Chapter 29
Aspiration
Risk, Prophylaxis, and Treatment
Geraldine O’Sullivan MD, FRCA, M. Shankar Hari Dip. Epi., MD, FRCA, FFICM
Chapter Outline
INCIDENCE, MORBIDITY, AND MORTALITY
GASTROESOPHAGEAL ANATOMY AND PHYSIOLOGY
RISK FACTORS FOR ASPIRATION PNEUMONITIS
RECOMMENDATIONS FOR CESAREAN DELIVERY
History
In 1848, Sir James Simpson first suggested aspiration as a cause of death during anesthesia. Hannah Greener, a 15-year old given chloroform for a toenail extraction, became cyanotic and “sputtered” during the anesthetic. A “rattling in her throat” then developed, and she soon died. Her physician administered water and brandy by mouth. Simpson1 contended that it was the aspiration of water and brandy, and not the adverse effects from the chloroform, that caused her death. In 1940, Hall published a report of 15 cases of aspiration, 14 of which occurred in mothers receiving inhalation anesthesia for a vaginal or cesarean delivery.2 Among the 14 obstetric cases, 5 mothers died.
Subsequently, Curtis Mendelson, in a landmark paper, reported a series of animal experiments that clearly described the clinical course and pathology of pulmonary acid aspiration.3 In the same paper, Mendelson also audited 44,016 deliveries at the New York Lying-In Hospital between 1932 and 1945. He identified 66 (0.15%) cases of aspiration, of which the aspirated material was recorded in 45 cases; 40 mothers aspirated liquid, and 5 aspirated solid food. Importantly, no mothers died of acid aspiration, but 2 mothers died of asphyxiation caused by the aspiration of solid food. At this time general anesthesia usually involved the inhalation of ether, often as Mendelson observed, by “a new and inexperienced intern.” Mendelson therefore advocated (1) the withholding of food during labor, (2) the greater use of regional anesthesia, (3) the administration of antacids, (4) the emptying of the stomach before administration of general anesthesia, and (5) the competent administration of general anesthesia. This advice became the foundation of obstetric anesthesia practice during subsequent decades.
Incidence, Morbidity, and Mortality
Maternal mortality from pulmonary aspiration of gastric contents has declined to almost negligible levels in the past 3 decades (Figure 29-1).4–6 This decline can probably be attributed to the following factors: (1) the greater use of neuraxial anesthesia; (2) the use of antacids, histamine-2 (H2) receptor antagonists, and/or proton-pump inhibitors; (3) the use of rapid-sequence induction of general anesthesia; (4) an improvement in the training of anesthesia providers; and (5) the establishment and enforcement of nil per os (NPO) policies. Arguably, the common use of neuraxial analgesic/anesthetic techniques, both during labor and for cesarean delivery, is the single most important factor in this remarkable decline in maternal mortality from pulmonary aspiration.
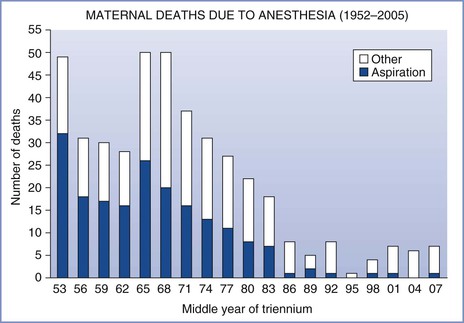
FIGURE 29-1 Maternal mortality from anesthesia and pulmonary aspiration in the United Kingdom, 1952-2008. (Compiled from data from references 4 through 6.)
The reported incidence of aspiration pneumonitis depends on the criteria used for making the diagnosis. The relative risk for aspiration in pregnant versus nonpregnant women can best be estimated from comparisons within single-study populations. Olsson et al.7 reported an overall incidence of aspiration of 1 in 2131 in the general population undergoing anesthesia and 1 in 661 in women undergoing cesarean delivery (i.e., a threefold higher aspiration risk). In two other surveys related to aspiration (one a retrospective review of 172,334 consecutive patients undergoing general anesthesia and the other a review of 133 cases of aspiration from the Australian Anaesthetic Incident Monitoring Study [AIMS]), there were no cases of pulmonary aspiration in women undergoing either elective or emergency cesarean delivery.8,9 However, in the latter two studies, emergency surgery was a significant predisposing factor for aspiration; this finding may be relevant for the practice of obstetric anesthesia, given that many obstetric surgical procedures are performed on an urgent or emergency basis. The AIMS study also implicated obesity as a significant risk factor for aspiration; others have noted that obesity is associated with an increased risk for maternal mortality.4,5
Morbidity and mortality associated with aspiration vary according to (1) the physical status of the patient, (2) the type and volume of aspirate, (3) the therapy administered, and (4) the criteria used for making the diagnosis. Since 1952, the Department of Health in the United Kingdom has published detailed triennial reports on all maternal deaths. Data from these reports, now administered by the body Mothers and Babies—Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK), indicate that death from pulmonary aspiration in obstetrics is vanishingly rare (see Figure 29-1).4–6 In the last five reports, which cover the 15-year period from 1994 to 2008, there were three maternal deaths from aspiration; one was an obese parturient, the second was a mother anesthetized 3 days after delivery, and the third was a women with a placenta previa who required an emergency cesarean delivery after eating a full meal and aspirated on emergence from general anesthesia. Although the number of anesthetics, particularly general anesthetics, administered to parturients during this 15-year period is unknown, there were approximately 10.5 million deliveries, indicating that the mortality rate from aspiration was less than 1 in 3.5 million deliveries.
Data on pulmonary aspiration in obstetrics in the United States are more difficult to evaluate. Despite the establishment of an ongoing National Pregnancy Mortality Surveillance System by the Centers for Disease Control and Prevention (CDC), it is often difficult to obtain adequate and detailed information about every maternal death. Prior to 1990, aspiration was the most common cause of anesthesia-related maternal death in the United States; it has been calculated that at that time there were 17 deaths related to general anesthesia for every one death related to regional anesthesia.10 By the early 1990s, this ratio had improved to 6 to 1. By 2002, death rates for both general and regional anesthesia were similar.11 However, mortality statistics are generally a poor predictor of maternal morbidity; several studies have indicated that perioperative aspiration can be associated with important morbidity in obstetric patients,12,13 and thus all possible measures still must be taken to prevent pulmonary aspiration in obstetric patients.
Gastroesophageal Anatomy and Physiology
Esophagus
In adults, the esophagus is approximately 25 cm long and the esophagogastric junction is approximately 40 cm from the incisor teeth. In humans, the proximal one third of the esophagus is composed of striated muscle but the distal end contains only smooth muscle. Muscular sphincters at both ends are normally closed. The cricopharyngeal or upper esophageal sphincter prevents the entry of air into the esophagus during respiration, and the gastroesophageal or lower esophageal sphincter prevents the reflux of gastric contents. The lower esophageal sphincter is characterized anatomically and manometrically as a 3-cm zone of specialized muscle that maintains tonic activity. The end-expiratory pressure in the sphincter is 8 to 20 mm Hg above the end-expiratory gastric pressure. The lower esophageal sphincter is kept in place by the phrenoesophageal ligament, which inserts into the esophagus approximately 3 cm above the diaphragmatic opening (Figure 29-2). The lower esophageal sphincter is not always closed; transient relaxations occur that account for the gastroesophageal reflux that healthy subjects experience.14
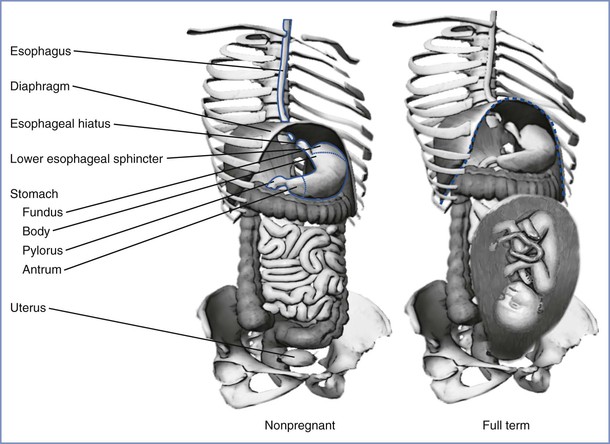
FIGURE 29-2 The stomach and its relationship to the diaphragm in nonpregnancy (left) and pregnancy (right). The stomach consists of a fundus, body, antrum, and pylorus. The function of the lower esophageal sphincter depends on the chronic contraction of circular muscle fibers, the wrapping of the esophagus by the crus of the diaphragm at the esophageal hiatus, and the length of the esophagus exposed to intra-abdominal pressure. The gravid uterus may encroach on the stomach and alter the effectiveness of the lower esophageal sphincter. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Gastrointestinal Motility
Differences in fasting and fed patterns of gut motility are now firmly established. During fasting, the main component of peristalsis is the migrating motor complex (MMC).15 Each MMC cycle lasts 90 to 120 minutes and comprises four phases: phase I has little or no electrical spike activity and thus no measurable contractions, phase II has intermittent spike activity, phase III has spikes of large amplitude and is associated with strong contractile activity, and phase IV is a brief period of intermittent activity leading back to phase I. The MMC first appears in the lower esophageal sphincter and stomach, followed by the duodenum, and finally the terminal ileum, at which time a new cycle begins in the lower esophageal sphincter and stomach. The phase of the MMC at the time of administration of certain drugs can affect absorption and thereby the onset of therapeutic effect.16 Eating abolishes the MMC and induces a pattern of intermittent spike activity that appears similar to that in phase II. The duration of the fed pattern is determined both by the calorie content and the type of nutrients in the meal.
The stomach, through the processes of receptive relaxation and gastric accommodation, can accept 1.0 to 1.5 L of food before intragastric pressure begins to increase. The contraction waves that propel food into the small intestine begin in the antrum. The pylorus closes midway through the contraction wave, allowing some fluid to exit into the duodenum but causing the remaining fluid to move retrograde toward the body of the stomach.17 The jet of fluid that exits the pylorus contains primarily liquid and fine particles. Large particles that lag behind are caught in the retrograde flow of fluid, which assists in their disintegration. Therefore, the manner by which individual components of a meal pass through the stomach depends on the particle size and the viscosity of the suspension. Small particles and fluids exit the stomach faster than larger particles.17 The outlet of the stomach—the pylorus—limits outflow by means of both its chronic tone and its anatomic position. The pylorus is higher than the most dependent portion of the stomach in both the supine and standing positions.17
Gastric Secretion
In one day, the stomach produces as much as 1500 mL of highly acidic fluid containing the proteolytic enzyme pepsin.18 Normal individuals can produce a peak acid output of 38 mmol/h.19 Acid is secreted at a low basal rate of approximately 10% of maximal output, even when the stomach is empty.19,20 There is diurnal variation in this basal rate of gastric acid secretion, with the lowest and highest outputs occurring in the morning and evening, respectively.
The stomach lining has two types of glands: pyloric and oxyntic. The pyloric glands contain chief cells, which secrete pepsinogen, the precursor for pepsin. The oxyntic glands contain the oxyntic cells, which secrete hydrochloric acid. Water molecules and carbon dioxide in the oxyntic cells combine to form carbonic acid, which dissociates into hydrogen ions and bicarbonate. The bicarbonate leaves the cell for the bloodstream, and the hydrogen ions are actively exchanged for potassium ions in the canaliculi connecting with the lumen of the oxyntic gland. The secretions of the oxyntic cell can contain a hydrochloric acid concentration as great as 160 mmol/L (pH 0.8).18 Proton pump inhibitors (PPIs) block the hydrogen ion pump on the canaliculi to decrease acid production.21
The pylorus contains G cells, which secrete gastrin into the bloodstream when stimulated by the vagus nerve, stomach distention, tactile stimuli, or chemical stimuli (e.g., amino acids, certain peptides). Gastrin binds to gastrin receptors on the oxyntic cell to stimulate the secretion of hydrochloric acid. Acetylcholine binds to muscarinic (M1) receptors on the oxyntic cell to cause an increase in intracellular calcium ion concentration, which results in hydrochloric acid secretion. Histamine potentiates the effects of both acetylcholine and gastrin by combining with H2 receptors on the oxyntic cell to increase the intracellular cyclic adenosine monophosphate concentration, leading to a dramatic increase in the production of acid.18 H2-receptor antagonists (e.g., ranitidine, famotidine) prevent histamine’s potentiation of acid production (Figure 29-3).
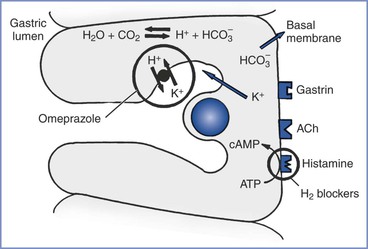
FIGURE 29-3 The oxyntic cell produces hydrogen ions that are secreted into the gastric lumen and bicarbonate ions that are secreted into the bloodstream. H2-receptor antagonists (e.g., ranitidine, famotidine) and proton-pump inhibitors (e.g., omeprazole) act on the oxyntic cell to reduce gastric acid secretion. H2-receptor antagonists block the histamine receptor on the basal membrane to decrease hydrogen ion production in the oxyntic cell. Omeprazole blocks the active transport of the hydrogen ions into the gastric lumen. ACh, acetylcholine; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CO2, carbon dioxide; H+, hydrogen ion; HCO3−, bicarbonate; H2O, water; K+, potassium.
Ingestion of Food
When a meal is eaten, the mechanisms that control the secretion of gastric juice and the motility and emptying of the stomach interact in a complex manner to coordinate the functions of the stomach. The response to eating is divided into three phases: cephalic, gastric, and intestinal. Chewing, tasting, and smelling cause an increase in the vagal stimulation of the stomach, which in turn increases gastric acid production. This represents the cephalic phase of digestion.18 In this phase, gastric acid output increases to approximately 55% of peak output.22 The gastric phase begins with the release of gastrin. Gastric acid secretion depends on antral distention, vagal activity, gastrin concentration, and the composition of the meal.20,22,23 Gastric acid secretion during a mixed-composition meal increases to approximately 80% of peak acid output.19 The intestinal phase begins with the movement of food into the small intestine and is largely inhibitory. Hormones (e.g., gastrin, cholecystokinin, secretin) and an enterogastric reflex further modulate gastric acid secretion and motility depending on the composition and volume of the food in the duodenum.18,24 This inhibition of gastric emptying by food in the duodenum enables the duodenal contents to be processed before more material is delivered from the stomach.
After the ingestion of a meal, gastric emptying depends on (1) the pre-meal volume, (2) the volume ingested, (3) the composition of the meal, (4) the size of the solids, (5) the amount of gastric secretion, (6) the physical characteristics of the stomach contents entering the duodenum, and (7) patient position.20,24–26 A mixture of liquids and solids passes through the stomach much more slowly than liquids alone. Gastric emptying is slowed by high lipid content, high caloric load, and large particle size.20,27,28 Thus, predicting an exact time for the passage of liquids and solids through the stomach is very difficult. For non-nutrient liquids (e.g., normal saline), the gastric volume decreases exponentially with respect to time.26 In one study, 90% of a 150-mL saline meal given to fasting adults in the sitting position passed through the stomach in a median time of 14 minutes; however, in adults in the left lateral position, the median time for gastric emptying was 28 minutes.25 In another study, 100% of a 500-mL saline meal given to fasting adults passed through the stomach within 2 hours, as determined by a polyethylene glycol marker.20 However, despite complete emptying of the saline test meal, the mean residual gastric volume at the end of 2 hours was 46 mL; this was because of greater secretion of gastric acid. Progressively less complete gastric emptying and higher mean residual gastric contents were observed with meals containing amino acids, glucose, and glucose with fat.20 These studies indicate that the volume and composition of the test meal, as well as the resulting gastric secretions, strongly affect gastric emptying and residual gastric content. For example, the subject described in Figure 29-4 responded to the test meal by secreting 800 mL of gastric juice and consequently the volume in the stomach remained high for almost 2 hours despite early, rapid emptying.29
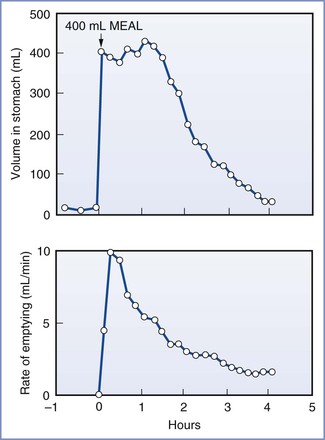
FIGURE 29-4 Volume of gastric contents and rate of gastric emptying in a subject eating a 400-mL meal of steak, bread, and vanilla ice cream. (From Malagelada JR, Longstreth GF, Summerskill WHJ, et al. Measurements of gastric functions during digestion of ordinary solid meals in man. Gastroenterology 1976; 70:203-10.)
Effects of Pregnancy on Gastric Function
Gastroesophageal reflux, resulting in heartburn, is a common complication of late pregnancy. Pregnancy compromises the integrity of the lower esophageal sphincter; it alters the anatomic relationship of the esophagus to the diaphragm and stomach, raises intragastric pressure, and in some women limits the ability of the lower esophageal sphincter to increase its tone.30–33 Progesterone, which relaxes smooth muscle, probably accounts for the inability of the lower esophageal sphincter to increase its tone.34 Lower esophageal pH monitoring has shown a higher incidence of reflux in pregnant women at term, even in those who are asymptomatic, than in nonpregnant controls. Therefore, at term gestation the pregnant woman who requires anesthesia should be regarded as having an incompetent lower esophageal sphincter. These physiologic changes return to their prepregnancy levels by 48 hours after delivery.33
Serial studies assessing gastric acidity during pregnancy have proved difficult to perform because pregnant women do not usually wish to swallow nasogastric tubes repeatedly for research purposes. However, in the most comprehensive study of gastric acid secretion during pregnancy, basal and histamine-augmented gastric acid secretion was measured in 10 controls and 30 pregnant women equally distributed throughout the three trimesters of pregnancy.35 No significant differences in basal gastric acid secretion were seen between the pregnant and nonpregnant women. However, when the women were divided into groups according to gestational age, the mean rate of gastric acid secretion was found to be reduced during the second trimester. The maximal response to histamine was significantly lower in women in the first and second trimesters than in women who were either not pregnant or in the third trimester of pregnancy.35
Assessment of gastric emptying during pregnancy and labor presents technical and ethical challenges, and a variety of techniques have been used (Table 29-1).36–60 Pregnancy does not significantly alter the rate of gastric emptying.39 In addition, gastric emptying was not found to be delayed in either obese or nonobese term pregnant women who ingested 300 mL of water after an overnight fast.56,57 However, management of obese parturients should take into account the possible presence of other associated problems in this group of patients (e.g., hiatal hernia or difficult airway). Gastric emptying appears to be normal in early labor but becomes delayed as labor advances49; the cause is uncertain. Pain is known to delay gastric emptying, but even when labor pain is abolished with epidural analgesia using a local anesthetic alone, the delay still occurs.60 Parenteral opioids cause a significant delay in gastric emptying, as do bolus doses of epidural and intrathecal opioids.40,45,49,51,58 Continuous epidural infusion of low-dose local anesthetic with fentanyl does not appear to delay gastric emptying until the total dose of fentanyl exceeds 100 µg.51
The plasma concentration of the gastrointestinal hormone motilin is decreased during pregnancy.61 Studies have shown either no change30,32,62 or an increase63 in the plasma concentration of gastrin.
Risk Factors for Aspiration Pneumonitis
Mendelson3 divided aspiration pneumonitis into two types: liquid and solid. Whereas the aspiration of solids could result in asphyxiation, Mendelson demonstrated that the sequelae from the aspiration of liquids were more severe clinically and pathologically when the liquid was highly acidic (Figure 29-5). His observations, together with the results from other investigations,64–72 suggest that the morbidity and mortality of aspiration depend on the following three variables: (1) the chemical nature of the aspirate, (2) the physical nature of the aspirate, and (3) the volume of the aspirate. Aspirates with a pH less than 2.5 cause a granulocytic reaction that continues beyond the acute phase.72 Aspiration of particulate material can engender a clinical picture with severity equal to or greater than that caused by the aspiration of acidic liquid.71 Aspiration of small volumes of neutral liquid results in a very low rate of mortality. However, aspiration of large volumes of neutral liquid results in a high mortality rate, presumably as a result of the disruption of surfactant by the large volume of liquid or from a mechanism similar to that seen in “near drowning.”67
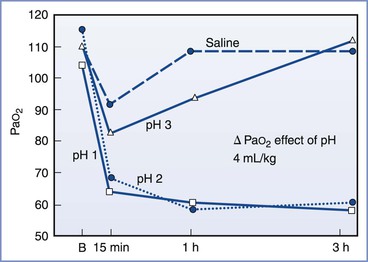
FIGURE 29-5 Relationship between acidity and PaO2. In this study, 4 mL/kg of fluid of varying pH was instilled into the tracheas of dogs. The severity of the hypoxemia correlated with the pH of the aspirate. A maximal decrease in PaO2 occurred with aspirates with a pH of less than 2.5. B, baseline. (From Awe WC, Fletcher WS, Jacob SW. The pathophysiology of aspiration pneumonitis. Surgery 1966; 60:232-9.)
Historically, anesthesia providers have considered a nonparticulate gastric fluid with a pH less than 2.5 and a gastric volume greater than 25 mL (i.e., 0.4 mL/kg) as risk factors for aspiration pneumonitis.64,69,70 No human study has directly addressed the relationship between preoperative fasting, gastric acidity and volume, and the risk for pulmonary aspiration during anesthesia.73,74 There appears to be a reasonable scientific basis using a gastric pH cut-off value of less than 2.5 as a risk factor. In animal experiments, the risk for aspiration pneumonitis clearly increased with decreasing pH of the tracheal aspirate.64,67 Awe et al.64 illustrated this concept in a graph of PaO2 versus time for aspirates of varying pH (see Figure 29-5).
Animal studies have also demonstrated that an increase in the volume of tracheal aspirate is associated with a higher risk for aspiration pneumonitis.67 However, the volume of aspirated material associated with risk has been disputed. The commonly accepted volume of 0.4 mL/kg (approximately 25 mL in a 70-kg adult) originated from an experiment in a single rhesus monkey in which 0.4 mL/kg of an acidic liquid was administered into the right mainstem bronchus and resulted in the animal’s death.70 The investigators made the assumption that this entire volume, if contained in the stomach, could be aspirated. However, Raidoo et al.75 demonstrated variability in the response of juvenile monkeys to different volumes of an acidic tracheal aspirate. Death was seen with aspirate volumes of 0.8 mL/kg and 1.0 mL/kg but not with volumes of 0.4 mL/kg and 0.6 mL/kg. Similarly, Plourde and Hardy76 refuted the assumption that all the gastric contents would be aspirated and demonstrated that gastric volumes of 0.4 mL/kg did not increase the risk for aspiration. Hence the gastric volume that puts a patient at risk for aspiration pneumonitis has not been determined. However, a reasonable goal of prophylactic therapy would be a gastric pH greater than 2.5 and a gastric volume as low as possible.
Pathophysiology
Aspiration pneumonitis (Mendelson’s syndrome) describes a chemical injury to the tracheobronchial tree and alveoli caused by the inhalation of sterile acidic gastric contents, whereas aspiration pneumonia may be regarded as an infectious process of the respiratory tract caused by the inhalation of oropharyngeal secretions that are colonized by pathogenic bacteria. Aspiration of gastric contents could therefore result in acid injury to the lung with or without bacterial and particulate matter–related effects.
Aspiration of acidic liquid injures the alveolar epithelium and results in an alveolar exudate composed of edema, albumin, fibrin, cellular debris, and red blood cells,3,69,71,72 whereas the aspiration of neutral, nonparticulate liquid leads to an alveolar exudate with minimal damage to the alveoli. The phospholipid and apoprotein composition of surfactant changes, exerting a negative effect on its surface-active properties.77 This effect leads to an increase in intraalveolar water and protein content and a loss of lung volume, resulting in a decrease in pulmonary compliance and intrapulmonary shunting of blood. The cellular debris and bronchial denuding cause bronchial obstruction. The exudative pulmonary edema, bronchial obstruction, reduced lung compliance, and shunting result in hypoxemia, increased pulmonary vascular resistance, and increased work of breathing. After the direct acid-mediated injury of the respiratory tract, an intense inflammatory response ensues from macrophage activation and secretion of cytokines, interleukins (IL) IL-1, IL-6, IL-8, and IL-10, and tumor necrosis factor-alpha (TNF-α).78 These inflammatory mediators lead to the chemotaxis, accumulation, and activation of neutrophils in the alveolar exudate, up-regulation of adhesion molecules within the pulmonary vasculature, and activation of the complement pathways. The neutrophils subsequently release oxidants, proteases, leukotrienes, and other proinflammatory molecules.78 Amplification of these inflammatory processes may result in the development of acute lung injury or acute respiratory distress syndrome (ARDS) (Figure 29-6).77–79
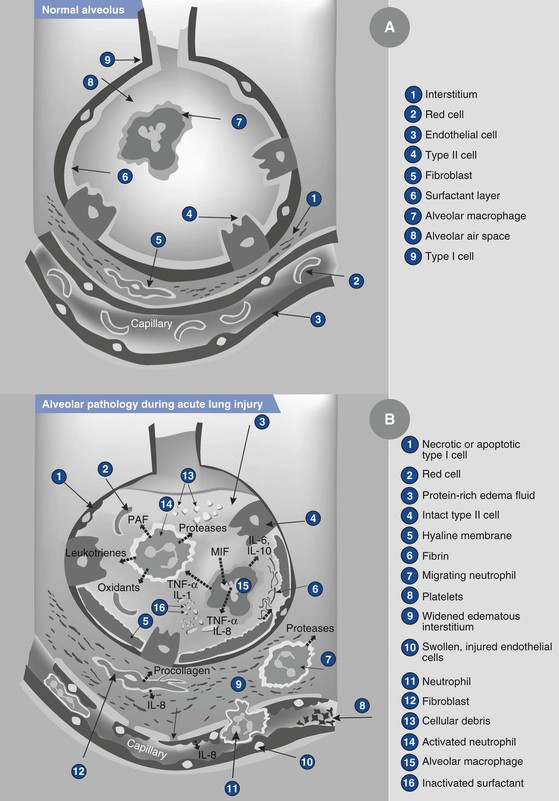
FIGURE 29-6 Illustration showing the normal alveolus (A) and the injured alveolus (B) during acute lung injury. In the acute phase of acute lung injury there is formation of protein-rich hyaline membranes on the denuded basement membrane. Neutrophils are marginating through the interstitium into the air space. Alveolar macrophages secrete interleukin (IL)-1, 6, 8, and 10, as well as tumor necrosis factor-alpha (TNF-α), that stimulate and activate neutrophils. Neutrophils release proinflammatory molecules (oxidants, proteases, leukotrienes, platelet-activating factor [PAF ]). The influx of protein-rich edema fluid into the alveolus has led to the inactivation of surfactant and, together with unresolved fibrin depositions, fibrin-rich hyaline membranes are formed. MIF, müllerian inhibiting factor. (From Dahlem P, van Aalderen WMC, Bos AP. Pediatric acute lung injury. Paediatr Respir Rev 2007; 8:348-62.)
The acidic contents of the stomach prevent the growth of bacteria under normal conditions. However, gastric contents may become colonized with pathogenic gram-negative bacteria in patients receiving antacid therapy or with enteral feeding tubes, gastroparesis, or intestinal obstruction. The bacterial content adds to the inflammatory response to acid aspiration.80
Aspiration of particulate matter in the supine position most commonly involves injury to the posterior segments of the upper lobes and the apical segments of the lower lobes, whereas aspiration in the semirecumbent or upright position typically leads to injury to the lower lobes. The right lower lobe is the most common site of aspiration injury because the right mainstem bronchus has larger and more vertical architecture compared with the left mainstem bronchus. Obstruction of the bronchus or bronchioles results in bronchial denudation and collapse of the bronchopulmonary segments. Persistent or unresolved collapse can lead to lung abscesses and cavitation.80
After the acute period, the process resolves through the proliferation and differentiation of surviving type II pneumocytes in the alveolar epithelial cells.78,79 The type II pneumocytes actively transport sodium out of the alveolus, and water follows passively. Soluble proteins are removed by paracellular diffusion and endocytosis, and insoluble proteins are removed by macrophages. Neutrophils are removed by programmed cell death and subsequent phagocytosis by macrophages. Type II pneumocytes gradually restore the normal composition of the surfactant. In a subset of patients with ARDS, the injury progresses to a fibrosing alveolitis—an accumulation of mesenchymal cells, their products, and new blood vessels.
Bronchospasm and disruption of surfactant likely account for the slight decrease in PaO2 and increase in shunting that are observed.72 Aspiration of large solid particles may cause atelectasis by obstructing large airways.3 Aspiration of smaller particulate matter causes an exudative neutrophilic response at the level of the bronchioles and alveolar ducts; the clinical picture is similar after the aspiration of acidic liquid.66,71,72
Clinical Course
In most cases of aspiration during anesthesia, the anesthesia provider witnesses regurgitation of gastric contents into the hypopharynx.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree