similar and the risks of OHSS were lower.254 Adding a GnRH antagonist to the treatment regimen can prevent premature LH surges and improve outcomes, but also increases costs. In a randomized trial, sequential clomiphene/gonadotropin stimulation and GnRH antagonist treatment yielded a pregnancy rate comparable to that achieved with a more aggressive standard treatment protocol,256 confirming the results of two earlier retrospective studies,257, 258 but contrasting with those of another observing lower pregnancy rates.259
clomiphene alone.251,252 and 253 Drug costs and monitoring requirements are moderately higher, but still substantially less than in standard stimulation regimens involving higher dose gonadotropin treatment after down-regulation with a long-acting GnRH agonist (described below).254,255 In one comparative trial, higher cancellation rates and lower pregnancy rates were observed in sequential clomiphene/gonadotropin cycles.255 In another, the sequential stimulation regimen yielded fewer oocytes and embryos, but pregnancy rates were similar and the risks of OHSS were lower.254 Adding a GnRH antagonist to the treatment regimen can prevent premature LH surges and improve outcomes, but also increases costs. In a randomized trial, sequential clomiphene/gonadotropin stimulation and GnRH antagonist treatment yielded a pregnancy rate comparable to that achieved with a more aggressive standard treatment protocol,256 confirming the results of two earlier retrospective studies,257, 258 but contrasting with those of another observing lower pregnancy rates.259
GnRH Agonist Down-Regulation Gonadotropin Stimulation—The “Long” Protocol
The introduction of long-acting GnRH agonists in the late 1980s revolutionized the approach to ovarian stimulation in ART by providing the means to suppress endogenous pituitary gonadotropin secretion and thereby prevent a premature LH surge during exogenous gonadotropin stimulation. Adjuvant treatment with a GnRH agonist eliminated the need for frequent serum LH measurements and assuaged fears of premature luteinization which previously had required cancellation of approximately 20% of all IVF cycles before oocyte retrieval.260,261 and 262 Because fewer than 2% of cycles are complicated by a premature LH surge after down-regulation with a GnRH agonist,263 stimulation could continue until follicles were larger and more mature. Numerous clinical trials subsequently demonstrated that egg yields and pregnancy rates were significantly higher than in cycles stimulated with exogenous gonadotropins alone.264,265 Moreover, GnRH agonist treatment offered the welcome additional advantage of scheduling flexibility, allowing programs to coordinate cycle starts for groups of women simply by varying the duration of GnRH agonist suppression. Not surprisingly, the “long protocol” quickly became the preferred ovarian stimulation regimen for all forms of ART. Its only disadvantages are that GnRH agonist treatment sometimes blunts the response to gonadotropin stimulation and increases the dose and duration of gondotropin therapy required to stimulate follicular development. The combined costs of the additional gonadotropins and the agonist itself increase the total cost of treatment substantially. Nevertheless, because GnRH agonists have more advantages than disadvantages, the long protocol became and has remained the standard ovarian stimulation regimen in IVF cycles.
In the typical cycle, GnRH agonist treatment begins during the midluteal phase, approximately 1 week after ovulation, at a time when endogenous gonadotropin levels are at or near their nadir and the acute release of stored pituitary gonadotropins in response to the agonist, known as the “flare” effect, is least likely to stimulate a new wave of follicular development.266,267 GnRH agonist treatment can be scheduled to begin on cycle day 21 (assuming a normal cycle of approximately 28 days duration), but most prefer to first con-firm that ovulation has occurred by measuring the serum progesterone concentration. In women who do not cycle predictably, oral contraceptives (OC) can be used to control the onset of menses, starting GnRH agonist treatment 1 week before their discontinuation. In the U.S., leuprolide acetate (administered by s.c. injection) is the most commonly used GnRH agonist. In Europe and elsewhere, buserelin acetate (administered by s.c. injection or intranasal spray) and triptorelin (administered subcutaneously) are more common268; all work equally well. For leuprolide, the usual treatment regimen begins with 1.0 mg daily for approximately 10 days or until onset of menses or gonadotropin stimulation, decreasing to
0.5 mg daily thereafter until hCG is administered. A single dose of a longer-acting depot form of GnRH agonist (leuprolide, goserelin) offers greater convenience, but evidence indicates the total dose and duration of gonadotropin stimulation required are increased significantly when depot forms of the agonists are used.269
0.5 mg daily thereafter until hCG is administered. A single dose of a longer-acting depot form of GnRH agonist (leuprolide, goserelin) offers greater convenience, but evidence indicates the total dose and duration of gonadotropin stimulation required are increased significantly when depot forms of the agonists are used.269
Gonadotropin stimulation begins after confirming that effective pituitary down-regulation has been achieved (serum estradiol level <30-40 pg/mL, no follicles >10 mm in diameter). Some women require longer durations of treatment to achieve suppression or may develop an ovarian cyst.260 The significance of an ovarian cyst has been controversial. Whereas some investigators have observed that baseline cysts are associated with a poorer response to gonadotropin stimulation, decreased numbers of oocytes and embryos, and lower overall IVF success rates,270,271 and 272 others have not.273,274,275,276 and 277 Overall, the weight of available evidence suggests that women who develop cysts or require longer durations of GnRH agonist treatment to achieve suppression are more likely to respond poorly to gonadotropin stimulation and less likely to achieve pregnancy. Cyst aspiration immediately before stimulation does not appear to adversely affect response278 and may even improve response in the aspirated ovary,279 but probably is not warranted in women with a normal contralateral ovary.
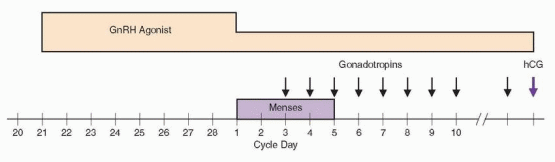 |
The initial dose of exogenous gonadotropins must be tailored to the needs of the individual woman. Typical starting doses range between 150 and 300 IU of urinary FSH (uFSH), recombinant FSH (rFSH), or urinary menotropins (hMG) daily, depending on age, the results of ovarian reserve testing, and the response observed in any previous stimulation cycles. Either a “step-up” (beginning with a low dose, increasing as necessary based on response) or a “step-down” (beginning with a higher dose, decreasing as necessary based on response) can be used, but the latter approach is generally preferred. All contemporary gonadotropin preparations, including hCG, can be administered subcutaneously.
Numerous clinical trials and meta-analyses have compared outcomes in cycles stimulated with uFSH, rFSH, or hMG, with or without GnRH agonist pretreatment, concluding that there is no compelling evidence to indicate the superiority of one gonadotropin preparation over others.280,281,282 and 283 However, a 2008 systematic review including seven trials comparing outcomes in cycles stimulated with rFSH or hMG, involving 2,159 patients, observed a significant increase in live birth rate with hMG (RR=1.18, CI=1.02-1.38); the pooled risk difference for live birth was 4%.284
Recombinant DNA technology has been used to develop a new longer-acting form of rFSH. Corifollitropin alpha is the product of a chimeric gene containing the sequences of the FSH-β subunit and the C-terminal peptide of the hCG-β subunit, which bears four O-linked glycosylation sites, and has a half-life three times longer than standard rFSH (95 vs. 32 hours).285,286 A single dose (100 μg for women <60 kg, 150 μg for those >60 kg) can induce and sustain multi-follicular growth for a week in women receiving ovarian stimulation for IVF. Corifollitropin has shown considerable promise in phase II trials, currently is being evaluated for safety and efficacy in large phase III trials, and is the first step
towards a new generation of recombinant gonadotropins.287 The first live birth resulting from treatment with corifollitropin was reported in 2003.288
towards a new generation of recombinant gonadotropins.287 The first live birth resulting from treatment with corifollitropin was reported in 2003.288
The low levels of LH secretion remaining after down-regulation with a GnRH agonist are sufficient to support normal follicular development in most women stimulated with uFSH or rFSH alone,289 because only about 1% of LH receptors must be occupied to sustain normal levels of steroidogenesis.290 However, in some women treated only with FSH, LH levels are markedly suppressed (<1 IU/L) and may be inadequate.291,292 In such cycles, the dose and duration of gonadotropin stimulation required is higher, peak estradiol levels are lower, and the numbers of oocytes and embryos may be reduced.293,294 Extremely low LH levels also may adversely affect fertilization, implantation, and pregnancy rates,295,296,297,298 and 299 and have been associated with a higher incidence of biochemical pregnancy and early pregnancy loss.300,301 A 2007 systematic review and meta-analysis including 11 trials comparing stimulation with rFSH alone or in combination with recombinant LH (rLH) after GnRH agonist-induced down-regulation in IVF and ICSI cycles observed no significant differences in clinical or ongoing pregnancy rates.302 However, in three trials including only poor responders, the pregnancy rate was higher in those receiving combined stimulation with rFSH and rLH.302 In sum, the evidence indicates there may be a subgroup of women who could benefit from supplemental rLH or hMG during ovarian stimulation. In the absence of any reliable method for identifying such women, and in light of recent evidence suggesting that use of hMG may increase live birth rates,284 many clinicians favor combined stimulation with FSH and hMG over stimulation with FSH alone.
The response to stimulation is monitored with serial measurements of serum estradiol and transvaginal ultrasonography. The first estradiol level usually is obtained after 3-5 days of stimulation to determine whether the chosen dose of gonadotropins requires adjustment. Thereafter, serum estradiol concentrations and sonography are obtained every 1-3 days, based on the quality of the response and the need to evaluate the impact of any further adjustments in the dose of gonadotropin treatment. In general, stimulation continues until at least two follicles measure 17-18 mm in mean diameter, when others typically measure 14-16 mm and the serum estradiol concentration reflects the overall size and maturity of the cohort. Most women require a total of 7-12 days of stimulation. It is important to emphasize that these parameters only approximate the goals of stimulation. In clinical practice, follicle measurements vary among observers and estradiol assays vary in their performance characteristics. Ultimately, each program must empirically establish its own thresholds, based on its own experience.
The endometrium is monitored during stimulation by measuring the endometrial thickness or “stripe” (the sum thickness of the two layers, measured in the mid-sagittal plane). Numerous studies have examined the prognostic value of endometrial thickness and pattern in ART cycles, but the issue remains unsettled. Many have suggested that results are best when endometrial thickness measures 8-9 mm or greater or appears “trilaminar,” and poor when the endometrium is less than 6-7 mm in thickness or appears homogeneous on the day of hCG administration.303,304,305,306,307 and 308 However, numerous others have failed to observe any clear correlation between endometrial thickness or appearance and outcomes.309,310,311,312,313 and 314 Some have suggested that excessive endometrial growth (>14 mm) also is a poor prognostic indicator,305,315 but that too has been refuted.316,317 Overall, although measurements of endometrial growth are routine, their utility remains unclear. Consequently, changes in stimulation regimens and cycle cancellations based on endometrial thickness or appearance alone are difficult to justify.318
When the cohort of ovarian follicles reaches maturity, hCG (5,000-10,000 IU) is administered to stimulate the final stages of follicular development. The equivalent dose of the recombinant form of hCG now available is 250 μg.319,320 A 2005 systematic review including seven trials comparing recombinant and urinary hCG observed no differences in clinical outcomes.321 The predictive value of the serum progesterone concentration
on the day of hCG administration has been debated vigorously, with some arguing that pregnancy rates were substantially lower when levels exceeded 0.9-1.0 ng/mL,322,323,324,325,326 and 327 and others refuting the contention.328,329,330,331 and 332 It is now clear that mildly increased progesterone levels are relatively common in women who respond well to gonadotropin stimulation and are a poor prognostic indicator only in poor responders.333 Whereas it may be tempting to delay hCG administration in poor responders to afford smaller follicles the opportunity to further mature, the strategy is not likely to succeed and may be detrimental.
on the day of hCG administration has been debated vigorously, with some arguing that pregnancy rates were substantially lower when levels exceeded 0.9-1.0 ng/mL,322,323,324,325,326 and 327 and others refuting the contention.328,329,330,331 and 332 It is now clear that mildly increased progesterone levels are relatively common in women who respond well to gonadotropin stimulation and are a poor prognostic indicator only in poor responders.333 Whereas it may be tempting to delay hCG administration in poor responders to afford smaller follicles the opportunity to further mature, the strategy is not likely to succeed and may be detrimental.
Approximately 7-18% of stimulation cycles are cancelled before oocyte retrieval, most for lack of adequate response, and some for excessive response.3 When the ovaries become grossly enlarged, containing large numbers of follicles of all sizes, and serum estradiol concentrations are markedly elevated (>5,000 pg/mL), the risk for OHSS increases substantially.334,335 and 336 Management options in “high responders” include all of the following:
Cycle cancellation.
“Coasting,” in which GnRH agonist treatment continues but without further gonadotropin stimulation for 1-3 days, administering hCG after estradiol levels moderate.
Proceeding with oocyte retrieval and fertilization but freezing all embryos in lieu of transfer.
Delaying transfer until 5 days after retrieval, while observing for clinical signs and symptoms of developing OHSS.
Canceling the cycle and starting anew using a more conservative stimulation regimen may ultimately decrease overall costs and maximize the chances for success.337 The prognosis for high responders in subsequent cycles is generally very good. Dual suppression with both an OC (one pill daily for 21 days or more) and a GnRH agonist (leuprolide 1.0 mg s.c. daily, beginning 1 week before discontinuation of contraceptive treatment) can attenuate the response to subsequent lower dose gonadotropin stimulation.338 Coasting allows larger follicles to continue growing but withdraws support from small and intermediate-sized follicles.339,340 Although approximately 20-30% of coasted cycles are ultimately cancelled, the strategy can help to reduce the risks for developing severe OHSS and avoid cancellation.339,341 Proceeding to oocyte retrieval and fertilization and freezing all embryos can salvage the cycle but avoid the greater risks of serious or prolonged OHSS observed in conception cycles.342,343 Delaying transfer until after symptoms abate and freezing all embryos when they persist is another option.344
The challenges presented by “poor responders” are far greater. Poor responders include women who develop few follicles (<3-5) despite high doses of gonadotropin stimulation or have relatively low peak estradiol levels (<500-1,000 pg.mL); there are no consensus criteria defining a poor responder. The prognosis is relatively poor for such women345,346 and 347 and the important decision centers on whether to attempt stimulation again using a different or more aggressive treatment regimen.348 Some of the more commonly employed options include the following:
The long protocol, beginning with higher doses of gonadotropin stimulation.
Decreasing the doses of GnRH agonist or discontinuing agonist treatment immediately before or soon after gonadotropin stimulation begins.
A short follicular phase GnRH agonist treatment regimen using a standard or micro-dose “flare” protocol (described below).
Using a GnRH antagonist (described below) instead of a long-acting agonist.
Higher doses of gonadotropin stimulation may generate a somewhat more vigorous follicular response, but doses greater than 450 IU daily generally have little or no additional benefit.349,350,351 and 352 Decreasing the dose or discontinuing GnRH agonist treatment early
or completely may help to improve the quality of response.353,354,355,356 and 357 A standard or microdose GnRH agonist “flare protocol” (described below) may stimulate an improved response in some poor responders.358,359 and 360 Stimulation regimens employing a GnRH antagonist instead of a long-acting agonist eliminate any suppressive effects of the agonist altogether.361 Other strategies have included efforts to increase androgen concentrations by treatment with dehydroepiandrosterone (DHEA)362 or an aromatase inhibitor,363 and the addition of growth hormone to the stimulation regimen.364 A 2009 systematic review and meta- analysis of randomized trials comparing different stimulation regimens in poor responders found insufficient evidence to support the routine use of any particular intervention.365 A 2010 systematic review including 10 trials involving eight different comparison groups reached the same conclusion.345
or completely may help to improve the quality of response.353,354,355,356 and 357 A standard or microdose GnRH agonist “flare protocol” (described below) may stimulate an improved response in some poor responders.358,359 and 360 Stimulation regimens employing a GnRH antagonist instead of a long-acting agonist eliminate any suppressive effects of the agonist altogether.361 Other strategies have included efforts to increase androgen concentrations by treatment with dehydroepiandrosterone (DHEA)362 or an aromatase inhibitor,363 and the addition of growth hormone to the stimulation regimen.364 A 2009 systematic review and meta- analysis of randomized trials comparing different stimulation regimens in poor responders found insufficient evidence to support the routine use of any particular intervention.365 A 2010 systematic review including 10 trials involving eight different comparison groups reached the same conclusion.345
GnRH Agonist “Flare” Gonadotropin Stimulation Protocol
The “short” or “flare” protocol is an alternative stimulation regimen designed to exploit both the brief initial agonistic phase of response to a GnRH agonist and the suppression that results from longer-term treatment.359,366 In a typical standard short protocol, leuprolide acetate (1.0 mg daily) is administered on cycle days 2-4, continuing thereafter at a reduced dose (0.5 mg daily), and gonadotropin stimulation (225-450 IU daily) begins on cycle day 3. Later adjustments in the dose of gonadotropin stimulation, if needed, are based on response and indications for hCG administration are the same as in the long protocol (described above).
An early meta-analysis including seven clinical trials comparing the short and long GnRH agonist treatment regimens determined that the two protocols yielded similar cancellation and pregnancy rates.264 A 2000 systematic review including 22 trials concluded that pregnancy rates achieved with the long protocol were superior to those using the flare regimen (OR=1.27, CI=1.04-1.56) overall,265 but the analysis did not control for diagnosis and other prognostic factors and results may not apply to all women, or to poor responders in particular. Whereas some have observed improved follicular response and lower cycle cancellation rates in poor responders treated with a flare protocol, pregnancy and live birth rates remained low.367,368 Decreased scheduling flexibility is a distinct disadvantage of the flare protocol, unless the onset of menses is controlled by preliminary treatment with an OC. The regimen also can result in a significant increase in serum progesterone and androgen levels, presumably resulting from late corpus luteum rescue,369,370 which may adversely affect oocyte quality and fertilization and pregnancy rates.371
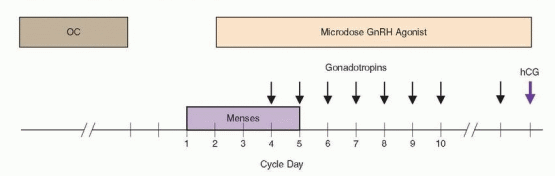
The “OC microdose GnRH agonist flare” stimulation regimen is a variation of the standard short protocol involving 14-21 days of preliminary ovarian suppression with an OC (one pill daily), followed by microdose leuprolide treatment (40 μg twice daily) beginning 3 days after discontinuation of OC treatment, and high-dose gonadotropin stimulation (300-450 IU daily) starting on day 3 of leuprolide therapy. Indications for later gonadotropin dose adjustments and hCG administration are the same as in other stimulation regimens. Its primary advantage over the standard short protocol is that it does not induce any increases in serum progesterone or androgen concentrations,360 possibly because the doses of GnRH agonist administered are much lower, but likely also because preliminary OC treatment all but eliminates the possibility there may be a corpus luteum left to respond.372,373 The OC-microdose GnRH agonist flare protocol may be useful in previous poor responders, in whom it can stimulate increased endogenous FSH release and may yield lower cancellation rates and higher peak serum estradiol levels, transfer rates, and pregnancy rates.360,374,375
 |
GnRH Antagonist Gonadotropin Stimulation Protocol
The introduction of GnRH antagonists into clinical practice provided another option for ovarian stimulation in ART. In contrast to the long-acting agonists, which first stimulate and later inhibit pituitary gonadotropin secretion by desensitizing gonadotropes to GnRH via receptor down-regulation, the antagonists block the GnRH receptor in a dose- dependent competitive fashion and have no similar flare effect376,377; gonadotropin suppression is almost immediate.
GnRH antagonists offer several potential advantages over agonists. First, the duration of treatment for an antagonist is substantially shorter than for an agonist. Since its only purpose is to prevent a premature endogenous LH surge and its effects are immediate, antagonist treatment can be postponed until later in follicular development (after 5-6 days of gonadotropin stimulation), after estradiol levels are already elevated, thereby eliminating the estrogen deficiency symptoms that may emerge in women treated with an agonist.378 Second, because any suppressive effects that agonists may exert on the ovarian response to gonadotropin stimulation also are eliminated, the total dose and duration of gonadotropin stimulation required is decreased.378,379 For the same reason, GnRH antagonist stimulation protocols may benefit women who are poor responders when treated with a standard long protocol.378,380 Third, by eliminating the flare effect of agonists, GnRH antagonists avoid the risk of stimulating development of a follicular cyst. Finally, the risk of severe OHSS associated with use of antagonists also appears lower than with agonists.381,382 and 383
GnRH antagonists have some potential disadvantages. When administered in small daily doses, strict compliance with the prescribed treatment regimen is essential.378 Antagonists suppress endogenous gonadotropin secretion more completely than agonists. Whereas the low levels of LH observed during agonist treatment are usually sufficient to support normal follicular steroidogenesis during stimulation with uFSH or rFSH, the even lower concentrations in women treated with an antagonist may not be. Indeed, serum estradiol levels may plateau or fall when antagonist treatment begins.299,378,384 Although follicular growth appears unaffected, most prefer to add or substitute a low dose of hMG (75 IU) at the same time if it was not already part of the stimulation regimen. Evidence also suggests that pregnancy rates in antagonist treatment cycles may be modestly lower than in cycles using agonists in the long protocol.385
The two GnRH antagonists available for clinical use, ganirelix and cetrorelix, are equally potent and effective. For both, the minimum effective dose to prevent a premature LH surge is 0.25 mg daily, administered subcutaneously.299,386 Either can be administered in a series of small daily doses (0.25 mg). The treatment protocol may be fixed and begin after 5-6 days of gonadotropin stimulation,299,386,387 or tailored to the response of the individual, starting
treatment when the lead follicle reaches approximately 13-14 mm in diameter.388,389 The individualized treatment regimen generally requires fewer total doses and may yield better overall results.388 Alternatively, a single larger dose of cetrorelix (3.0 mg) will effectively prevent an LH surge for 96 hours. If given on day 6-7 of stimulation, the interval of effective suppression will encompass the day of hCG administration in most women (75-90%); the remainder may receive additional daily doses (0.25 mg) as needed, ending on the day of hCG treatment.390,391 and 392 The single dose antagonist treatment regimen also can be withheld until the lead follicle reaches 13-14 mm in diameter.393
treatment when the lead follicle reaches approximately 13-14 mm in diameter.388,389 The individualized treatment regimen generally requires fewer total doses and may yield better overall results.388 Alternatively, a single larger dose of cetrorelix (3.0 mg) will effectively prevent an LH surge for 96 hours. If given on day 6-7 of stimulation, the interval of effective suppression will encompass the day of hCG administration in most women (75-90%); the remainder may receive additional daily doses (0.25 mg) as needed, ending on the day of hCG treatment.390,391 and 392 The single dose antagonist treatment regimen also can be withheld until the lead follicle reaches 13-14 mm in diameter.393
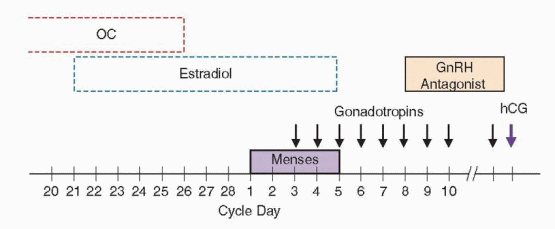
A common variation of the antagonist stimulation regimen uses preliminary treatment with an OC to control the onset of menses, typically ending approximately 5 days before the scheduled start, which also may help to synchronize the follicular cohort before stimulation begins. Another variation advocated for poor responders uses micronized estradiol (2 mg twice daily, administered orally, beginning on day 21 of the preceding cycle) to suppress FSH during the late luteal phase for the same purpose, ending on the day before gonadotroins stimulation begins,393,394 or continuing through the first 3 days of gonadotropin stimulation.347 The improved follicular dynamics observed are similar to those achieved by down-regulation with a GnRH agonist in the long protocol. The rebound increase in endogenous FSH levels that follows the discontinuation of estradiol treatment also may synergize with exogenous gonadotropins to promote multifollicular development.395,396
 |
Results of a number of early trials comparing a fixed antagonist treatment protocol to the standard long protocol suggested that the two stimulation regimens yielded similar pregnancy rates.379,391,397,398 However, a 2006 systematic review and meta-analysis including 27 trials comparing different antagonist stimulation protocols with the long GnRH agonist protocol observed a significantly lower clinical pregnancy rate (OR=0.84, CI=0.72-0.97) and ongoing pregnancy/live birth rate (OR=0.82, CI=0.69-0.98). Overall, the total dose and duration of gonadotropin stimulation required, peak serum estradiol levels, and the number of follicles and oocytes were lower in antagonist cycles.
The explanation for the modestly lower pregnancy rates observed in antagonist treatment cycles is not clear. It is possible, but unlikely, that GnRH antagonists may have adverse effects on oocytes, embryos, or the endometrium.399,400 It is far more likely that early results reflected inexperience and improved with time and further refinements in the treatment regimen like those described above. Many of the advantages originally envisioned for GnRH antagonists already have been realized. Whether antagonists ultimately will replace agonists and become the standard ovarian stimulation regimen in ART cycles remains to be seen, but their place in the therapeutic arsenal already is firmly established.
Women with polycystic ovary syndrome (PCOS) characteristically exhibit high tonic LH secretion and are predisposed to premature LH surges when treated with standard ovulation induction regimens. Women with PCOS also are at increased risk for developing OHSS when aggressively stimulated with exogenous gonadotropins. Whereas both GnRH agonists and antagonists can suppress elevated circulating LH concentrations, the smaller
follicular cohorts observed in antagonist cycles may help to reduce the risk of OHSS in women with PCOS who tend to be high responders. The use of antagonists, rather than agonists, provides the opportunity to use an agonist instead of hCG to induce final oocyte maturation, thereby possibly further decreasing the risk of OHSS.401 Whereas a single bolus injection of an agonist (leuprolide 0.5 mg, triptorelin 0.2 mg) triggers a physiologic LH surge that lasts less than 24 hours, hCG levels remain elevated for several days and stimulate markedly higher estradiol and progesterone concentrations.402
follicular cohorts observed in antagonist cycles may help to reduce the risk of OHSS in women with PCOS who tend to be high responders. The use of antagonists, rather than agonists, provides the opportunity to use an agonist instead of hCG to induce final oocyte maturation, thereby possibly further decreasing the risk of OHSS.401 Whereas a single bolus injection of an agonist (leuprolide 0.5 mg, triptorelin 0.2 mg) triggers a physiologic LH surge that lasts less than 24 hours, hCG levels remain elevated for several days and stimulate markedly higher estradiol and progesterone concentrations.402
The antagonist treatment regimens currently in use have potential disadvantages for women with PCOS. Their tonically elevated LH levels will remain high until antagonist treatment begins. Consequently, LH levels may rise prematurely, particularly if antagonist treatment is withheld until the lead follicle reaches 14 mm or more. Moreover, evidence indicates that increased LH exposure during early follicular development may be detrimental and predispose to lower pregnancy rates.403,404,405 and 406 In theory, pretreatment with an OC might prove quite useful by suppressing LH and androgen levels before stimulation begins, decreasing exposure during early follicular development and the risk of rising LH levels before antagonist treatment starts. Preliminary OC suppression and later antagonist treatment may help to limit the follicular response to gonadotropin stimulation while preserving the option to use an agonist to trigger final oocyte maturation. These considerations simply serve to illustrate that GnRH antagonists are not a panacea and are not necessarily the best choice even for women with PCOS.
Antagonist stimulation protocols are advocated for poor responders, primarily because they avoid the suppressive effects that agonists can have on follicular response and can prevent the premature LH surges observed commonly in women stimulated with gonadotropins alone.407 However, evidence is insufficient to indicate they yield results consistently better than other stimulation regimens.345,365
Oocyte Retrieval
Oocyte retrieval is generally performed approximately 34-36 hours after hCG administration. Modestly longer intervals do not substantially increase the risk of ovulation or adversely affect oocyte quality, fertilization rates, or overall results in GnRH agonist downregulated stimulation cycles,408,409,410 and 411 but earlier retrieval may yield fewer mature oocytes.412
Whereas oocyte retrieval once was performed via laparoscopy, transvaginal aspiration guided by ultrasonography under intravenous sedation is now the standard technique. Deep sedation (propofol) is most common, but most women tolerate the procedure very well with “conscious sedation” using short-acting narcotics (fentanyl) and benzodiazepines (midazolam), administered in small doses, as needed. There is no compelling evidence to indicate any difference in patient satisfaction or outcomes.413 Constant monitoring by automated blood pressure recordings and pulse oximetry is essential to ensure that the proper plane of sedation is maintained and not exceeded. Specific reversal agents for narcotics (naloxone) and benzodiazepines (flumazenil) should be readily available.
Prophylactic antibiotic treatment (doxycycline 100 mg or cefoxitin 2 g), administered intravenously 30-60 minutes before retrieval is common but controversial because of the low incidence of infectious complications following retrieval (0.3-0.6%).414,415 Alternatively, oral antibiotics may be started immediately following the procedure (tetracycline, doxycycline), reserving prophylactic intravenous antibiotics for women at increased risk for infection (history of pelvic inflammatory disease, endometrioma).
Antiseptics (povidine iodine) are toxic to oocytes and limited evidence suggests their use may be associated with lower pregnancy rates.416 When used to prepare the vagina before retrieval, thorough irrigation with sterile saline should follow, but repeated irrigation with saline alone is generally sufficient to cleanse the vagina. The bladder can become distended as a result of intravenous fluid administration, but can be drained immediately before retrieval; an indwelling catheter is unnecessary.
A vaginal probe (5-7 MHz) in a sterile plastic sheath with an attached needle guide is used to image the ovaries and to align the guide with the follicles in their largest diameter. A specially designed disposable 16-17 gauge needle is used to enter each follicle, in turn, and to aspirate the follicular fluid and oocytes. At the proper vacuum pressure (approximately 100 mm Hg), the follicle walls collapse but do not obstruct the needle lumen. Whereas some have observed that flushing and re-aspiration of follicles using a double-lumen needle can increase oocyte yield,417 it is generally unnecessary and increases both operating time and analgesic requirements.418 Efforts to minimize the arc swept by the needle within the ovary help to limit discomfort and ovarian trauma. In general, all follicles within the ovaries greater than 10 mm in diameter can be aspirated with no more than one to three separate entries on each side. Flushing the needle and attached tubing with media after each withdrawal helps to maximize oocyte yield. Abdominal pressure can sometimes stabilize a mobile ovary or move an ovary into a more convenient location for aspiration. Ovaries adherent to the posterior uterus often are more easily approached from the contralateral side but may be difficult to enter without traversing a portion of the uterus.419 It may be more prudent to simply abandon some follicles, particularly when the number of oocytes already retrieved is sufficient.
The “empty follicle syndrome,” characterized by a failure to retrieve oocytes despite apparently normal multi-follicular development, occurs in up to 0.5-1% of cycles.420,421 and 422 The phenomenon can be observed when hCG is administered later than scheduled423 or forgotten altogether,421 and might rarely result from reduced biological activity in some lots of commercially prepared hCG.424,425 and 426 The serum hCG concentration 36 hours after injection generally ranges between 100 and 300 IU/L.425
Serious complications of oocyte retrieval are uncommon. Limited vaginal hemorrhage from a puncture site is relatively common (8%) and usually can be controlled with a brief interval of direct pressure, but sometimes may require a suture.415 Acute hemorrhage from the ovary and hemorrhage or hematomas resulting from injury to the uterine, ovarian, or iliac vessels are rare (0.04-0.07%).415 The incidence of postoperative pelvic infections is quite low even without prophylactic antibiotic treatment (0.3-0.6%) and almost half present as tubo-ovarian abscesses, 1-6 weeks after retrieval.414,415 Women with ovarian endometriomas and those with past history of salpingitis are at highest risk.66,67,427,428 Other rare reported complications include the rupture of a dermoid cyst,429 laceration of a sacral vein,430 and lumbo-sacral osteomyelitis.431 Potential complications include pelvic infection, adnexal torsion, and even vertebral osteomyelitis.432
Oocyte Maturation
Up to 20-30% of retrieved oocytes may be immature at the time of retrieval, reflecting the varying size and maturity of follicles in the cohort at the time hCG is administered. An accurate assessment of oocyte maturity is important to the timing of fertilization, even more so when ICSI is to be performed.
Like the LH surge in natural cycles, hCG triggers the resumption of meiosis in primary ooyctes previously arrested at prophase I of the first meiotic division. Oocyte maturity
generally can be judged by the expansion of the cumulus mass, radiance of the corona cells, the size and cohesiveness of granulosa cells, and the shape and color of the oocyte. When the cumulus mass is removed, as it is in preparation for ICSI, the oocyte can be further evaluated according to the presence or absence of the first polar body and germinal vesicle (nuclear membrane).
generally can be judged by the expansion of the cumulus mass, radiance of the corona cells, the size and cohesiveness of granulosa cells, and the shape and color of the oocyte. When the cumulus mass is removed, as it is in preparation for ICSI, the oocyte can be further evaluated according to the presence or absence of the first polar body and germinal vesicle (nuclear membrane).
A mature (metaphase II) oocyte has extruded the first polar body and is in the resting phase of meiosis II. The cumulus cells are typically expanded and luteinized and the corona radiata exhibits a sunburst pattern. A metaphase I oocyte of intermediate maturity has no polar body and denser cumulus cells, but the germinal vesicle and nucleolus have faded. Metaphase I oocytes require additional time in culture before fertilization and must be examined periodically to document extrusion of the first polar body. A prophase I oocyte is grossly immature and exhibits a compact corona containing relatively few cumulus cells and a prominent germinal vesicle and nucleolus; dissolution of the germinal vesicle signals the resumption of meiosis I.
In Vitro Maturation
Human oocytes reach full size (100-200 μm) during the early antral stage of follicular development. The ability of an oocyte to resume and complete meiosis relates to follicular diameter.433 Although immature oocytes collected from small antral follicles can mature with time in culture (the majority within 46-48 hours), even those that reach meiosis II do not necessarily acquire developmental competence, which requires synchronous maturation of both the nucleus and cytoplasm. Consequently, although they frequently fertilize, immature oocytes yield embryos that often develop poorly and exhibit low implantation potential.434,435 Nuclear maturation involves germinal vesicle breakdown, normally induced by the LH surge, followed by resumption of meiosis and, finally, extrusion of the first polar body. Cytoplasmic maturation is more difficult to define, but involves a number of factors that prepare the cytoplasm for fertilization and subsequent embryonic development.436 Epigenetic processes are involved in both nuclear and cytoplasmic maturation and influence development after fertilization.437,438
Technically, the term in vitro maturation (IVM) describes the maturation of immature oocytes in culture after their retrieval from follicles not exposed to exogenous LH or hCG in vivo. In efforts to improve the relatively low efficiency of classical IVM, new methods involving preliminary “follicular priming” have been developed.439,440 and 441 One method involves FSH treatment for 3-6 days, followed by retrieval on cycle day 9-10. Another involves a single injection of hCG (10,000 IU), administered when the largest follicle reaches 10-12 mm in size and 36 hours before retrieval. A third method combines the two techniques, involving sequential treatment with FSH and hCG before oocyte retrieval.
Numerous studies have explored methods for IVM using oocytes obtained from normal women442,443,444,445,446 and 447 and from women with PCOS.442,443,448,449,450,451,452,453 and 454 Studies examining the effects of follicular priming in vivo have yielded inconsistent results, but embryos derived from primed oocytes generally have yielded higher implantation and pregnancy rates than those derived from oocytes collected from unstimulated antral follicles.450,452,454 In one large trial examining the efficiency of IVM in women with normal ovaries, 400 women were randomly allocated to receive no priming or priming with hCG, FSH, or FSH and hCG.455 The overall maturation rate and total number of available metaphase II oocytes were significantly higher in the groups receiving hCG than in those not receiving hCG. The overall clinical pregnancy rate per transfer was 18.3% and the implantation rate was 10.6%. Among the groups, the clinical pregnancy rate was higher in the group receiving both FSH and hCG priming (30%) than in all others.455
The best timing and method for efficient retrieval of immature oocytes from small follicles (<10 mm in diameter) have not been established.447,452,456 Aspiration of follicles greater than 13 mm in size generally has yielded fewer oocytes, possibly because such follicles are already atretic.457 A variety of aspiration vacuum pressures (80-300 mm Hg) and needles (16-20 guage) has been described.447,458 Whereas evidence is insufficient to warrant recommendation of any one method, extremely high vacuum pressures appear to adversely affect oocyte development in vitro.459 The culture media composition that best supports IVM and the best method for fertilization of oocytes subjected to IVM also remain to be established; ICSI has achieved higher fertilization rates, but embryos derived from oocytes fertilized by conventional methods have exhibited higher implantation rates and yielded higher clinical pregnancy rates,443 suggesting that ICSI is not required.
Although the clinical pregnancy rates achieved in IVM trials have been reasonably good, they do not approach those of standard IVF and have been achieved by transfer of a larger number of embryos. Implantation rates for embryos derived from IVM oocytes (5-22%) also are lower than those expected in similar women (age <35 years) receiving treatment with conventional IVF (34%).3 Although the number of children derived from oocytes subjected to IVM is still quite small, preventing confident conclusions, the incidence of malformations and developmental abnormalities has thus far not differed from those in children resulting from traditional IVF or ICSI.
In summary, the results achieved thus far with IVM after follicular priming in vivo suggest the methods have real clinical promise. However, numerous questions must be answered before IVM can be recommended for wider clinical application.460 Women with polycystic ovary syndrome (PCOS) having large numbers of antral follicles and greatest risk for developing ovarian hyperstimulation syndrome (OHSS) represent one population that could benefit from IVM, because purposeful retrieval of immature oocytes would require fewer days of gonadotropin stimulation. Women with cancer represent another, because many require immediate treatment that does afford them the time to pursue established methods of fertility preservation, which require ovarian stimulation, oocyte retrieval and oocyte or embryo cryopreservation.
Fertilization
Fertilization can be achieved by conventional microinsemination or by ICSI when there is a known or suspected male factor and poor or failed fertilization is a concern. In fact, male factor infertility is the one most common diagnosis among couples who undergo IVF. In the U.S. national ART summary for 2007, 18% of all cycles were performed for male factor indications and a male factor was one of multiple infertility factors in another 18% of cycles.3
A semen sample should be obtained by masturbation immediately before or after retrieval. The two methods most commonly used for sperm preparation before fertilization, the “swim-up” procedure and density gradient centrifugation, are described in detail in Chapter 30. Whereas both methods can successfully isolate a population of highly motile sperm for insemination, density gradient centrifugation also appears to select sperm with normal morphology and is widely regarded as the better choice when semen parameters are abnormal.461,462,463 and 464 The isolated sperm are then incubated in media supplemented with a high concentration of protein for 0.5-4.0 hours to achieve capacitation.
In general, each oocyte is incubated with 50-100 thousand motile sperm for an interval of 12-18 hours at 37°C in 5% carbon dioxide in air at 98% relative humidity. The acrosome
reaction, which enables sperm to penetrate the zona pellucida, is initiated by contact between the sperm and the zona. In turn, sperm penetration triggers the cortical reaction which involves exocytosis of cortical granules from the ooplasm and renders the zona pellucida relatively refractory to penetration by more than a single sperm (polyspermy). Conventional IVF typically achieves fertilization rates ranging between 50% and 70%.
reaction, which enables sperm to penetrate the zona pellucida, is initiated by contact between the sperm and the zona. In turn, sperm penetration triggers the cortical reaction which involves exocytosis of cortical granules from the ooplasm and renders the zona pellucida relatively refractory to penetration by more than a single sperm (polyspermy). Conventional IVF typically achieves fertilization rates ranging between 50% and 70%.
Sperm penetration also activates the oocyte and stimulates the second meiotic division, resulting in segregation of chromatids between the oocyte and the second polar body. Oocytes are evaluated for evidence of fertilization at approximately 18 hours after insemination. A normally fertilized oocyte exhibits two distinct pronuclei, one derived from the oocyte and the other from the sperm, and two polar bodies in the peri-vitelline space. The zygotes must be carefully inspected for the presence of extra pronuclei because polyploid embryos may cleave normally and go unrecognized at later stages of development. Polyploidy can be observed in up to 5-10% of embryos overall, but is far more prevalent in immature oocytes (up to 30%) than in mature oocytes (1-2%).465,466 Besides polyspermy, polyploidy may result from digyny (fertilization of a diploid oocyte), due to meiotic spindle errors or failure to extrude a polar body, which are more commonly associated with immature, aging, or postmature oocytes.467,468 The fertilization process requires approximately 24 hours and ends with the first mitotic division (cleavage).
Past failure of fertilization or severe male factor infertility requires ICSI, which yields pregnancy rates in couples with male factor infertility that compare favorably with those in couples without a male factor.469 In the absence of a male factor, ICSI offers no clinical advantage over conventional IVF470,471; in fact, evidence suggests that standard IVF yields higher implantation and clinical pregnancy rates.3,470
When there is no ejaculate (aspermia) or only rare or no sperm (azoospermia) in the ejaculate, a variety of methods can be used to retrieve sperm for fertilization. Donor sperm also can be used, by design or as a contingency should efforts to retrieve sperm on the day of oocyte retrieval fail. Men with ejaculatory failure have no ejaculate or retrograde ejaculation. Ejaculatory failure may result from neurologic dysfunction or injury to the sympathetic outflow tracts that control emission and ejaculation (spinal cord injury, diabetes mellitus, multiple sclerosis, retroperitoneal surgery) or can be psychogenic in origin. Azoospermia may relate to ductal obstruction (obstructive azoospermia) or result from Sertoli cell-only syndrome, maturation arrest, or hypospermatogenesis (non-obstructive azoospermia). The diagnostic evaluation for aspermic and azoospermic men is described in detail in Chapter 30.
Sperm Retrieval Techniques
In the past, men with non-obstructive azoospermia were considered sterile and untreatable by any means other than the use of donor sperm. However, testis biopsy specimens in such men often demonstrate sperm,472 suggesting low level production of sperm that do not survive epididymal transit to reach the ejaculate.473 Whereas conventional wisdom was that sperm must traverse the male reproductive tract to acquire the ability to fertilize an oocyte, success with ICSI using epididymal or testicular sperm has demonstrated otherwise. Even grossly immature sperm (round spermatid nuclear injection; ROSNI) have been used to achieve fertilization, albeit with limited success.474
It is important to emphasize again that genetic evaluation and counseling are indicated for men with severe seminal abnormalities before their sperm are used for ICSI. Men with congenital bilateral absence of the vas deferens (CBAVD) or less severe forms of vasal aplasia, and their female partners, should be screened for cystic fibrosis gene mutations
before any attempts at pregnancy via ART to determine the risk for transmitting cystic fibrosis or CBAVD to offspring.475,476 and 477 Men with non-obstructive azoospermia or severe oligospermia (less than 5 million/mL) should be offered karyotyping and screening for Y chromosome microdeletions.477
before any attempts at pregnancy via ART to determine the risk for transmitting cystic fibrosis or CBAVD to offspring.475,476 and 477 Men with non-obstructive azoospermia or severe oligospermia (less than 5 million/mL) should be offered karyotyping and screening for Y chromosome microdeletions.477
Sperm Recovery in Men with Retrograde Ejaculation
Men with documented retrograde ejaculation may be treated with sympathomimetics directed at control of the internal sphincter (imipramine 25 mg twice daily or 50 mg at bedtime, pseudoephedrine 60 mg, ephedrine 25-50 mg four times daily, phenylpropanolamine 50-75 mg twice daily). When medical treatment proves unsuccessful, sperm can be recovered directly from the bladder after masturbation; best results are achieved when urine pH and osmolality (300-380 mOsm/L) are carefully controlled by alkalinizing the urine (sodium bicarbonate 650 mg four times daily, beginning 1-2 days before collection) and controlling fluid intake.478,479 Alternatively, the bladder can be filled with buffered medium immediately before ejaculation.
Vibratory Stimulation and Electroejaculation
In men with psychogenic ejaculatory failure or spinal cord injuries below the T6 level, vibratory stimulation often can succeed in producing an ejaculate. Rectal probe electrical stimulation (electroejaculation) is recommended for men who fail vibratory stimulation and those with previous retroperitoneal surgery.480,481 Induced ejaculations may be retrograde and further require the procedures described above. Because electroejaculates frequently exhibit asthenospermia and teratospermia, ICSI is often necessary.
Epididymal Sperm Aspiration
Sperm can be obtained by microsurgical epididymal sperm aspiration (MESA) at the time of vasoepididymostomy or as an isolated procedure in men with CBAVD or uncorrectable obstructions. The technique involves incision of an isolated dilated tubule, gradually moving more proximally, if necessary, until sperm are obtained.482,483 Sperm are collected into a micropipette by capillary action with gentle compression of the testis and epididymis and flushed into a container with a small volume of IVF culture medium. Recovered sperm are cryopreserved in multiple aliquots for use in IVF cycles, if required.484
Percutaneous epididymal sperm aspiration using a fine needle also has been used successfully to obtain sperm and achieve pregnancy,485,486 but the technique is less reliable, the small quantities of sperm obtained are sometimes inadequate to allow cryopreservation, and the pregnancy rates achieved generally have been lower than with the open technique.
Testicular Sperm Extraction and Aspiration
In men with non-obstructive azoospermia and those in whom epididymal sperm aspiration techniques fail or do not apply, sperm can be retrieved using any of three other techniques. Open microsurgical testicular sperm extraction (TESE) yields the greatest number of sperm with potential for cryopreservation. Percutaneous core biopsy or aspiration of the
testis has also been described but is most applicable in men with normal spermatogenesis and obstructive azoospermia.487,488
testis has also been described but is most applicable in men with normal spermatogenesis and obstructive azoospermia.487,488
Using the preferred open microsurgical technique, sperm can be retrieved from the majority of men, even those with non-obstructive azoospermia. Magnification minimizes the risk of injury to the testicular blood supply, increases the probability of retrieving a blood-free biopsy specimen, and allows identification of larger caliber tubules that are more likely to yield sperm.489,490 Normal pregnancies have been achieved even in those with congenital or acquired testicular failure,491 post-chemotherapy azoospermia,492 and Klinefelter syndrome.493
In men with non-obstructive azoospermia, TESE is best performed on the day of or day before oocyte retrieval, when possible, and no earlier than approximately 6 months after any previous biopsy or TESE procedure, for several reasons. First, up to one-third of men with apparent non-obstructive azoospermia may exhibit sperm in their ejaculate on the day of planned retrieval and will not require TESE.494 Second, sperm retrieved from men with non-obstructive azoospermia may not be motile or even viable after cryopreservation and thawing and ICSI using immotile sperm may yield poorer results than when performed with motile sperm.495 Finally, the likelihood of successful retrieval of viable sperm for ICSI is significantly reduced when TESE is performed soon after a testis biopsy or previous TESE.495 Matched donor sperm should be available in case they are needed, because TESE yields viable sperm in only about half of men with non-obstructive azoospermia.489,496,497 When TESE cannot be performed near the time of oocyte retrieval, elective TESE can be performed and the recovered sperm cryopreserved; the risk of having no viable sperm after thawing is real but relatively small, and donor sperm can be used if needed.498,499 and 500
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


