Assessment of Fetal Well-Being
Catherine Y. Spong
In the beginning of the 19th century, reports on the presence of fetal heart tones were published, and nearly 150 years later, continuous fetal heart rate (FHR) monitoring became a reality. By 1998, electronic fetal monitoring was used in 84% of all U.S. births, regardless of whether the primary caregiver was a physician or a midwife. With the advent of these technologies, fetal monitoring is implemented in nearly all pregnancies, either in the antepartum or intrapartum period. The challenge of fetal surveillance is to identify those fetuses whose physiologic defense mechanisms are compromised in order to be able to act before decompensation has occurred. The goal is to prevent fetal and neonatal morbidity and especially mortality.
Why Perform Fetal Monitoring?
Since its inception, the primary objective of FHR monitoring has been to identify the fetus in distress so that measures might be taken in time to avert permanent fetal damage or death. However, a clear consensus regarding the definition of fetal distress has not been established. It has been described as “a condition in which fetal physiology is so altered as to make death or permanent injury a probability within a relatively short period of time” and is usually considered to denote disruption of normal fetal oxygenation, ranging from mild hypoxia to profound fetal asphyxia. The term hypoxia refers to the reduction of tissue oxygen supply below physiologic levels. Asphyxia, derived from the Greek word meaning “a stopping of the pulse,” implies a combination of hypoxia and metabolic acidosis. Historically, the clinical diagnosis of birth asphyxia has been based on findings such as meconium-stained amniotic fluid, abnormal FHR patterns, low Apgar scores, abnormal blood gases, and neonatal neurologic abnormalities. When present together, these findings are highly suggestive of a recent asphyxial insult. Isolated abnormalities, however, correlate poorly with birth-related asphyxia and subsequent neurologic impairment. In 2002, the American College of Obstetricians and Gynecologists Task Force on Neonatal Encephalopathy and Cerebral Palsy stated the criteria to define an acute intrapartum event sufficient to cause cerebral palsy (CP).
Essential criteria (must meet all four):
Evidence of a metabolic acidosis in fetal umbilical cord arterial blood obtained at delivery (pH <7 and base deficit >12 mmol/L).
Early onset of severe or moderate neonatal encephalopathy in infants born at 34 or more weeks gestation.
CP of the spastic quadriplegic or dyskinetic type.
Exclusion of other identifiable etiologies such as trauma, coagulation disorders, infectious conditions, or genetic disorders.
Criteria that collectively suggest an intrapartum timing (within close proximity to labor and delivery, e.g., 0–48 hours) but are nonspecific to asphyxial insults:
A sentinel (signal) hypoxic event occurring immediately before or during labor.
A sudden and sustained fetal bradycardia or the absence of FHR variability in the presence of persistent, late, or variable decelerations, usually after a hypoxic sentinel event when the pattern was previously normal.
Apgar scores of 0–3 beyond 5 minutes.
Onset of multisystem involvement within 72 hours of birth.
Early imaging study showing evidence of acute nonfocal cerebral abnormality.
At the cellular level, asphyxia triggers a cascade of events, including membrane depolarization, disruption of energy metabolism, altered neurotransmission, ion shifts, protease activation, free radical production, and phospholipid degradation. Profound and prolonged asphyxia may result in cell death and, eventually, death of the organism. Sublethal asphyxia may lead to multiorgan system dysfunction. Severe asphyxial brain injury may lead to long-term neurodevelopmental impairment.
Cerebral palsy is a major disorder of neurodevelopment, defined as “a chronic disability, characterized by aberrant control of movement and posture, appearing early in life and not the result of recognized progressive disease.” It may be accompanied by mental retardation (41%), seizures (23%), or cortical visual impairment. The insult responsible for the development of CP may occur at any time during the prenatal, perinatal, or postnatal periods. Unlike other major neurodevelopmental disorders, the relationship between CP and abnormal or difficult birth has long been recognized, publicly dating back to a treatise presented by William John Little in 1862. In 1943, Windle demonstrated clinical and histopathologic evidence of neural damage in experimentally asphyxiated fetal guinea pigs. He later reported the effects of prolonged anoxia on fetal rhesus monkeys. Total anoxia for less than 8 minutes did not produce consistent injury, whereas anoxia for more than 10 minutes invariably resulted in neuropathology. There were no survivors beyond 20 to 25 minutes of anoxia. The pattern of injury produced by prolonged anoxia, however, did not correlate with the cerebral injury, mental retardation, and spasticity seen in CP. Later work demonstrated that prolonged partial asphyxia in monkeys produced acidosis, late FHR decelerations, and neuropathologic defects consistent with the findings in the common forms of CP. In addition to lesions in the thalamus and basal ganglia, prolonged partial asphyxia caused generalized cerebral necrosis or focal necrosis in the parasagittal regions and the border zones between the parietal and occipital lobes.
Although early studies created and fostered the assumption that birth-related asphyxia was the primary cause of CP, recent evidence challenges this assumption. In 1986, Nelson and Ellenberg reported a multivariate analysis of risk in 189 cases of CP. After accounting for major congenital malformations, low birth weight, microcephaly, and alternative explanations for the disorder, they were able to attribute only 9% of CP cases to birth asphyxia. Others have reached similar conclusions. Although birth asphyxia nearly tripled the odds of developing CP, only 8.2% of CP cases were potentially attributable to birth asphyxia.
As early as the 19th century, researchers using auscultation recognized that certain FHR patterns were associated with adverse perinatal outcome. The introduction of direct electronic fetal monitoring and fetal scalp blood sampling in the 1960s provided tools for evaluating the fetus. FHR decelerations have been found to be correlated with fetal acidosis. Fetuses with no decelerations, early decelerations, or mild variable decelerations had average scalp pH values greater than or equal to 7.29, whereas those with severe variable or late decelerations had pH values less than or equal to 7.15. In addition, FHR variability was found to be correlated with scalp pH values, as fetuses with normal FHR variability had higher scalp pH values than those with decreased variability. The absence of FHR accelerations was correlated with poor perinatal outcome, and the presence of FHR accelerations has been shown to predict normal scalp pH values. With the development of indirect monitoring techniques, the experience derived from direct intrapartum monitoring became applicable to the antepartum period, leading to the development of antepartum testing.
Antepartum fetal monitoring has the goal of identifying the fetus at risk, allowing sufficient time to intervene before permanent injury or death occur. Intrapartum fetal monitoring should be able to identify three groups of fetuses:
The fetus that is not affected by labor
The fetus that is negatively affected by labor but has enough reserve to compensate fully and is in no immediate danger
The fetus that is negatively affected and lacks the reserve to compensate, thus uses its key resources to survive and is in danger for morbidity/mortality.
It is the third group that would most benefit from intervention.
Who Should Be Monitored?
Antepartum fetal monitoring typically is offered to patients at increased risk of fetal or neonatal morbidity/mortality. These include maternal medical complications, fetal conditions, and pregnancy complications. Overall, the studies supporting the methods of testing, the timing, and initiation are extremely varied; thus, absolute guidelines based on scientific evidence cannot be established. However, recommended gestational ages for initiation of testing, timing, and methods for specific maternal conditions and the supportive evidence can be found in Table 10.1.
Intrapartum fetal monitoring has remained controversial since its inception. The FHR may be evaluated by auscultation or by electronic monitoring. Auscultation is typically performed with a DeLee stethoscope or Doppler ultrasound. Electronic monitoring can be either performed externally or internally. External monitors use a Doppler device with computerized logic to interpret and count the signals. Internal monitoring uses a fetal electrode that records the fetal electrocardiogram (ECG). Well-controlled studies have shown the equivalence of intermittent auscultation to continuous fetal heart monitoring when
auscultation was performed at specific intervals with a 1:1 nurse-to-patient ratio. The intensity of monitoring is based on risk factors, with more intensive surveillance required for high-risk pregnancies.
auscultation was performed at specific intervals with a 1:1 nurse-to-patient ratio. The intensity of monitoring is based on risk factors, with more intensive surveillance required for high-risk pregnancies.
TABLE 10.1 General Guidelines for Initiation of Antenatal Testing | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
What Can We Monitor?
Fetal Heart Rate
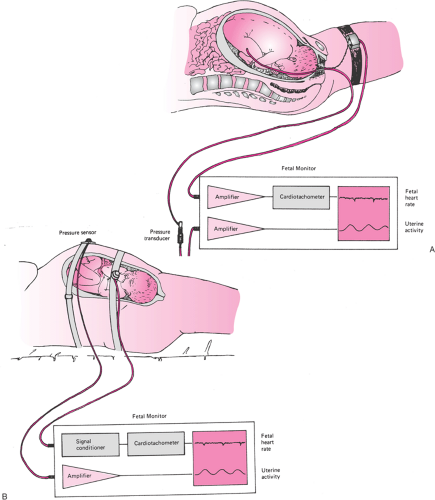
The FHR can be monitored and recorded indirectly through the use of an ultrasound transducer or directly via a subcutaneous ECG electrode placed on the fetus (Fig. 10.1). The indirect method can be used throughout pregnancy and has no contraindications. Using the indirect method, ultrasound waves originating from the transducer penetrate the tissues and are reflected by tissue interfaces. Waves reflected from the moving structures of the fetal heart return to the transducer and are translated into electrical signals. In the direct method, the subcutaneously placed ECG electrode detects electrical impulses originating in the fetal heart. Amplified signals are processed by a cardiotachometer, comparing each incoming QRS complex to the one immediately preceding it. The interval between the two complexes is used to calculate a heart rate. The process is repeated, with each cardiac cycle yielding a graphic beat-to-beat display of the FHR. The direct method requires rupture of the fetal membranes for placement of the ECG electrode. In addition, in light of the potential risk of infection, the
electrode for direct assessment only should be used when the benefits outweigh this risk.
electrode for direct assessment only should be used when the benefits outweigh this risk.
Uterine Activity
Similar to FHR, the detection and measurement of uterine activity can be performed indirectly or directly (Fig. 10.1). Indirect assessment of uterine activity is performed with a pressure transducer (tocodynamometer) applied snugly to the maternal abdomen over the uterine fundus. Uterine contractions exert pressure on the abdominal wall that is transmitted to the tocodynamometer. Changes in pressure are converted into signals and plotted on the uterine activity graph. The indirect method is noninvasive and can be performed at any time during pregnancy. The limitations of the indirect method are that the readout can be used only to determine contraction frequency, not strength of the contraction (a tightly fit belt will record larger contractions than a loosely fitting or misplaced belt), and the ability to detect contractions in extremely obese women may be difficult. Direct assessment of uterine activity employs a thin, flexible intrauterine pressure catheter (IUPC) placed transcervically into the amniotic cavity. Intrauterine pressure is transmitted from the amniotic fluid through the fluid-filled IUPC to a pressure transducer. The transducer converts pressure measurements into electrical signals, and continuous pressure readings are displayed on the uterine activity graph. The direct assessment is invasive and requires ruptured membranes. The direct method allows the readout of both the frequency and the strength of the uterine contractions. This can be especially useful in the evaluation and assessment of patients with prolonged labor.
Fetal State (Tone/Breathing/Movements)
Using real-time ultrasound, the state of the fetus can be evaluated. Typically included in this evaluation are assessments of fetal tone, movements, and breathing. Fetal voiding and swallowing also can be evaluated. Specific assessments of fetal tone, breathing, and movements are discussed later in the section Biophysical Profile.
Amniotic Fluid Volume
The volume of amniotic fluid is a measure of fetal well-being. By the second trimester, the predominant source of amniotic fluid is fetal urine. The level of amniotic fluid is thought to represent “long-term” fetal well-being. A compromised fetus will preferentially shunt blood to the major organs, such as the central nervous system [CNS] and adrenals, and away from others, such as the kidney. Decreased fetal renal perfusion results in a decrease in fetal renal function and subsequent oligohydramnios. The amniotic fluid can be assessed ultrasonographically. There are a number of methods to quantitate the volume, including the amniotic fluid index (AFI), single deepest pocket, two-dimensional pocket, and a subjective assessment.
How Do We Monitor?
Equipment
The fetal monitor tracing is a continuous paper strip composed of two cartesian graphs. The FHR tracing is displayed on the upper graph, with time on the x-axis and heart rate on the y-axis (range 30 to 240 beats per minute). Uterine activity is displayed on the lower graph, with time on the x-axis and pressure on the y-axis (range 0 to 100 mm Hg). Heart rate and uterine activity are plotted separately on the heat-sensitive paper by two thermal pens. On both grids, fine vertical lines represent 10-second intervals, and heavy lines denote 1-minute intervals. In the United States, the standard paper speed is 3 cm per minute.
Interpretation of the Fetal Monitor Tracing
Analysis of the fetal monitor strip requires a systematic approach. First, the FHR is analyzed with respect to (a) the baseline, (b) variability, and (c) periodic patterns, including FHR accelerations and decelerations (Table 10.2). Uterine activity is evaluated with attention to the frequency, duration, and strength of contractions as well as the baseline uterine tone between contractions.
TABLE 10.2 Fetal Monitor Interpretation | |
|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree