Background
Preterm birth remains a major cause of neonatal morbidity and mortality worldwide. Short cervical length (CL) as measured by transvaginal ultrasound (TVU) in the second trimester represents the single most predictive risk factor for spontaneous preterm birth. Previous studies have addressed, in part, the limitations of TVU availability by utilizing a cervicometer to screen patients for short cervix, identifying those patients who may not benefit from TVU CL screening. In view of the prior studies indicating that a cervicometer measurement may have a high negative predictive value (NPV) for a sonographically short cervix, we sought to identify the ideal cervicometer threshold value in a prospective, multicenter study.
Objective
The primary objective was to determine the cervicometer CL measurement threshold that provides a high NPV for the identification of patients who are highly unlikely to have a TVU CL measurement ≤20 and ≤25 mm and, therefore, may forego TVU.
Study Design
This prospective study, executed in 5 US centers, included 401 women ≥18 years of age who provided written informed consent to undergo CL measurement in the mid trimester. All women underwent both cervicometer- and TVU-measured CLs by individuals blinded to results of the other measurement. Both measurements were performed at 17-23 weeks’ gestation (visit 1) and repeated at 24-29 weeks’ gestation (visit 2). All TVU measurement images were reviewed by a central reader. Test characteristics and receiver operating characteristic curves were created to determine and confirm the optimal cervicometer CL threshold, maximizing the NPV.
Results
In all, 358 subjects were evaluable at visit 1 and 267 at visit 2. At visit 1, the average TVU CL was 38.7 ± 7.6 mm and the average cervicometer CL was 30.3 ± 8.8 mm. Similar measurements were seen at visit 2. Receiver operating characteristic curves were utilized to graphically identify a cervicometer CL threshold of 30 mm that maximized sensitivity while minimizing the false-positive rate. The 30-mm cervicometer CL threshold provided a 98-100% NPV and 0.0 negative likelihood ratio for identification of women who have a low likelihood to have a sonographic short cervix (ie, transvaginal CL ≤20 mm or ≤25 mm). The 17-23 weeks′ gestation 30-mm cervicometer CL threshold has 100% sensitivity, 45-46% specificity, and 1.8 and 0.0 positive and negative likelihood ratios to predict sonographic CL ≤20 and ≤25 mm.
Conclusion
Cervicometer CL screening successfully identifies women at low risk for short transvaginal CL. Use of a 30-mm threshold by cervicometer CL measurement confers a 98-100% NPV, with high sensitivity and moderate specificity to predict a TVU short CL. Cervicometer measurement of CL may permit almost 50% of women to avoid TVU.
Introduction
Preterm birth (PTB) remains a major cause of neonatal morbidity and mortality worldwide. Short cervical length (CL) as measured by transvaginal ultrasound (TVU) in the second trimester represents the single most predictive risk factor for spontaneous PTB, with the relative risk exceeding that of a prior spontaneous PTB. A series of studies evaluating the effectiveness of vaginal progesterone has provided evidence that the high rate of PTB in women with a short CL in the second trimester can be substantially reduced and neonatal outcomes improved in a cost-effective manner. A compelling case has been made that universal midtrimester CL screening meets World Health Organization criteria for a good screening test. The Society for Maternal-Fetal Medicine and American Congress of Obstetricians and Gynecologists endorse the use of vaginal progesterone to prevent PTB in women with a second-trimester short CL, and support a protocol of universal second-trimester CL screening in singleton pregnancies. Universal CL screening with TVU has been implemented at some centers, but poses cost, access, resource (eg, sonographer, equipment), and time limitations.
Previous studies have addressed, in part, the limitations of TVU availability by utilizing a cervicometer to screen patients for short cervix, identifying those patients who may not benefit from TVU CL screening. Cervicometer measurements of the left and right cervical portio length are highly consistent, and measurements have an excellent sensitivity and negative predictive value (NPV) in identifying patients with TVU short CL. In view of the prior studies indicating that a cervicometer measurement may have a high NPV for a sonographically short cervix, we sought to identify the ideal cervicometer threshold value in a prospective, multicenter study.
Materials and Methods
Our study population included women at least 18 years of age from 17-23 weeks’ gestation at enrollment with a singleton pregnancy; no known anomalies of the lower uterine segment, cervix, or vagina; and no history of cervical surgery. Exclusion criteria for enrollment included cerclage (in a prior or the present pregnancy), fetal demise, cervical dilatation ≥3 cm, active herpetic lesions, known HIV infection, therapeutic reduction of multiple gestation, or evidence of preterm labor (ie, treatment tocolytics, hospitalization for suspected preterm labor) in the current pregnancy. All enrollees signed written, informed consent. Nonrandomized clinical trial of a measurement device in a healthy population did not require registration with clinicaltrials.gov .
Study participants were scheduled for 2 CL evaluation study visits, and received their usual prenatal care from their provider. The initial study visit (visit 1) occurred at 17 0/7-23 0/7 weeks’ gestation, typically the day of enrollment, and the follow-up study visit (visit 2) at 24 0/7-29 0/7 weeks’ gestation, with a minimum of 28 days between visits. At visit 1, maternal history was obtained (age, race, weight, height, progesterone use in current pregnancy, and use of tobacco, alcohol, and recreational drugs), in addition to the standard obstetrical and gynecological history and subsequently updated at visit 2.
A standardized TVU imaging protocol consistent with the Perinatal Quality Foundation CL Education and Review program was utilized across all 5 sites and every sonographer was required to submit certification images to a central reader (J.K.B.) prior to assessing any study subjects. At each of the 2 study visits, a TVU to assess CL was performed, and 6 images meeting protocol-specified requirements were obtained: 3 prior to fundal pressure and 3 following fundal pressure. The sonographer then reported the shortest CL of each set of 3 images. To further ensure consistency, images were reviewed by the central reader; if necessary, the central reader (blinded to cervicometer results and clinical outcome) was able to correct the sonographer assessment of CL based on image quality, consistency, and accuracy.
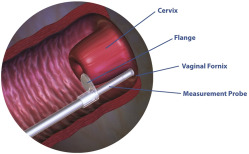
For each subject, a separate nurse or physician examiner, blinded to the TVU measurements, performed the cervicometer measurements. Each user of the cervicometer was trained in person by the same trainer (F.J.K.) using the cervicometer and a device that replicated 3 cervices of varying lengths. Users were instructed on the positioning of device, proper placement of the flange, locking of the device, and reading of the measurement. Users were required to successfully measure the 3 mock cervices within a 1-mm margin of error in the presence of the trainer. The CerviLenz cervicometer device (Cervilenz Inc, Chagrin Falls, OH) is a single-use (disposable) device intended to measure CL from the external os to the lateral vaginal fornix during pregnancy ( Figure 1 ). It is composed of a handle with a button lock, a measurement probe with calibrated markings in millimeters, and an outer slideable sleeve with a flange. While the patient’s bladder was emptied prior to TVU, there was no specific protocol requirement to empty it prior to the speculum examination and cervicometer measurement. The cervicometer examiner inserted a vaginal speculum, typically for 1-4 minutes, with the subject in conventional lithotomy position and measured vaginal CL in both the right and left lateral fornix (3 and 9 o-clock) positions. If the values differed by >3 mm, the process was repeated. If repeat values again differed by >3 mm, presumably due to anatomical differences of the depth of the fornices or the vaginal portion of the cervix, the subject was excluded from the study. The cervicometer and TVU measurements were performed within 2 hours of each other. Delivery outcomes were recorded, with preterm delivery defined as delivery at <37 weeks (results reported separately). If a subject delivered at a site other than the study site where she was enrolled in this study, the study site attempted to obtain the delivery medical records. Any adverse effects observed or reported by the subject were recorded. Patient satisfaction with the use of each measurement method was not specifically addressed.
Analyses were performed using the shortest recorded cervicometer measurement and the shortest measurement obtained for the TVU (with or without fundal pressure). CL measurements obtained outside of the protocol gestational age (GA) windows at each visit–specified as 17-23 and 24-29 weeks’ GA–were not included in the analyses. The number of certified sonographers involved in the study at each site ranged from 4-8 (median 6). The number of trained cervicometer examiners at each site ranged from 1-9 (median 2).
Statistical analyses were performed independent of the study investigators and sponsor by InClin Inc (San Mateo, CA). Clinical operations and additional statistical support were provided by Blue Phoenix Biopharmaceutical Consulting (Dexter, MI). Receiver operating characteristic (ROC) curves were generated for prediction of TVU CL ≤25 mm and ≤20 mm, as both TVU threshold values have been utilized as criteria for treatment initiation (ie, cerclage, vaginal progesterone). The ROC curves were used to graphically identify inflection points of potential cervicometer CL thresholds above which women may be able to avoid having a TVU CL, maximizing sensitivity while minimizing the false-positive rate. The test characteristics (sensitivity, specificity, NPV and positive predictive value [PPV], and likelihood ratios) of the cervicometer thresholds, along with 2-sided exact 95% confidence intervals, were calculated using the Clopper-Pearson method. We identified cervicometer thresholds that maximized sensitivity and NPV so as to minimize the chance of a woman with a short TVU CL being missed when evaluated by cervicometer (false negatives). The NPV observed at each cervicometer threshold was calculated, along with the 2-sided 95% confidence interval and the P value from the binomial proportion test (at an α level of 0.05) of the H o : NPV = 80% vs H a : NPV ≠ 80% hypothesis. Descriptive statistics for cervicometer and TVU CL measurements were assessed for subjects by GA at measurement. As both cervicometer and TVU CL measures at each visit were normally distributed, data are presented as mean ± SD. However, differences between cervicometer and TVU measurements (accuracy) and changes in cervicometer and TVU measures from visit 1-2 were not normally distributed and are presented as median and interquartile range (IQR). The change in cervicometer measurements from visit 1-2 was calculated for all subjects in the evaluable population, using the shortest CL observed at each visit.
For a 2-sided test at α = 0.05, a total of 257 subjects were required to provide 80% power to test the hypothesis that the cervicometer NPV relative to TVU (<25 mm) is equal to 80%. The overall sample size was increased to 400 subjects to account for a potential 20% screen failure and early withdrawal rate, as well as the desire to include at least 10 subjects with a short CL (TVU CL ≤25 mm), given the expected 3% short cervix rate. Of these 400 patients, we specifically recruited 100 with a previous PTB <37 weeks to increase the expected number of patients with a short CL (TVU CL ≤25 mm).
The study was approved by individual institutional review boards for 5 locations, including 4 university-affiliated obstetric centers (Thomas Jefferson University, Philadelphia, PA; Yale University, New Haven, CT; Spectrum Health Hospital, Grand Rapids, MI; and Christiana Care Health Services, Newark, DE) and 1 private maternal-fetal medicine consulting practice (Regional Obstetrical Consultants, Chattanooga, TN). All 5 institutions offered universal CL screening by TVU.
Materials and Methods
Our study population included women at least 18 years of age from 17-23 weeks’ gestation at enrollment with a singleton pregnancy; no known anomalies of the lower uterine segment, cervix, or vagina; and no history of cervical surgery. Exclusion criteria for enrollment included cerclage (in a prior or the present pregnancy), fetal demise, cervical dilatation ≥3 cm, active herpetic lesions, known HIV infection, therapeutic reduction of multiple gestation, or evidence of preterm labor (ie, treatment tocolytics, hospitalization for suspected preterm labor) in the current pregnancy. All enrollees signed written, informed consent. Nonrandomized clinical trial of a measurement device in a healthy population did not require registration with clinicaltrials.gov .
Study participants were scheduled for 2 CL evaluation study visits, and received their usual prenatal care from their provider. The initial study visit (visit 1) occurred at 17 0/7-23 0/7 weeks’ gestation, typically the day of enrollment, and the follow-up study visit (visit 2) at 24 0/7-29 0/7 weeks’ gestation, with a minimum of 28 days between visits. At visit 1, maternal history was obtained (age, race, weight, height, progesterone use in current pregnancy, and use of tobacco, alcohol, and recreational drugs), in addition to the standard obstetrical and gynecological history and subsequently updated at visit 2.
A standardized TVU imaging protocol consistent with the Perinatal Quality Foundation CL Education and Review program was utilized across all 5 sites and every sonographer was required to submit certification images to a central reader (J.K.B.) prior to assessing any study subjects. At each of the 2 study visits, a TVU to assess CL was performed, and 6 images meeting protocol-specified requirements were obtained: 3 prior to fundal pressure and 3 following fundal pressure. The sonographer then reported the shortest CL of each set of 3 images. To further ensure consistency, images were reviewed by the central reader; if necessary, the central reader (blinded to cervicometer results and clinical outcome) was able to correct the sonographer assessment of CL based on image quality, consistency, and accuracy.
For each subject, a separate nurse or physician examiner, blinded to the TVU measurements, performed the cervicometer measurements. Each user of the cervicometer was trained in person by the same trainer (F.J.K.) using the cervicometer and a device that replicated 3 cervices of varying lengths. Users were instructed on the positioning of device, proper placement of the flange, locking of the device, and reading of the measurement. Users were required to successfully measure the 3 mock cervices within a 1-mm margin of error in the presence of the trainer. The CerviLenz cervicometer device (Cervilenz Inc, Chagrin Falls, OH) is a single-use (disposable) device intended to measure CL from the external os to the lateral vaginal fornix during pregnancy ( Figure 1 ). It is composed of a handle with a button lock, a measurement probe with calibrated markings in millimeters, and an outer slideable sleeve with a flange. While the patient’s bladder was emptied prior to TVU, there was no specific protocol requirement to empty it prior to the speculum examination and cervicometer measurement. The cervicometer examiner inserted a vaginal speculum, typically for 1-4 minutes, with the subject in conventional lithotomy position and measured vaginal CL in both the right and left lateral fornix (3 and 9 o-clock) positions. If the values differed by >3 mm, the process was repeated. If repeat values again differed by >3 mm, presumably due to anatomical differences of the depth of the fornices or the vaginal portion of the cervix, the subject was excluded from the study. The cervicometer and TVU measurements were performed within 2 hours of each other. Delivery outcomes were recorded, with preterm delivery defined as delivery at <37 weeks (results reported separately). If a subject delivered at a site other than the study site where she was enrolled in this study, the study site attempted to obtain the delivery medical records. Any adverse effects observed or reported by the subject were recorded. Patient satisfaction with the use of each measurement method was not specifically addressed.




