Chapter 38
Antepartum and Postpartum Hemorrhage
Barbara M. Scavone MD
Chapter Outline
Obstetric hemorrhage is the most common cause of maternal mortality worldwide, accounting for 25% of maternal deaths.1 The World Health Organization estimates that severe hemorrhage complicates 10.5% of live births globally and carries with it a case-fatality rate of 1%.1 The rates of maternal death and death due to hemorrhage vary widely throughout various regions of the world (see Chapter 40).1–3 In the United States, hemorrhage accounts for 12.5% of pregnancy-related deaths (1.8 pregnancy-related deaths due to hemorrhage per 100,000 live births).4 Data from the United Kingdom indicate death from peripartum hemorrhage occurs in 0.39 per 100,000 maternities.5 Hemorrhage is the most common cause for admission of an obstetric patient to an intensive care unit and is a risk factor for myocardial ischemia and infarction and stroke.6–8 A 2010 investigation demonstrated that organ dysfunction complicates 16% of cases of major obstetric hemorrhage (defined as transfusion of 5 or more units of packed red blood cells [PRBCs]).9
Evidence indicates hemorrhage rates and severe morbidity due to hemorrhage are increasing in the United States and other high-resource countries, owing primarily to increases in postpartum, rather than antepartum, hemorrhage.10–13 The explanation for this acceleration is not entirely clear but appears to be related to rising rates of postpartum uterine atony as well as increases in abnormal placentation coincident with the rise in cesarean delivery rates.10,11,14,15
The majority of hemorrhage-related adverse outcomes are considered preventable.5,16,17 Common provider-related shortcomings include failure to recognize risk factors, failure to accurately estimate the extent of blood loss, and failure to initiate treatment in a timely fashion. It is essential that clinicians develop an appreciation for the rapidity with which obstetric patients can become unstable, and that anesthesiologists—often the only physicians on the labor and delivery unit with specific training in resuscitation and critical care—become involved early in the care of bleeding patients. Timely and effective communication among all obstetric caregivers is imperative.
Mechanisms of Hemostasis
Uterine contraction, stimulated by endogenous oxytocic substances released after delivery, represents the primary mechanism for controlling blood loss at parturition. Uterine tetany creates shearing forces that cleave the placenta from the uterine wall through the layer of the uterine decidua (see Figure 4-3). In addition, uterine contraction constricts the spiral arteries and placental veins spanning the myometrium and supplying the placental bed.
After disruption of vascular integrity, mechanisms of coagulation include (1) platelet aggregation and plug formation, (2) local vasoconstriction, (3) clot polymerization, and (4) fibrous tissue fortification of the clot. Platelet activation and aggregation occur rapidly after endothelial damage. Activated platelets release adenosine diphosphate (ADP), serotonin, catecholamines, and other factors that promote local vasoconstriction and hemostasis. These factors also activate the coagulation cascade. The end result of the cascade is conversion of fibrinogen to fibrin and stabilization of the blood clot (see Chapter 44).
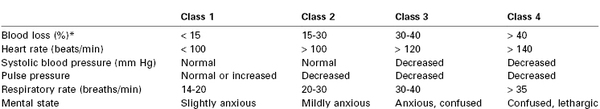
Anesthesia providers, obstetricians, midwives, and labor nurses frequently underestimate blood loss at delivery.18 Heavy bleeding is associated with larger errors in estimated blood loss, and this underestimation may lead to inadequate replacement of intravascular volume.18 Tachycardia and hypotension are late signs of hypovolemia, particularly in healthy young patients (Table 38-1); therefore, constant vigilance is necessary to ensure accurate estimation of blood loss and adequate resuscitation. Fluid and transfusion therapy is best guided by continual reassessment of maternal vital signs, urine output, hemoglobin concentration, and acid-base balance.
Antepartum Hemorrhage
Antepartum vaginal bleeding may occur in as many as 25% of pregnant women; fortunately, only a fraction of these patients experience life-threatening hemorrhage.19 The majority of cases occur during the first trimester. The causes of antepartum hemorrhage range from cervicitis to abnormalities in placentation, including placenta previa and placental abruption. The greatest threat of antepartum hemorrhage is not to the mother but to her fetus. Several decades ago, vaginal bleeding during the second and third trimesters was associated with perinatal mortality rates as high as 80%. More recent data suggest that antepartum bleeding secondary to placenta previa and placental abruption is responsible for perinatal mortality rates of 2.3% and 12%, respectively.20–22
Placenta Previa
Placenta previa is present when the placenta implants in advance of the fetal presenting part. Further classification can be made on the basis of the relationship between the placenta and the cervical os. A total placenta previa completely covers the cervical os. A partial placenta previa covers part but not all of the os. A marginal placenta previa lies within 2 cm of, but does not cover, the cervical os (Figure 38-1).
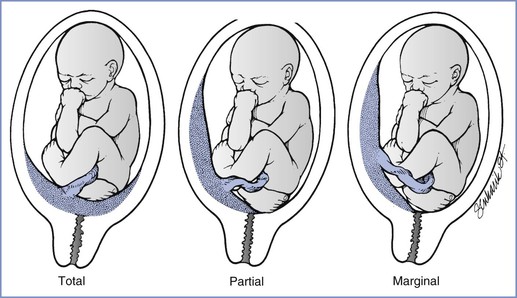
FIGURE 38-1 Three variations of placenta previa. (From Benedetti TJ. Obstetric hemorrhage. In Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4th edition. New York, Churchill Livingstone, 2001:516.)
Epidemiology
The incidence of placenta previa is 4.0 per 1000 pregnancies.23,24 The exact cause is unclear, but prior uterine trauma (e.g., scar from prior cesarean delivery) is a common element. The placenta may implant in the scarred area, which typically includes the lower uterine segment. Conditions associated with placenta previa include multiparity, advanced maternal age, smoking history, male fetus, previous cesarean delivery or other uterine surgery, and previous placenta previa.23 The presence of placenta previa increases the likelihood that the patient will require a peripartum hysterectomy.25
Diagnosis
Transvaginal ultrasonography has become the gold standard for diagnosis of placenta previa; the distance from the placental edge to the internal os is measured and predicts the likelihood of antepartum hemorrhage and need for cesarean delivery.26,27 Advances in ultrasonography have made the double setup examination (i.e., vaginal examination with all personnel ready for immediate cesarean delivery) nearly obsolete in modern obstetric practice. Magnetic resonance imaging (MRI) is also useful for the diagnosis of placenta previa, but its use is not practical in most cases of antepartum hemorrhage.
The classic clinical sign of placenta previa is painless vaginal bleeding during the second or third trimester. The first episode of bleeding typically occurs preterm and is not related to any particular inciting event. The lack of abdominal pain and/or absence of abnormal uterine tone helps distinguish this event from placental abruption. The absence of these factors does not exclude abruption, however, and patients with placenta previa are at risk for coexisting placental abruption.24
Obstetric Management
Obstetric management is based on the severity of vaginal bleeding and the maturity and status of the fetus. Active labor, persistent bleeding, a mature fetus (≥ 36 weeks’ gestational age), or nonreassuring fetal status should prompt delivery.28 The fetus is at risk from two distinct pathophysiologic processes: (1) progressive or sudden placental separation that causes uteroplacental insufficiency and (2) preterm delivery and its sequelae. The first episode of bleeding characteristically stops spontaneously and rarely causes maternal shock or fetal compromise. Expectant management in the hospital has been shown to prolong pregnancy by an average of 4 weeks after the initial bleeding episode.28 Maternal vital signs are assessed frequently, and the hemoglobin concentration is checked at regular intervals. Fetal evaluation involves frequent performance of a nonstress test or biophysical profile, ultrasonographic assessment of fetal growth, and fetal lung maturity studies as indicated. Hemorrhage may be prevented by limitations on physical activity and avoidance of vaginal examinations and coitus, although the evidence supporting these measures is limited.
Outpatient management has resulted in good outcome in carefully selected patients.29 Outpatient management is reserved for stable patients without bleeding in the previous 48 hours who have both telephone access and the ability to be transported quickly to the hospital. Expectant management requires immediate access to a medical center with 24-hour obstetric and anesthesia coverage and a neonatal intensive care unit.28
In most cases of placenta previa diagnosed between 24 and 34 weeks’ gestation, a corticosteroid (e.g., betamethasone) is administered to accelerate fetal lung maturity.28 A significant number of patients with placenta previa have preterm labor, which may provoke bleeding. Obstetricians may administer tocolytic therapy to decrease preterm contractions, with the goal to stabilize antepartum bleeding. Ritodrine has been shown to prolong pregnancy in women with placenta previa, but no studies have confirmed any decrease in the frequency or severity of vaginal bleeding.28,30 Obstetricians must balance the potential cardiovascular consequences of tocolytic therapy in the event of maternal hemorrhage against the consequences of preterm delivery. Tocolytic therapy is not recommended for patients with uncontrolled hemorrhage or those in whom placental abruption is suspected. Although expectant management reduces the risk for prematurity, it does not eliminate it, and prematurity remains the most common cause of neonatal mortality and morbidity, especially if bleeding begins before 20 weeks’ gestation.31
Fetuses of women with placenta previa may be at risk for other complications, including asymmetric fetal growth restriction (also known as intrauterine growth restriction).32 Several factors may account for the association between placenta previa and fetal growth restriction. First, the lower uterine segment may be less vascular than normal sites of placental implantation. Second, the placenta often is adherent to an area of fibrosis tissue. Third, patients with placenta previa have a higher incidence of first-trimester bleeding, which may promote a partial placental separation, reducing the surface area for placental exchange. Fourth, although the blood loss from placenta previa is almost entirely maternal, trauma to the placenta with vaginal examination or coitus may result in some fetal blood loss, which could retard fetal growth.32 Some studies have reported a higher incidence of congenital anomalies in the fetuses of women with placenta previa.20
Anesthetic Management
All patients admitted with antepartum vaginal bleeding should be evaluated by an anesthesia provider on arrival. Special consideration should be given to the airway examination, intravascular volume assessment, and history of previous cesarean delivery or other procedures that create a uterine scar. Volume resuscitation should be initiated using a non–dextrose-containing balanced salt solution (e.g., lactated Ringer’s, normal saline). Women with placenta previa may remain hospitalized for some time prior to delivery, and at least one intravenous catheter should be maintained if bleeding is recurrent or imminent delivery is anticipated. For women without recurrent bleeding, consideration may be given to either deferring venous access entirely or placing a peripherally-inserted central catheter (PICC) for drug administration so that other peripheral veins are preserved for large-bore intravenous catheter access in the event of hemorrhagic emergency. Hemoglobin concentration measurement may be indicated after a bleeding episode. A blood type and screen, and for women who are actively bleeding, a blood type and crossmatch, should be maintained. The American Association of Blood Banks (AABB) recommends repeating such tests every 3 days in pregnant women owing to the small but finite risk for developing a new alloantibody during pregnancy.33 This recommendation is written into the U.S. Code of Federal Regulations.34 The use of lower-extremity sequential compression devices may decrease the risk for venous thromboembolism in patients on bed rest. Pharmacologic prophylaxis is not commonly used because of the risk for bleeding.
Double Setup Examination.
The accuracy of ultrasonography for the identification of placenta previa has almost eliminated the need for double setup examination, but a few patients still require it (e.g., morbidly obese patients who cannot be adequately evaluated with ultrasonography). The examination is performed in the operating room. All members of the obstetric care team, including the anesthesia provider, obstetrician, and pediatrician, make full preparation for cesarean delivery. Full preparation consists of application of maternal monitors, insertion of two large-bore intravenous cannulas, administration of a nonparticulate antacid, and sterile preparation and draping of the abdomen. The obstetrician subsequently performs a careful vaginal examination. A cesarean delivery is performed if significant bleeding occurs or if the obstetrician confirms the presence of total placenta previa in a woman with a mature fetus.
Cesarean Delivery.
Experts recommend that women with a placental edge-to-internal os distance greater than 1 cm be offered a trial of labor; the risk for antepartum hemorrhage and need for cesarean delivery during labor are low in this setting.26 Parturients with total previa, placental edge-to-internal os distance less than 1 cm, and/or significant bleeding will require abdominal delivery, as will some patients with nonreassuring fetal status. The choice of anesthetic technique depends on the indication and urgency for delivery, the severity of maternal hypovolemia, and the obstetric history (e.g., prior cesarean delivery).
Surveys of obstetric anesthesiologists show that neuraxial anesthesia is preferred in patients with placenta previa without active bleeding or intravascular volume deficit.35 Patients who have placenta previa—without active preoperative bleeding—remain at risk for increased intraoperative blood loss for at least three reasons. First, the obstetrician may injure an anteriorly located placenta during uterine incision. Second, after delivery, the lower uterine segment implantation site, lacking uterine muscle compared with the fundus, does not contract as well as the normal fundal implantation site. Third, a patient with placenta previa is at increased risk for placenta accreta, especially if there is a history of previous cesarean delivery (Table 38-2).25 For these reasons, two large-bore intravenous cannulas should be placed before the start of either elective or emergency cesarean delivery. No consensus exists on the need for blood product availability in these patients, but it seems prudent to order at least a blood type and screen. If preoperative imaging indicates the possibility of a placenta accreta, preparation for massive blood loss should be undertaken (see later discussion).
TABLE 38-2
Risk for Placenta Accreta in Patients with Placenta Previa: Relationship to Number of Prior Cesarean Deliveries
| Number of Prior Cesarean Deliveries | % of Patients with Placenta Accreta |
| 0 | 3 |
| 1 | 11 |
| 2 | 40 |
| 3 | 61 |
| 4 or more | 67 |
Modified from Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol 2006; 107:1226-3.
A randomized controlled trial comparing epidural with general anesthesia for cesarean delivery in women with placenta previa in the absence of active bleeding demonstrated that epidural anesthesia was associated with (1) more stable blood pressure after delivery and (2) lower transfusion rates and transfusion volumes with similar hematocrit measurements the day after surgery.36 Operative times, estimated blood loss, urine output, and neonatal Apgar scores were similar in the two groups.36 Combined spinal-epidural anesthesia, or even single-shot spinal anesthesia, is considered acceptable for patients without active bleeding provided that there is both a low risk for placenta accreta and a low risk for difficult airway management should intraoperative conversion to general anesthesia become necessary.
Patients with placenta previa and active preoperative bleeding represent a significant challenge for the anesthesia care team. Frequently, such patients have just presented to the hospital and there is minimal time for evaluation. In these cases, patient evaluation, resuscitation, and preparation for operative delivery all proceed simultaneously. Because the placental site is the source of hemorrhage, the bleeding may continue unabated until the placenta is removed and the uterus contracts. Preoperative evaluation requires careful assessment of the parturient’s airway and intravascular volume. Two large-bore intravenous catheters should be placed, and blood products should be ordered as necessary. Blood administration sets, fluid warmers, and equipment for invasive monitoring should be immediately available. Initially, non–dextrose-containing crystalloid or colloid is infused rapidly. In some cases, the patient requires transfusion before completion of the blood crossmatch, and type-specific blood or type O, Rh-negative blood must be administered.
Rapid-sequence induction of general anesthesia is the preferred technique for bleeding patients. The choice of intravenous induction agent depends on the degree of cardiovascular instability. In patients with severe hypovolemic shock, tracheal intubation may be accomplished without an induction agent, although this situation is rare. A low dose of propofol should be administered in women with ongoing hemorrhage; in the case of severe ongoing hemorrhage it may be best to avoid propofol. Ketamine and etomidate are useful alternative induction agents for hemodynamically unstable patients. Ketamine 0.5 to 1.0 mg/kg has an excellent record of safety and efficacy in obstetric anesthesia practice. Emergence phenomena such as hallucinations and nightmares are uncommon when the dose does not exceed 1 mg/kg. Ketamine may cause myocardial depression, which may result in hypotension in patients with severe hypovolemia. Etomidate 0.3 mg/kg causes minimal cardiac depression and is safe for use in obstetric patients.37 A low dose is appropriate in patients with severe hemorrhage. Disadvantages of etomidate include venous irritation, myoclonus, and possible adrenal suppression.38
The agent(s) chosen for maintenance of anesthesia depends on maternal cardiovascular stability. In patients with modest bleeding and no fetal compromise, 50% nitrous oxide in oxygen can be administered with a low concentration of a volatile halogenated agent before delivery to prevent maternal awareness. The concentration of nitrous oxide can be reduced or omitted in cases of severe maternal hemorrhage or fetal compromise. In these cases, scopolamine or a benzodiazepine such as midazolam may be administered to ensure amnesia.
Oxytocin should be administered by intravenous infusion immediately after delivery. The relatively amuscular lower uterine segment implantation site does not contract as efficiently as the uterine fundus. If bleeding continues, it may be best to discontinue the volatile halogenated agent after delivery and to substitute 70% nitrous oxide and an intravenous opioid or ketamine. These drugs, along with small doses of midazolam, can be administered without causing significant uterine relaxation or cardiovascular depression. A low-dose infusion of propofol and/or ketamine may be considered, with the caution that propofol causes decreased uterine contractility in a dose-dependent manner.39,40 Some anesthesia providers contend that bispectral index (BIS) monitoring may be useful in lowering the risk for intraoperative awareness in cases in which the volatile anesthetic agent has been discontinued, although this issue is a matter of some dispute.
If the placenta does not separate easily, a placenta accreta may exist. In such cases, massive blood loss and the need for cesarean hysterectomy should be anticipated (see later discussion). The need for invasive hemodynamic monitoring varies among patients. An indwelling arterial catheter is useful for patients with hemodynamic instability or for those who require frequent determination of hematocrit and blood gas measurements. Coagulopathy rarely occurs with placenta previa.
Placental Abruption
Placental abruption is defined as complete or partial separation of the placenta from the decidua basalis before delivery of the fetus. Maternal hemorrhage may be revealed by vaginal bleeding or may be concealed behind the placenta. Fetal compromise occurs because of the loss of placental surface area for maternal-fetal exchange of oxygen and nutrients.41
Epidemiology
Placental abruption complicates 0.4% to 1.0% of pregnancies, and the incidence is increasing, particularly among African-American women in the United States.21,42–44 The causes are not well understood, but several conditions are known risk factors for abruption (Box 38-1).42,43 Patients hospitalized for both acute and chronic respiratory diseases are at risk for placental abruption for unclear reasons.45
Diagnosis
The classic presentation of abruption consists of vaginal bleeding, uterine tenderness, and increased uterine activity; however, not all of these symptoms are always present. In cases of concealed abruption, vaginal bleeding may be absent and gross underestimation of maternal hypovolemia can occur. Bleeding may be painless.41 In some cases, abruption may manifest as idiopathic preterm labor. Patients may have a variety of nonreassuring fetal heart rate (FHR) patterns, including bradycardia, late or variable decelerations, and/or loss of variability.41 The diagnosis of placental abruption is primarily clinical, but in a subset of cases, ultrasonography may help confirm it. Ultrasonography is highly specific for placental abruption (96%), but it is not very sensitive (24%).46 It is also useful for determining placental location, which can exclude placenta previa as a cause of vaginal bleeding.41 The ultrasonographic examination can ascertain whether retroplacental or subchorionic hematoma is present. Normal findings do not exclude the diagnosis of placental abruption.
Pathophysiology
Complications of placental abruption include hemorrhagic shock, coagulopathy, and fetal compromise or demise. One third of coagulopathies in pregnancy are attributable to abruption, and coagulopathy is associated with fetal demise.47 Placental tissue displays tissue factor and other procoagulant substances on cell membranes, and it is surmised that when bleeding at the decidual-placental interface (i.e., abruption) occurs, these thromboplastic substances are released into the central circulation, resulting in consumptive coagulopathy and disseminated intravascular coagulation (DIC).48
Although some cases of abruption occur acutely (e.g., in the setting of trauma), many abruptions complicate chronic, long-standing placental abnormalities. Investigators have noted strong associations between abruption, fetal growth restriction, and preeclampsia, and all three conditions share similar risk factors.49 Naeye et al.50 prospectively studied more than 53,000 deliveries and found that decidual necrosis at the placental margin and large placental infarcts were the most common abnormalities among patients who suffered placental abruption and fetal demise. Infants who died had 14% less placental weight, 8% less body weight, and 3% shorter body length than surviving control infants of the same gestational age. In addition, histologic evidence of shallow trophoblastic invasion of the spiral arteries supports the conclusion that “ischemic placental disease” may underlie chronic placental hypoxia, leading to preeclampsia, fetal growth restriction, and abruption.49
The major risks for the fetus are hypoxia and prematurity. Fetal oxygenation depends on adequate maternal oxygen-carrying capacity, uteroplacental blood flow, and transplacental exchange. Separation of all or part of the placenta reduces gas exchange surface area and can lead to fetal death. The risk for intrauterine fetal demise increases as the detachment area increases, particularly when the location of bleeding is retroplacental rather than subchorionic.41,51,52 Inadequate transplacental oxygen exchange is exacerbated by maternal hypotension, which decreases uteroplacental blood flow. Ananth and Wilcox21 reviewed outcomes for 7.5 million pregnancies in the United States; the perinatal mortality rate associated with placental abruption was 12%. The high mortality rate is due in large part to the fact that infants of mothers with placental abruption are five times more likely to be delivered preterm.21
Obstetric Management
If the diagnosis of abruption is suspected, the practitioner should insert a large-bore intravenous catheter and obtain blood for assessment of hematocrit, coagulation status, and type and crossmatch. When assessing volume status, the clinician must remain aware of the possibility of hemorrhage concealed behind the placenta. Placement of a urethral catheter to monitor urine output may help the physician assess the adequacy of renal perfusion. The definitive treatment is delivery of the infant and placenta, but the degree of maternal and fetal compromise and estimated gestational age determine the timing and route of delivery.41 If the fetus is at or near term and both maternal and fetal status are reassuring, vaginal delivery may be appropriate. If the patient is preterm, the extent of abruption is minimal, and the mother and fetus show no signs of compromise, the patient may be hospitalized and the pregnancy allowed to continue to optimize fetal maturation. The obstetrician may administer a corticosteroid to promote fetal lung maturity. If the mother develops hemodynamic instability or coagulopathy, or fetal status becomes nonreassuring, urgent cesarean delivery may become necessary. Vaginal delivery is preferred for patients with intrauterine fetal demise.41
Anesthetic Management
The anesthesia provider should consider the severity of the abruption and the urgency of delivery in planning anesthetic management.
Labor and Vaginal Delivery.
Neuraxial labor analgesia may be offered in the setting of abruption provided that hypovolemia has been treated and coagulation status is normal. The appropriateness of neuraxial analgesia with its accompanying sympathectomy in patients at risk for extension of abruption and further hemorrhage has been questioned; however, the risk that neuraxial analgesia will worsen hemorrhage-associated tachycardia and hypotension can be mitigated by appropriate intravascular volume replacement and use of vasopressors. Close monitoring is required for evidence of further bleeding and changes in intravascular volume status. A coagulopathic patient may present for vaginal delivery, particularly in the setting of fetal demise. In this case, intravenous patient-controlled opioid analgesia should be offered.
Cesarean Delivery.
Similar anesthetic considerations pertain to the administration of neuraxial anesthesia for cesarean delivery. Spinal, combined-spinal epidural, or epidural anesthesia may be administered in stable patients in whom intravascular volume status is adequate and coagulation studies are normal. General anesthesia is preferred for most cases of urgent cesarean delivery accompanied by unstable maternal status, a nonreassuring FHR pattern, or both. Propofol may precipitate severe hypotension in patients with unrecognized hypovolemia; ketamine and etomidate may represent better options for the patient with unknown or decreased intravascular volume.
Aggressive volume resuscitation is critical. Either crystalloid or colloid may be used; the choice is less important than adequate restoration of intravascular volume. In cases of severe hemorrhage, insertion of an intra-arterial catheter may aid prompt recognition of hypotension and allow for frequent blood sampling and assessment of anemia and coagulation status. Patients with abruption are at risk for persistent hemorrhage after delivery from uterine atony or coagulopathy; after delivery, oxytocin should be infused promptly to prevent uterine atony. Persistent uterine atony requires the administration of other uterotonic drugs (see later discussion). Red blood cells (RBCs) and coagulation factors should be replaced as indicated by laboratory studies. Experts recommend aggressive monitoring and early replacement of coagulation factors, especially fibrinogen, to minimize the developing coagulopathy.53
Most parturients recover quickly and completely after delivery. A minority of postpartum patients, notably those who have prolonged hypotension or coagulopathy, and who need massive blood volume and blood product replacement, are best monitored in a multidisciplinary intensive care unit.
Uterine Rupture
Epidemiology
Rupture of the gravid uterus can be disastrous for both the mother and the fetus. Fortunately, it does not occur often. Previous uterine surgery (e.g., cesarean delivery or myomectomy) increases the risk, but the incidence of true uterine rupture after cesarean delivery is still low, occurring at a rate of less than 1%.54,55 Uterine rupture is rare in the primigravid woman or the woman with an unscarred uterus, but it does occur.55 Box 38-2 lists additional conditions that have been associated with uterine rupture.55,56 Very rarely, uterine rupture occurs without explanation.57
Rupture of a previous uterine scar may occur in the absence of labor. In a review of records from a large multistate hospital system, nearly half of all true uterine ruptures occurred in the absence of a history of cesarean delivery, and 22% of ruptures occurred in the absence of labor.58 Lydon-Rochelle et al.59 undertook a population-based retrospective analysis of more than 20,000 women who had undergone one previous cesarean delivery. The risk for rupture among nonlaboring women was 1.6 per 1000. Among women in spontaneous labor the risk increased approximately threefold to 5.2 per 1000; among women undergoing induction of labor the risk increased nearly fivefold to 7.7 per 1000; and among women undergoing prostaglandin induction the risk increased almost 16-fold to 24.5 per 1000. Additional risk factors for uterine rupture during a trial of labor after cesarean (TOLAC) include post-term gestation (≥ 42 weeks), birth weight greater than 4000 g, maternal age older than 35 years, and maternal height greater than 164 cm.54
Because of variation in nomenclature and severity, accurate determination of maternal and fetal morbidity secondary to uterine rupture is difficult. The most common variety of uterine scar disruption is separation or dehiscence, some cases of which are asymptomatic. Uterine scar dehiscence is defined as a uterine wall defect that does not result in excessive hemorrhage or FHR abnormalities and does not require emergency cesarean delivery or postpartum laparotomy. In contrast, uterine rupture, less common than dehiscence, refers to a uterine wall defect with maternal hemorrhage and/or fetal compromise sufficient to require emergency cesarean delivery or postpartum laparotomy.
The rupture of a classical uterine incision scar (a vertical incision involving the muscular uterine fundus) is associated with greater morbidity and mortality than rupture of a low transverse uterine incision scar because the anterior uterine wall is highly vascular and may include the area of placental implantation. Lateral extension of the rupture can involve the major uterine vessels and is typically associated with massive bleeding. Maternal death secondary to uterine rupture is rare, although there were three deaths attributed to uterine rupture in the 2006 to 2008 triennial report from the United Kingdom.5 In Sweden between 1983 and 2001, Kaczmarczyk et al.54 estimated that the neonatal mortality rate associated with uterine rupture was approximately 5%.
Diagnosis
The variable presentation of uterine rupture may cause diagnostic difficulty. Abdominal pain and an abnormal FHR pattern are the two most common presenting signs of uterine rupture,60 but neither is 100% sensitive. One retrospective study reported the occurrence of abdominal pain in 17% of patients; an FHR abnormality was the first sign of uterine rupture in 87% of patients (see Chapter 19).61 Other presenting signs include vaginal bleeding, uterine hypertonia, cessation of labor, maternal hypotension, loss of the fetal station, decrease in cervical dilation, or a change in fetal presentation. Breakthrough pain during neuraxial labor analgesia may also indicate uterine rupture.62
Obstetric Management
Treatment options for uterine rupture include repair of the uterus, arterial ligation, and hysterectomy. Uterine repair is appropriate for most cases of separation of a prior low transverse uterine scar and for some cases of rupture of a classical incision. However, the risk for rupture in a future pregnancy remains. A disadvantage of arterial ligation is that it may not control the bleeding and may delay definitive treatment. Hysterectomy may be required for some cases of uterine rupture.56
Anesthetic Management
Patient evaluation and resuscitation are initiated while the patient is being prepared for emergency laparotomy. If rupture has occurred antepartum, fetal compromise is likely. General anesthesia is often necessary, except in some stable patients with preexisting epidural labor analgesia. Aggressive volume replacement is essential, and transfusion may be necessary. Urine output should be monitored. Invasive hemodynamic monitoring may be appropriate if there is uncertainty about the intravascular volume status.
Vasa Previa
Vasa previa is defined as the velamentous insertion of the fetal vessels over the cervical os (i.e., the fetal vessels traverse the fetal membranes ahead of the fetal presenting part). Thus, the fetal vessels are not protected by the placenta or the umbilical cord. Rupture of the membranes is often accompanied by tearing of a fetal vessel, which may lead to exsanguination of the fetus.
Epidemiology
Vasa previa occurs rarely (1 in 2500 to 1 in 5000 deliveries).28 Because it involves the loss of fetal blood, vasa previa is associated with a high fetal mortality rate (nearly 60% if vasa previa is unrecognized).63 The blood volume of the fetus at term is approximately 80 to 100 mL/kg. Therefore, the amount of blood that can be lost without fetal death is small. In addition, the vulnerable fetal vessels may be compressed by the fetal presenting part, resulting in fetal hypoxia and death. Risk factors for vasa previa include the presence of placenta previa or low-lying placenta in the second trimester, placental accessory lobes, in vitro fertilization, and multiple gestation.28
Diagnosis
Ultrasonography can be used to visualize the velamentous insertion of the vessels, and transvaginal color Doppler imaging can confirm the diagnosis.28,63 Vasa previa should be suspected whenever bleeding occurs with rupture of membranes, particularly if the rupture is accompanied by FHR decelerations or fetal bradycardia. Hemorrhage can also occur without rupture of membranes, making the diagnosis more difficult. Rarely, vasa previa can be diagnosed via digital cervical examination or amnioscopy. The diagnosis of vasa previa can be confirmed through examination of the shed blood for evidence of fetal hemoglobin (e.g., Kleihauer-Betke test); however, when bleeding occurs, the emergency nature of vasa previa usually precludes such diagnostic confirmation.
Obstetric Management
Prenatal diagnosis confers a neonatal survival benefit. Oyelese et al.63 conducted a retrospective study of 155 pregnancies complicated by vasa previa. Neonatal mortality was 3% when the vasa previa was diagnosed antenatally and 56% when it was not. The authors recommended ultrasonographic examination with transvaginal color Doppler in patients at risk for vasa previa. The management of vasa previa is directed solely toward ensuring fetal survival. Some authors advocate hospitalization of the patient between 30 and 32 weeks’ gestation to ensure prompt delivery if rupture of membranes should occur; consideration should be given to the administration of a corticosteroid to promote fetal lung maturity.28 Timing of delivery reflects a balance between the risks of preterm delivery and the risk for vessel rupture if the pregnancy is allowed to continue. Robinson and Grobman64 compared delivery timing strategies for women with vasa previa and calculated that the best fetal outcomes occurred with elective delivery between 34 and 35 weeks’ gestation. They further determined that confirmation of fetal lung maturity via amniocentesis was not necessary.64
Ruptured vasa previa is a true obstetric emergency that requires immediate delivery of the fetus, almost always by the abdominal route. Neonatal resuscitation requires immediate attention to neonatal volume replacement with colloid, balanced salt solutions, and blood.
Anesthetic Management
The choice of anesthetic technique depends on the urgency of the cesarean delivery. In many cases, general anesthesia is necessary for prompt delivery.
Postpartum Hemorrhage
Conflicting definitions of postpartum hemorrhage exist; however, the most commonly accepted definition is more than 500 mL blood loss after vaginal delivery or more than 1000 mL after cesarean delivery.1,65 These values may have low clinical utility because they are only slightly higher than the average blood loss for each type of delivery. Postpartum hemorrhage can also be inferred clinically (albeit retrospectively) from a 10% decrease in hematocrit from admission to the postpartum period or the need to administer PRBCs owing to postpartum blood loss.
Postpartum hemorrhage is the most common cause of maternal mortality worldwide and an important contributor to maternal death in the United States.1,2,4 The incidence of postpartum hemorrhage varies widely throughout different regions of the world2; in the United States the current rate of postpartum hemorrhage is approximately 3%.10,11 Postpartum hemorrhage, severe postpartum hemorrhage, and the attendant morbidity and mortality from hemorrhage are increasing in incidence.10,11 Between 1994 and 2006 the transfusion rate for postpartum hemorrhage more than doubled.10 The explanation for this acceleration is not entirely clear but appears to be related to rising rates of postpartum uterine atony as well as increases in the incidence of abnormal placentation, both coincident with the rise in cesarean delivery rates.10,11,14,15 Other factors may include the rising rates of obstetric interventions, such as induction and augmentation of labor,66–68 and the increasing prevalence of obesity,69–71 multiple gestation,67,72 hypertensive diseases of pregnancy,73 and advanced maternal age.12,74 However, the rising prevalence of these risk factors does not entirely explain the upward trend in postpartum hemorrhage that has been observed.10,11
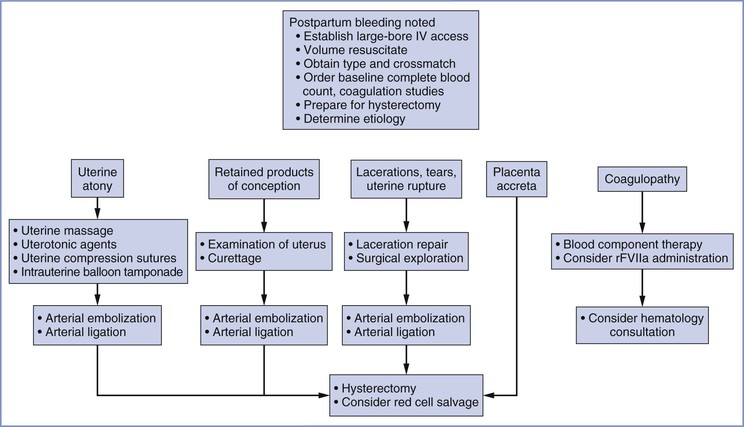
Primary postpartum hemorrhage occurs during the first 24 hours, and secondary postpartum hemorrhage occurs between 24 hours and 6 weeks after delivery.75 Primary postpartum hemorrhage is more likely to result in maternal morbidity or mortality. Figure 38-2 provides an overview of the obstetric management of postpartum hemorrhage.
Uterine Atony
Epidemiology
Uterine atony is the most common cause of severe postpartum hemorrhage, accounting for approximately 80% of cases.10,11 In addition to normal hemostatic mechanisms, postpartum hemostasis involves the release of endogenous uterotonic agents—primarily oxytocin and prostaglandins—that contract the uterus and constrict uterine vessels. Uterine atony represents a failure of this process. In addition, parturients with obstetric hemorrhage may have uterine arteries that are relatively unresponsive to vasoconstrictor substances.76 Box 38-3 lists conditions associated with uterine atony.
Diagnosis
A soft, poorly contractile uterus and vaginal bleeding are the most common findings in patients with uterine atony. The absence of vaginal bleeding does not exclude this disorder because the atonic, engorged uterus may contain more than 1000 mL of blood. Unrecognized bleeding may manifest initially as tachycardia; worsening hypovolemia eventually leads to hypotension (see Table 38-1).
Obstetric and Anesthetic Management
Prophylaxis.
The American College of Obstetricians and Gynecologists (ACOG) recommends prophylactic administration of uterotonic agents to prevent uterine atony.75 Active management of the third stage of labor, including uterine massage and oxytocin administration, decreases blood loss and transfusion requirements compared with expectant management.77,78
Oxytocin is the first-line drug for prophylaxis and treatment of uterine atony after delivery of a third-trimester pregnancy. The number of high-affinity receptors for oxytocin increases greatly near term; alternative uterotonics are more effective in the first and second trimesters of pregnancy. Endogenous oxytocin is a 9-amino acid polypeptide produced in the posterior pituitary. The exogenous form of the drug (Pitocin, Syntocinon) is a synthetic preparation with a rapid onset and short half-life. Unfortunately, exogenous oxytocin can be associated with serious side effects, including tachycardia, hypotension, myocardial ischemia, and, rarely, death, especially in hypovolemic or other hemodynamically compromised women79–83; many of these adverse effects are directly related to the dose of oxytocin.84,85 Preeclamptic women may be less able to tolerate high doses of oxytocin than healthy women.86 In addition, high doses of oxytocin administered concomitantly with large volumes of intravenous fluids, especially those containing free water, can lead to hyponatremia, seizures, and coma because of oxytocin’s structural similarity to vasopressin.87
The dose of oxytocin required to generate satisfactory uterine tone after delivery is lower than previously thought (see Chapter 26). In a study of nonlaboring women undergoing elective cesarean delivery, the ED90 of bolus dose oxytocin for satisfactory uterine tone within 3 minutes of delivery was 0.35 international units (IU)88; The ED90 was approximately 3 IU in laboring women undergoing cesarean delivery for labor arrest after labor augmentation with oxytocin.89 The ED90 of oxytocin administered via infusion without a bolus dose in nonlaboring women was approximately 0.3 IU/min for 1 hour.90 Munn et al.91 randomized women undergoing a cesarean delivery during labor to receive a prophylactic infusion of oxytocin at 2.67 IU/min or 0.33 IU/min for 30 minutes after delivery; the higher dose was associated with less need for secondary uterotonics (19% versus 39%, respectively; P < .001); however, the high dose may be associated with clinically significant tachycardia and hypotension (see later discussion).
Oxytocin is rapidly metabolized by hepatic oxytocinases and cleared in the urine and bile, resulting in a half-life of less than 6 minutes. Consequently, a prolonged intravenous infusion may be more effective than bolus administration in preventing uterine atony. In an international randomized, controlled trial, Sheehan et al.92 found that the addition of a 4-hour maintenance infusion of 0.17 IU/min (after an initial 5-IU bolus dose) decreased the need for secondary uterotonics compared with a 5-IU bolus dose alone.92 King et al.93 studied women at high risk for postcesarean uterine atony and demonstrated that administering a 5-IU bolus of oxytocin before a 1.3-IU/min infusion did not provide benefit compared with an infusion without a bolus. Administration of phenylephrine with oxytocin can mitigate the adverse hemodynamic consequences of oxytocin,94 but phenylephrine may not be necessary as long as an oxytocin bolus dose is avoided and the infusion rate is maintained below 1 IU/min, the threshold at which hemodynamic consequences become apparent.84
Data demonstrating lower oxytocin dose requirements than previously assumed and awareness of the dangers of high-dose administration call into question the common practice of injecting 10 to 40 IU of oxytocin into a 1-liter crystalloid solution and infusing the solution at an unspecified rate, often “wide open” (i.e., gravity-dependent flow). The doses administered with this method may approach those achieved with bolus administration. At my institution, my colleagues and I administer prophylactic oxytocin at a rate of 0.3 IU/min (the ED90) and increase the rate to 0.6 IU/min (twice the ED90) if there is inadequate response. The maximum beneficial oxytocin infusion rate to treat persistent uterine atony is unknown.
Carbetocin is an alternative synthetic oxytocin-receptor agonist available in Canada, the United Kingdom, and other developed countries but not the United States. A meta-analysis comparing carbetocin with oxytocin suggests that carbetocin reduces the need for secondary uterotonics95; this difference may reflect the fact that equipotent dosing regimens have not been determined. Carbetocin has a longer duration of action than oxytocin; therefore, prolonged infusion is not necessary.
Treatment.
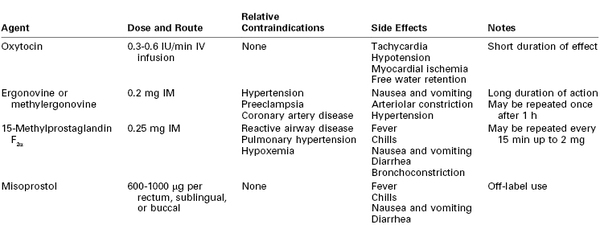
Despite preventive measures, postpartum uterine atony may occur. A multidisciplinary response to atony is imperative. General resuscitative measures include (1) additional large-bore intravenous access, (2) intravenous administration of crystalloid and colloid solutions and vasopressors, (3) laboratory determination of hemoglobin concentration or hematocrit and assessment of coagulation status, and (4) blood bank preparation of blood products for transfusion. Bimanual compression and massage of the uterus and continued infusion of oxytocin may be helpful in restoring uterine tone. Unfortunately, few high-quality data exist to guide therapy if these management strategies fail; current practice relies on expert opinion and clinical judgment. In the case of inadequate response to oxytocin, additional uterotonic agents should be employed. Three classes of drugs are currently available for the treatment of uterine atony: oxytocin, ergot alkaloids, and prostaglandins (Table 38-3).
The ergot alkaloids comprise one class of drugs used for the treatment of uterine atony. The natural ergot alkaloids are produced by a fungus that commonly infests rye and other grains. Ergonovine and methylergonovine (a semisynthetic preparation) are the two ergot alkaloids currently available for use; their pharmacologic profiles are identical. Ergot alkaloids are unstable unless they are refrigerated.96 Both drugs are dispensed in ampules containing 0.2 mg. They have a rapid onset when administered via the intramuscular route. Bolus intravenous administration is not recommended. The uterotonic effect usually lasts for 2 to 4 hours.
Both drugs rapidly produce tetanic uterine contractions and for this reason are restricted to postpartum use. The mechanism of action is poorly understood, but the uterotonic effect is most likely mediated by alpha-adrenergic receptor stimulation.97 Parenteral administration of an ergot alkaloid is associated with a high incidence of nausea and vomiting.98 Administration by any route may cause serious cardiovascular system derangements, including vasoconstriction, hypertension,98 myocardial ischemia and infarction due to coronary vasospasm,99–101 cerebrovascular accidents,102 seizures,102 and even death.99,103 Patients at greatest risk are those with preexisting hypertension; however, sudden and marked hypertension may also occur in previously normotensive patients. The combination of an ergot alkaloid followed by a vasopressor has been reported to lead to exaggerated hypertension.104 Relative contraindications to the use of ergot alkaloids include hypertension, preeclampsia, peripheral vascular disease, and ischemic heart disease. Treatment of ergot-induced vasoconstriction and hypertension may require administration of a potent vasodilator such as nitroglycerin or sodium nitroprusside. Blood pressure and the electrocardiogram should be monitored closely after administration.
Prostaglandins of the E and F families have gained wide acceptance as escalation therapy when high-dose oxytocin is inadequate. Concentrations of endogenous prostaglandins increase during labor, but levels do not peak until the time of placental separation. It is hypothesized that uterine atony may represent a failure of prostaglandin concentrations to increase during the third stage of labor in some women.105,106 Prostaglandins increase myometrial intracellular free calcium concentration,107 ultimately leading to an increase in myosin light-chain kinase activity. Common side effects noted after administration of prostaglandins include fever, chills, diarrhea, nausea, and vomiting.108,109
A prostaglandin commonly used for the treatment of refractory uterine atony is 15-methyl prostaglandin F2α, or carboprost; its administration may succeed in controlling hemorrhage when all other pharmacologic treatments have failed.108,110 The recommended dose is 0.25 mg (250 µg) administered intramuscularly, which may be repeated every 15 to 30 minutes; the total dose should not exceed 2 mg (eight doses). Unfortunately, this valuable agent may precipitate bronchospasm, abnormal ventilation-perfusion ratio, increased intrapulmonary shunt fraction, and hypoxemia in susceptible patients.111,112
Misoprostol is a prostaglandin E1 analogue that has been used successfully for cervical ripening and induction of labor. Misoprostol is thermostable in tropical conditions and does not require intravenous access for administration; prophylactic misoprostol administration reduced the incidence of postpartum hemorrhage compared with placebo.113 These characteristics make it an attractive alternative to oxytocin and ergot alkaloids in low-resource areas, where the rate of maternal mortality from hemorrhage is high1,2,113; however, parenteral oxytocin is more effective for postpartum hemorrhage prophylaxis than misoprostol (relative risk [RR] of hemorrhage, 1.34; 95% confidence interval [CI], 1.16 to 1.55).113
Whether misoprostol can also decrease bleeding in patients with postpartum uterine atony unresponsive to conventional uterotonics is unclear.114 A large international randomized controlled trial failed to identify any benefit of misoprostol 600 µg administered sublingually in addition to oxytocin for treatment of postpartum hemorrhage.109 A second randomized controlled trial suggested that misoprostol may be less effective than the combined administration of ergometrine and oxytocin for the treatment of postpartum hemorrhage.115 A dose of 600 to 1000 µg per rectum is commonly administered; administration via the oral, buccal, and sublingual routes has been described.75,113,114 Like other prostaglandins, misoprostol may be associated with fever, chills, nausea, vomiting, and diarrhea.109,113,114 Misoprostol may have a more favorable side effect profile than ergonovine or 15-methyl prostaglandin F2α in patients with hypertension and/or reactive airway disease.
If hemorrhage and atony persist despite aggressive administration of multiple classes of uterotonic drugs, invasive techniques must be considered. Invasive techniques include intrauterine balloon tamponade, uterine compression sutures, embolization of the arteries supplying the uterus, surgical ligation of arteries, and cesarean hysterectomy (see later discussion).
Genital Trauma
The most common childbirth injuries are lacerations and hematomas of the perineum, vagina, and cervix. Most injuries have minimal consequence, but some puerperal lacerations and hematomas are associated with significant hemorrhage, either immediate or delayed.116 Prompt recognition and treatment can minimize morbidity and mortality.116 Genital tract lacerations should be suspected in all patients who have vaginal bleeding despite a firm, contracted uterus. The cervix and vagina must be inspected carefully in these patients. Computed tomography (CT) and/or MRI may be useful in detecting the presence, location, and extent of suspected hematoma.117 Pelvic hematomas may be divided into four types: vaginal, vulvar, vulvovaginal, and retroperitoneal.116
Vaginal hematomas result from soft tissue injury during delivery, and they may involve bleeding from the descending branch of the uterine artery.116,118 The use of forceps or vacuum extraction increases the risk.118 A study in Sweden of all cases of vaginal hematoma from 1987 to 2000 found a prevalence of approximately 1 in 1240 deliveries.119 The investigators identified nulliparity, advanced maternal age, and neonatal birth weight exceeding 4000 g as risk factors for vaginal hematoma. Other risk factors may include prolonged second stage of labor, multiple gestation, preeclampsia, and vulvovaginal varicosities.116
Vulvar hematomas commonly involve branches of the pudendal artery.116 Injury is usually suggested by extreme pain or clinical manifestations of hypovolemia secondary to blood loss.116 Small vaginal or vulvar hematomas that are not enlarging may be observed and treated conservatively with ice packs and oral analgesics. Large hematomas should be incised and evacuated. Bleeding vessels should be ligated. Often no specific bleeding source can be identified. The successful use of arterial embolization to decrease bleeding and aid in surgical management of genital tract hematomas has recently been reported.118 Volume resuscitation and transfusion may be necessary.116
Retroperitoneal hematomas are the least common and most dangerous hematomas associated with childbirth. A retroperitoneal hemorrhage occurs after laceration of one of the branches of the hypogastric artery. Injury typically occurs during cesarean delivery or rarely after rupture of a low transverse uterine scar during labor. These hematomas may be large and may extend as far as the kidneys.
The symptoms of concealed bleeding depend on the size of the hematoma and the rate at which it forms. In some instances, abrupt hypotension may be the first sign of bleeding. The diagnosis of a retroperitoneal hematoma must be considered whenever a postpartum patient has an unexpected decrease in hematocrit or unexplained tachycardia and hypotension. Other signs and symptoms are restlessness, lower abdominal pain, a tender mass above the inguinal ligament that displaces a firm uterus to the contralateral side, and vaginal bleeding with hypotension out of proportion to the external blood loss. Ileus, unilateral leg edema, urinary retention, and hematuria also may occur.116 A high index of suspicion is needed; in obese women it may be especially difficult to examine the abdomen for signs of retroperitoneal hematoma.
Occasionally, a retroperitoneal hematoma may be self-limiting and need no surgical intervention. Life-threatening hematomas require exploratory laparotomy and ligation of the hypogastric vessels. Fliegner120 reported that 38 of 39 patients with a broad ligament hematoma received a blood transfusion. The average amount administered was 4000 mL. Eight (21%) of the patients required a hysterectomy.
Anesthetic Management
Choice of anesthetic technique for the repair of genital lacerations and evacuation of pelvic hematomas depends on the affected area, surgical requirements, volume/hemodynamic status of the patient, and urgency of the procedure. Local infiltration and a small dose of intravenous opioid suffice for drainage of some vulvar hematomas; however, repair of extensive lacerations and drainage of vaginal hematomas require significant levels of analgesia or anesthesia. Pudendal nerve block may not be technically feasible because of anatomic distortion or severe pain from the hematoma. Low doses of ketamine (10-mg boluses, not exceeding a total dose of 0.5 mg/kg) may suffice to produce sedation and analgesia with minimal risk for altering airway reflexes. Spinal or epidural anesthesia may be necessary, although the clinician should exercise caution initiating (or extending) a neuraxial block in a hypovolemic patient. In some cases, general anesthesia with tracheal intubation may be necessary. Exploratory laparotomy for a retroperitoneal hematoma typically requires the administration of general anesthesia.
Retained Placenta
Retained placenta is defined as failure to deliver the placenta completely within 30 minutes after delivery of the infant and occurs in approximately 3% of vaginal deliveries.121,122 Retained placenta is a leading cause of both primary and secondary postpartum hemorrhage. The risk for postpartum hemorrhage increases significantly if the interval between delivery of the infant and the placenta exceeds 30 minutes.121 The severity of bleeding ranges from minimal to severe and can be life-threatening and require transfusion.122 Risk factors for retained placenta include history of retained placenta, preterm delivery, oxytocin use during labor, preeclampsia, and nulliparity.121,122
Obstetric Management
Treatment of retained placenta during the early postpartum period often involves manual removal and inspection of the placenta. Curettage may be required. After removal of the placenta, uterine tone should be augmented with oxytocin and the patient should be observed for evidence of recurrent hemorrhage.
Anesthetic Management
Choice of anesthetic technique depends on the degree of hemorrhage. In some cases, the administration of small amounts of sedatives and analgesics is adequate to allow examination and manual placental extraction by a skilled obstetrician. Neuraxial anesthesia may be considered in patients who are not bleeding severely and who are hemodynamically stable. This may be accomplished with either administration of additional local anesthetic through an existing labor epidural catheter or initiation of spinal anesthesia. General anesthesia sometimes becomes necessary, particularly in patients who are hemodynamically unstable.
In some cases, the obstetrician requires uterine relaxation to facilitate manual removal of the placenta. Historically, anesthesia providers have performed rapid-sequence induction of general anesthesia, followed by the administration of a high dose of volatile halogenated agent to relax the uterus. Equipotent doses of halothane, sevoflurane, and desflurane depress uterine contractility equally and in a dose-dependent manner. In a study of isolated human uterine muscle, an equi-anesthetic concentration of isoflurane was a less effective uterine relaxant than the other volatile agents.123 Uterine contractility is decreased by 50% with administration of approximately 1.5 minimum alveolar concentration (MAC) of a volatile anesthetic agent.123 The induction of general anesthesia and administration of a volatile halogenated agent results in rapid onset of uterine relaxation, and discontinuation of the volatile agent results in rapid offset when uterine relaxation is no longer necessary. However, induction of general anesthesia in a parturient entails risk for failed ventilation, failed tracheal intubation, and/or aspiration of gastric contents.
Alternatively, nitroglycerin may be administered for uterine relaxation. Nitroglycerin provides a rapid onset of reliable smooth muscle relaxation and a short plasma half-life (2 to 3 minutes).124,125 Nitroglycerin has been administered for various obstetric emergencies without clinically significant side effects.125 Peng et al.126 described successful removal of retained placenta in 15 parturients after administration of intravenous nitroglycerin 500 µg. DeSimone et al.127 used a substantially smaller dose of nitroglycerin (50 to 100 µg) with similar results; all patients were managed successfully without the need for induction of general anesthesia. Nitroglycerin may also be administered sublingually via spray or tablet. A double-blind, randomized, controlled study compared sublingual nitroglycerin with placebo for management of retained placenta128
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree