● INTRODUCTION
Anomalies of systemic and pulmonary venous connections can occur as isolated anomalies or in association with simple (atrial septal defect) or complex cardiac malformations (heterotaxy syndrome). Prenatal detection of venous anomalies increased in the last several years, primarily due to the advent of high-resolution grayscale and color Doppler ultrasound (1–7). Systemic venous malformations include anomalies of the inferior vena cava (IVC), superior vena cava (SVC), and coronary sinus (5, 7). Persistent left SVC (LSVC) and interruption of the IVC with azygos continuation are the two most common systemic venous malformations in fetal and postnatal series (5, 7). The diagnoses of anomalies of the pulmonary venous system, including total or partial anomalous venous connections, are infrequently performed prenatally (8). The recognition of such abnormalities, however, is critical in order to optimize neonatal care. Anomalies of the fetal abdominal venous system, such as ductus venosus, hepatic or umbilical vein abnormalities, and rare systemic venous malformations, are not discussed in this chapter. For further reading, see Chapter 10 and review articles (5, 6, 9–12).
● PERSISTENT LEFT SUPERIOR VENA CAVA
Definition, Spectrum of Disease, and Incidence
In the embryo, the involution of the LSVC, which becomes the ligament of Marshall, follows the development of the left brachiocephalic (or innominate) vein at the 7th week of gestation (see also Chapter 3). Persistent LSVC is thought to result from failure of the left anterior and common cardinal veins to involute. The persistent LSVC, or simply called LSVC, starts at the junction of the left jugular and subclavian veins, runs anterior to the aortic arch and left pulmonary artery and on the lateral border of the left atrium (Fig. 31.1) (5, 7). The LSVC joins the coronary sinus in the posterior left atrioventricular groove (Fig. 31.1) and drains into the right atrium in 92% of cases or into the left atrium in the remainder of cases when the coronary sinus is partially or completely unroofed. The LSVC is the most common variant of the thoracic venous system and is reported in about 0.3% to 0.5% of the population (13–15). LSVC coexists with congenital heart disease in up to 5% to 9% of infants and up to 9% of fetuses with cardiac malformations (3, 4, 13–15). Cardiac malformations that are commonly associated with LSVC include heterotaxy syndrome, left ventricular tract obstructive defects, and conotruncal malformations (3, 4, 16, 17). The right SVC can also be absent in association with LSVC (18, 19).
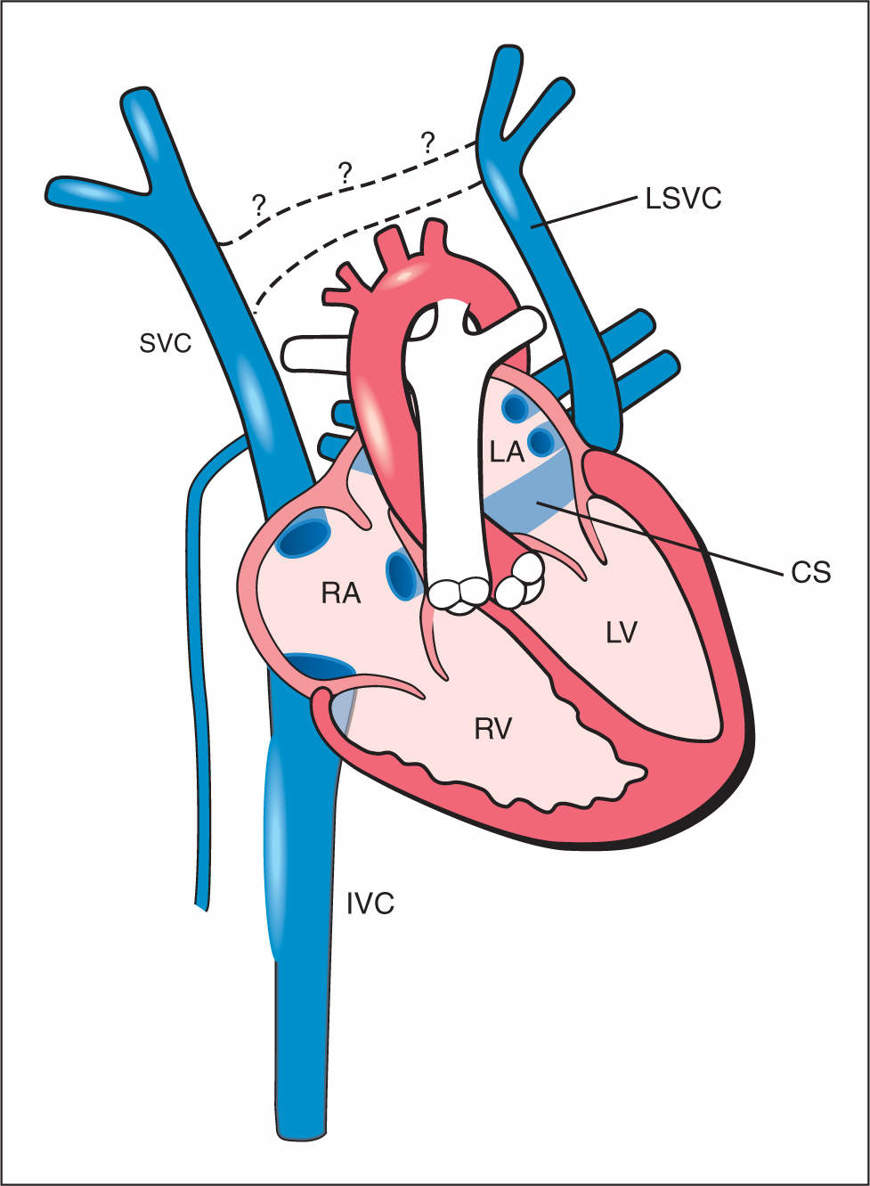
Figure 31.1: Schematic drawing of the persistent left superior vena cava (LSVC). Note the anterior course of the LSVC and its drainage into the coronary sinus (CS). The dilated CS, the right superior vena cava (SVC) and the inferior vena cava (IVC) drain into the right atrium (RA). Note the absence of the bridging vein, the left brachiocephalic vein (dashed lines with question marks), a common finding in LSVC. LA, left atrium; LV, left ventricle; RV, right ventricle.
Ultrasound Findings
Gray Scale
The diagnosis of a LSVC may be easy to make if the operator is aware of the anatomic course of the LSVC and its diagnostic planes in the chest. Identifying a LSVC can be achieved in four transverse planes and in one longitudinal plane (Fig. 31.2). A LSVC can be found as an isolated finding, but it can be also part of a cardiac anomaly. Aortic coarctation is often accompanied by a LSVC (see Chapter 23), and a LSVC can be associated with complex anomalies in the spectrum of isomerism (see Chapter 30). In the four-chamber view, LSVC can be identified in cross section at the left border of the left atrium (Fig. 31.3). Occasionally in this plane, the left atrium and ventricle appear narrower than the right side (Fig. 31.3B, see also Fig. 23.4) and the inflow tract across the mitral valve can become narrow with the LSVC bulging into the left atrium (Fig. 31.3B). This ventricular disproportion can be a normal variant but can also be a sign for an associated aortic coarctation.
In a plane slightly inferior to the four-chamber view, a dilated coronary sinus can be noted in the region of the mitral valve (Fig. 31.4) (16). Under normal conditions, the coronary sinus has a diameter of 1 to 3 mm, courses perpendicular to the interatrial septum, and opens into the posterior wall of the right atrium. In the presence of a LSVC with or without associated cardiac abnormality, the coronary sinus is dilated and measures between 3 and 7 mm or more in diameter (Fig. 31.4) (7). In the three-vessel-trachea view, the LSVC is seen as a fourth vessel located left and anterior to the pulmonary artery (Figs. 31.5 to 31.7) (5, 7). In the rare condition of an associated absence of the right SVC with a LSVC, only three vessels can be seen at the three-vessel-trachea view (Fig. 31.6B). In the three-vessel-trachea view plane, other hints for additional cardiac malformations can be visualized as well (see Chapters 9 and 10) and Figure 31.7 shows two examples. A more cranial plane to the three-vessel-trachea view shows the LSVC and right SVC without the demonstration of the left brachiocephalic vein, which is absent in almost all cases where both LSVC and right SVC persist (7, 20) (Fig. 31.8). Finally, a left parasagittal plane of the thorax and neck can demonstrate the LSVC draining into the coronary sinus (Fig. 31.9). This view is often difficult to obtain with grayscale mode but it is easier with color Doppler.
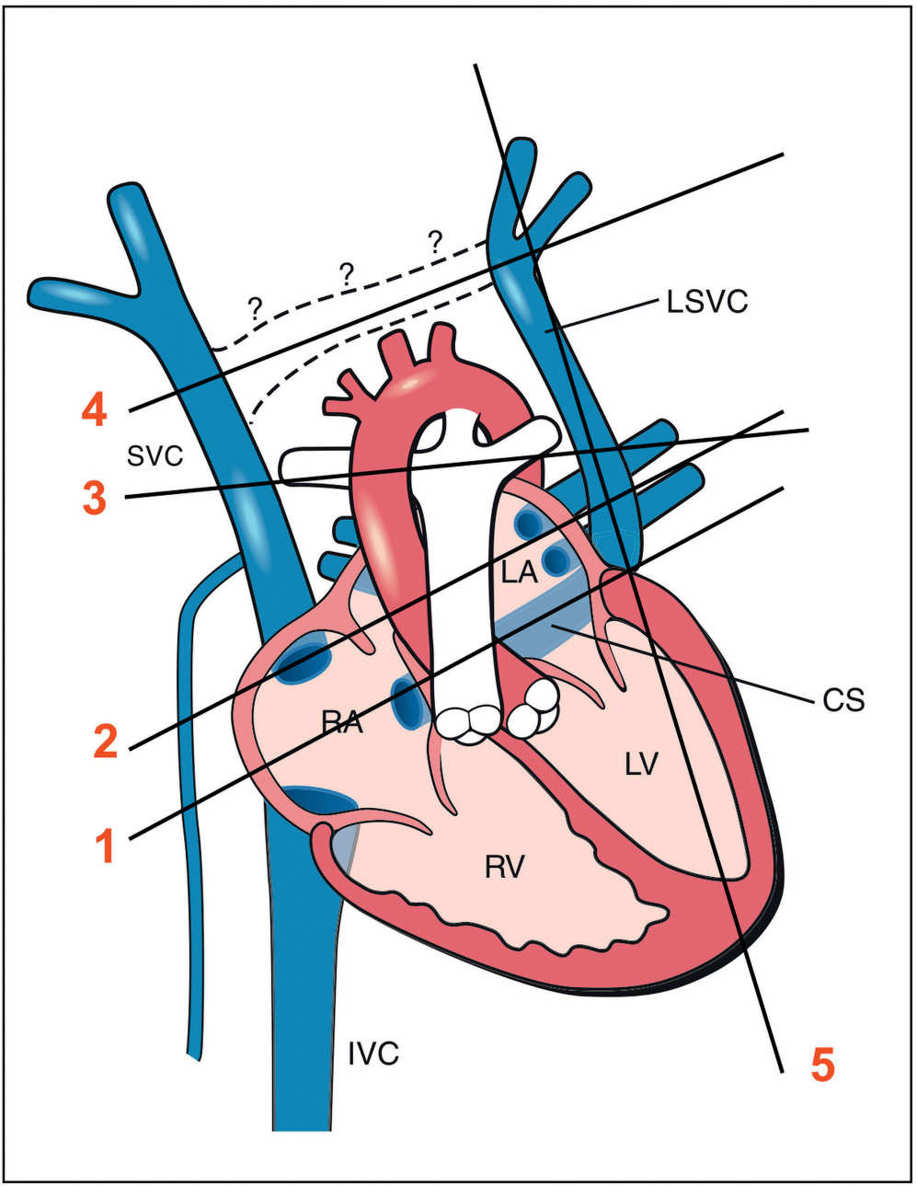
Figure 31.2: Schematic drawing of the left superior vena cava (LSVC), with four transverse lines (lines 1–4) and one parasagittal line (line 5) corresponding, respectively, to the five planes (planes 1–5) where the LSVC can be demonstrated. Plane 1 is the coronary sinus (CS) plane showing a dilated coronary sinus, which is the common site of draining of the LSVC (see Fig. 31.4). Plane 2 corresponds to the four-chamber view plane revealing a cross section of the LSVC on the lateral border of the left atrium (LA) (see Fig. 31.3). Plane 3 is the three-vessel-trachea view with the LSVC shown as the fourth vessel to the left to the pulmonary artery (see Fig. 31.5). Plane 4 is an axial view of the upper mediastinum showing the superior vena cava (SVC) and the LSVC with the absence of the connecting left brachiocephalic vein (see Fig. 31.8). Plane 5 is a parasagittal view showing the drainage of the LSVC into the coronary sinus (see Fig. 31.9). RA, right atrium; RV, right ventricle; LV, left ventricle; IVC, inferior vena cava.
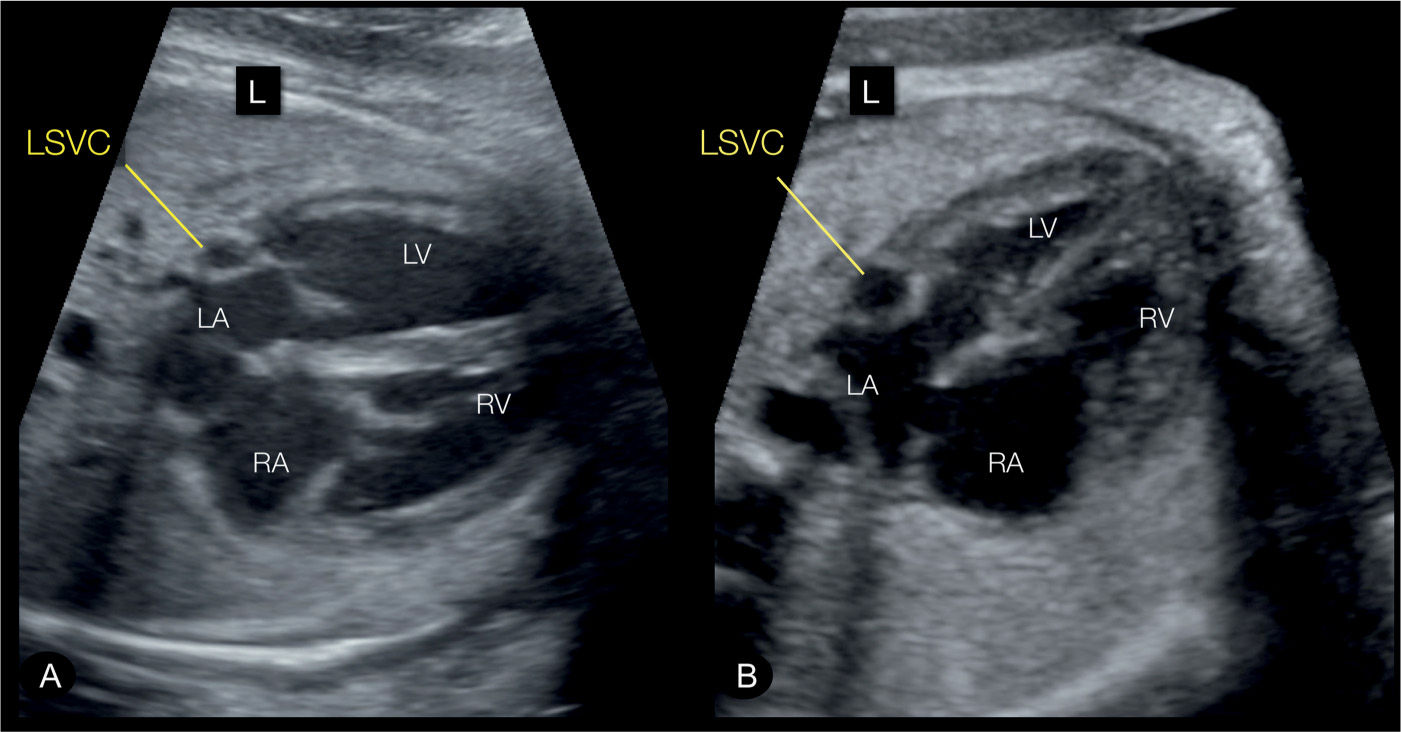
Figure 31.3: Transverse (A) and apical (B) four-chamber views in two fetuses with persistent left superior vena cava (LSVC). The four-chamber views correspond to plane 2 in Figure 31.2. The LSVC can be identified in cross section at the left border of the left atrium (LA) (labeled). RA, right atrium; RV, right ventricle; LV, left ventricle; L, left.
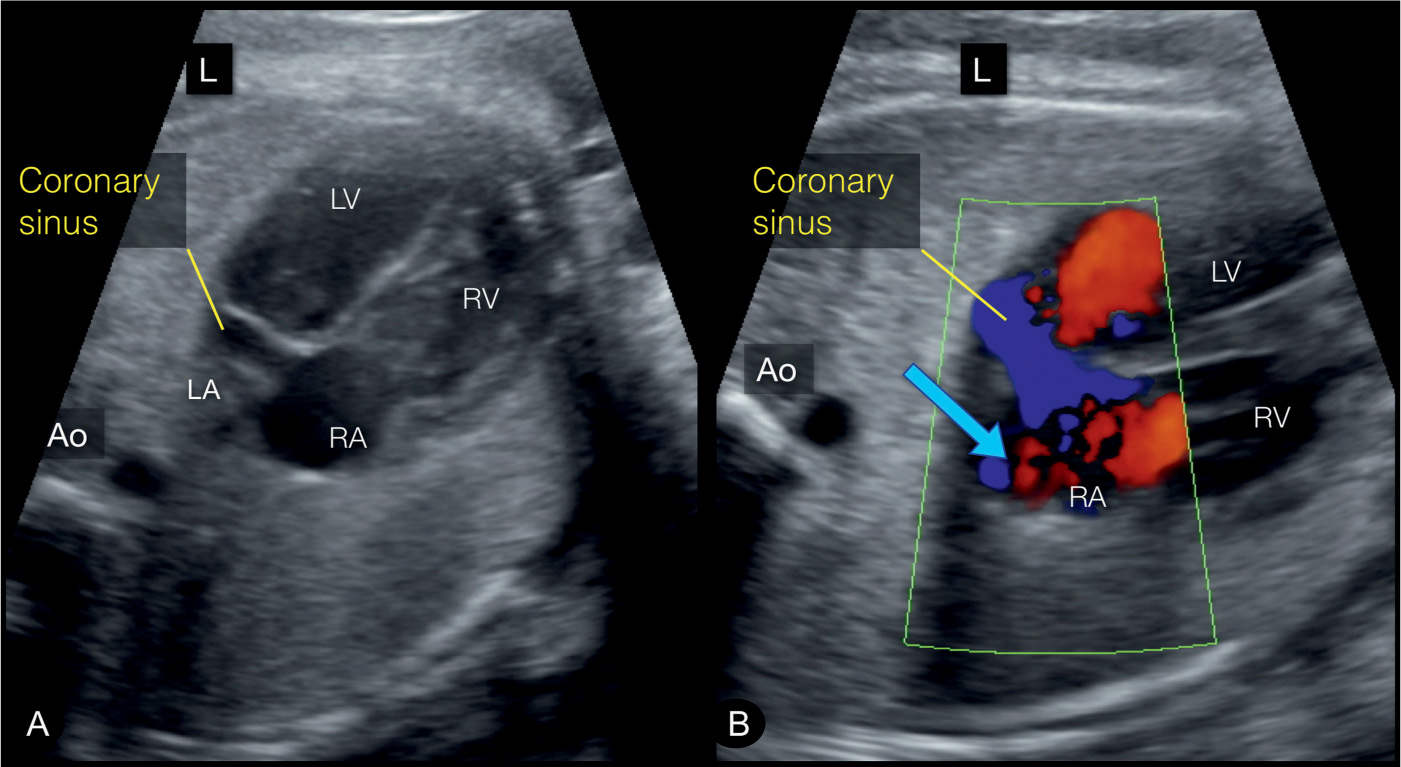
Figure 31.4: In the coronary sinus plane, which is slightly inferior to the four-chamber view, a dilated coronary sinus can be well identified (Figures A and B). It is the common site of draining of the LSVC. Note on color Doppler (B) blood flow within the coronary sinus in a left-to-right direction (blue arrow), which is in opposite direction to the flow across the foramen ovale (right-to-left). This view corresponds to plane 1 in Figure 31.2. Ao, aorta; LV, left ventricle; RV, right ventricle; RA, right atrium; LA, left atrium; L, left.
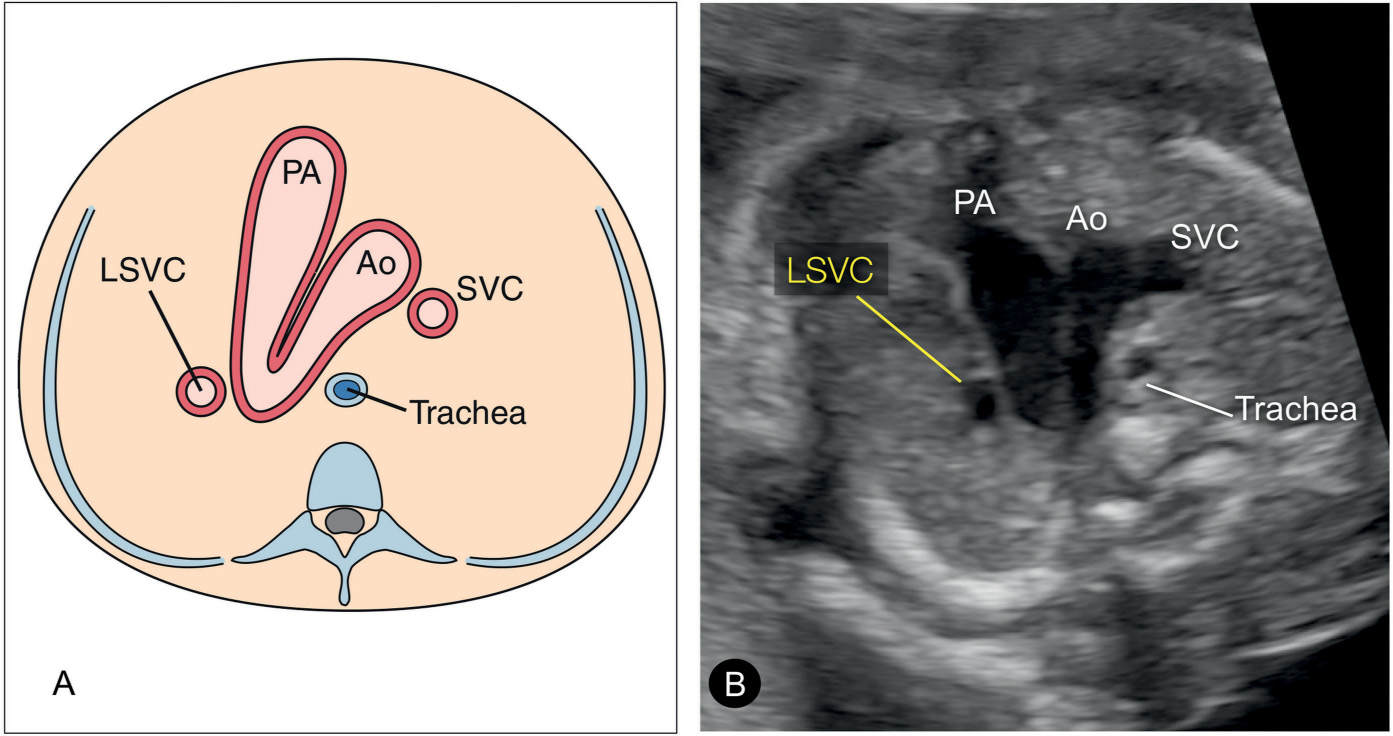
Figure 31.5: Schematic drawing (A) and corresponding ultrasound image (B) of the three-vessel-trachea view in a fetus with persistent left superior vena cava (LSVC). In this plane, the LSVC is noted as a fourth vessel to the left of the pulmonary artery (PA). This view corresponds to plane 3 in Figure 31.2. Ao, aorta; SVC, superior vena cava.
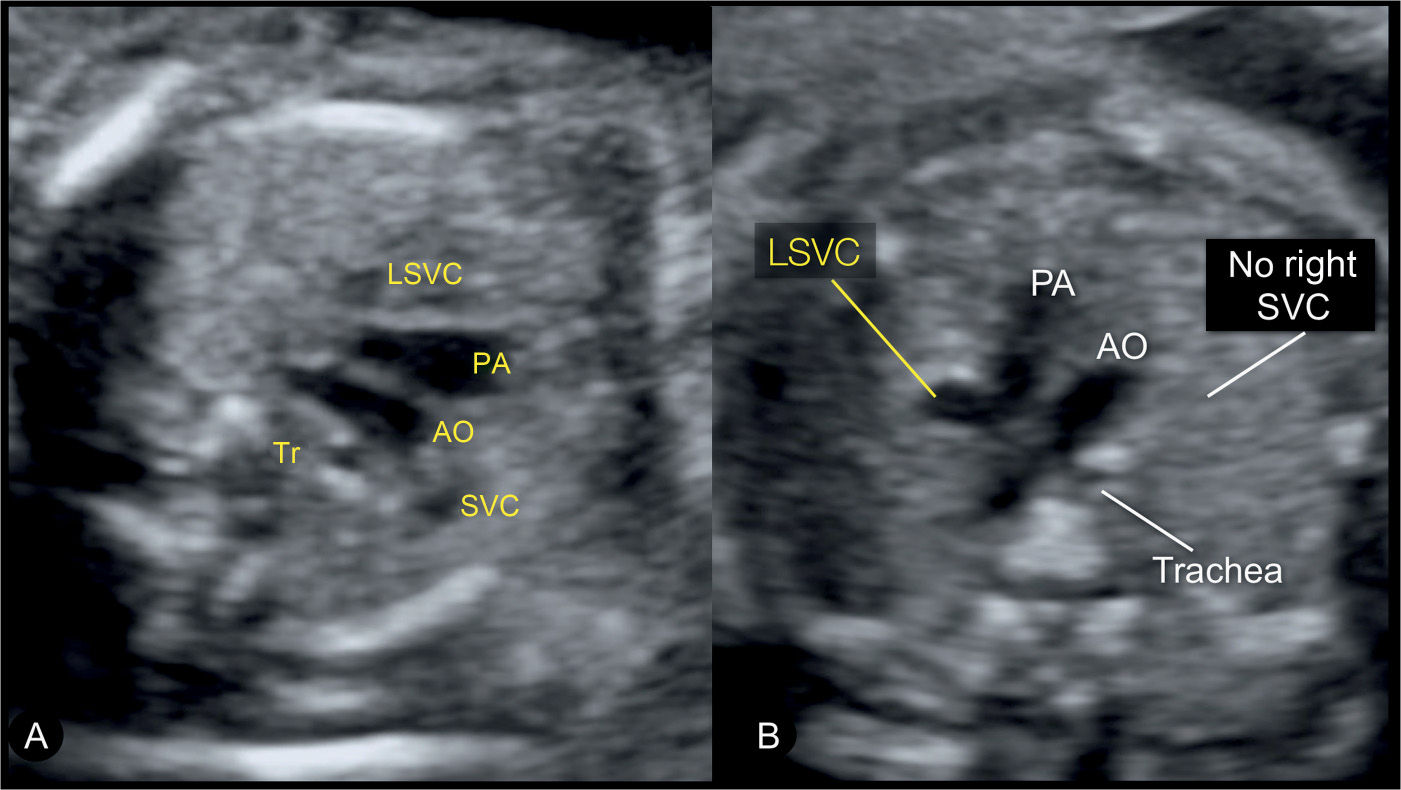
Figure 31.6: Three-vessel-trachea view in two fetuses with left superior vena cava (LSVC). The three-vessel-trachea view corresponds to plane 3 in Figure 31.2. In this view, the LSVC is identified as an additional vessel on the left side of the pulmonary artery (PA). In A, the fetus has a right superior vena cava (SVC) and a LSVC and thus four vessels are recognized in this plane. In B, the fetus has a LSVC with an absent right SVC, and thus only three vessels are seen. AO, aorta; Tr, trachea.
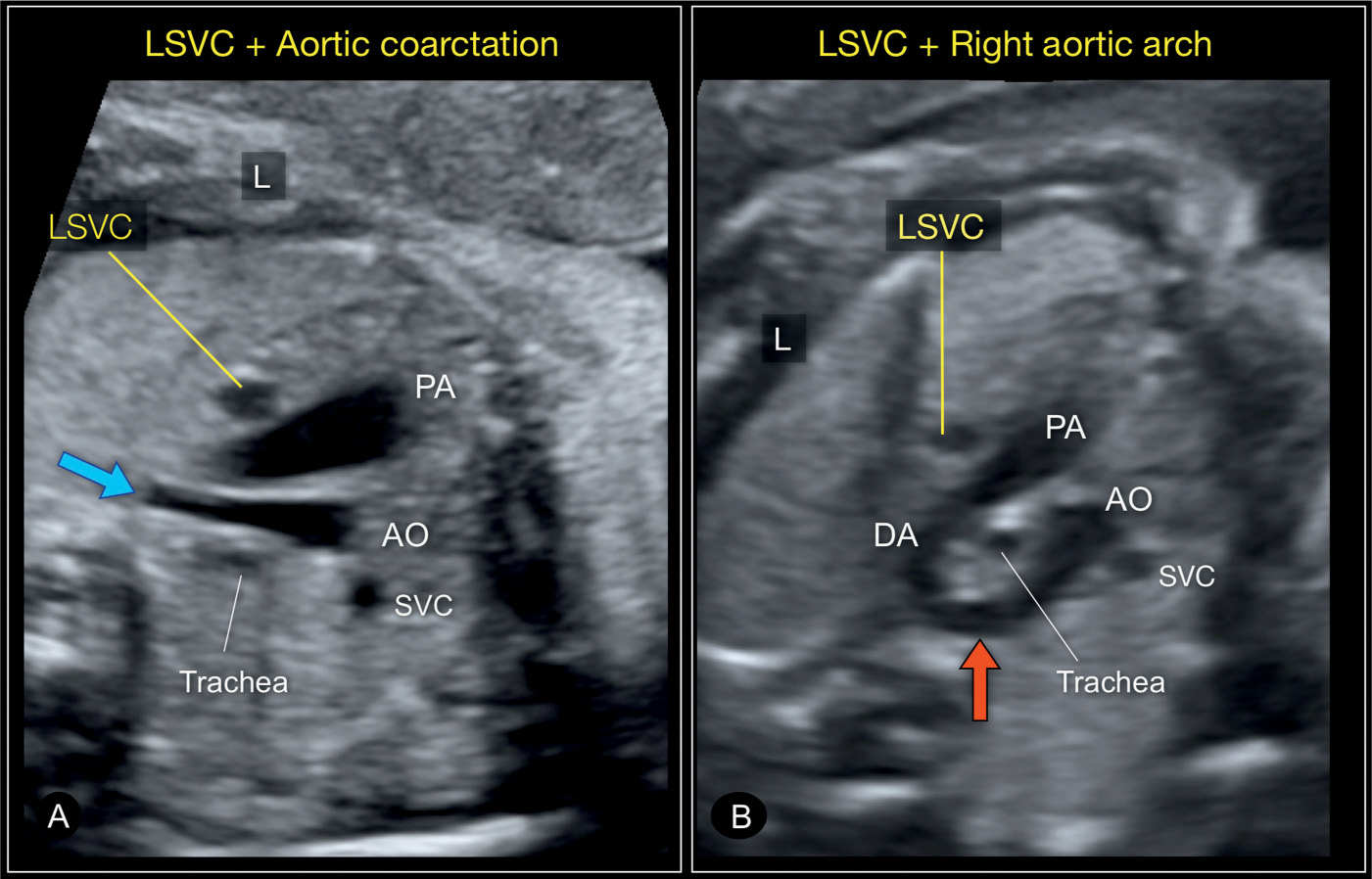
Figure 31.7: Three-vessel-trachea views in two fetuses with left superior vena cava (LSVC) in combination with a heart anomaly. In A, the fetus has an aortic coarctation with a narrow transverse and distal aortic arch (blue arrow). In B, the fetus has a right aortic arch (red arrow), a left ductus arteriosus (DA) with the U-sign (see Chapter 29) surrounding the trachea. A right superior vena cava (SVC) is present as well in both fetuses. AO, aorta; PA, pulmonary artery; L, left.
Color Doppler
Color Doppler is not essential for the diagnosis of LSVC but may help in confirming the diagnosis and ruling out additional findings. In the four-chamber plane, color Doppler can visualize the size of the blood inflow into the left ventricle and compare it to that of the right ventricle (Fig. 31.10). Color Doppler applied to the dilated coronary sinus shows blood flow toward the right atrium (Figs. 31.4 and 18.6), a good differentiating ultrasound feature from blood flow across an atrial septum primum defect (see Chapter 18 and Fig. 18.7). In a left parasagittal plane, direction of blood flow in the LSVC toward the heart can be confirmed (Fig. 31.11). Color Doppler can also help in identifying or confirming the absence of a left brachiocephalic (innominate) vein between the LSVC and right SVC.
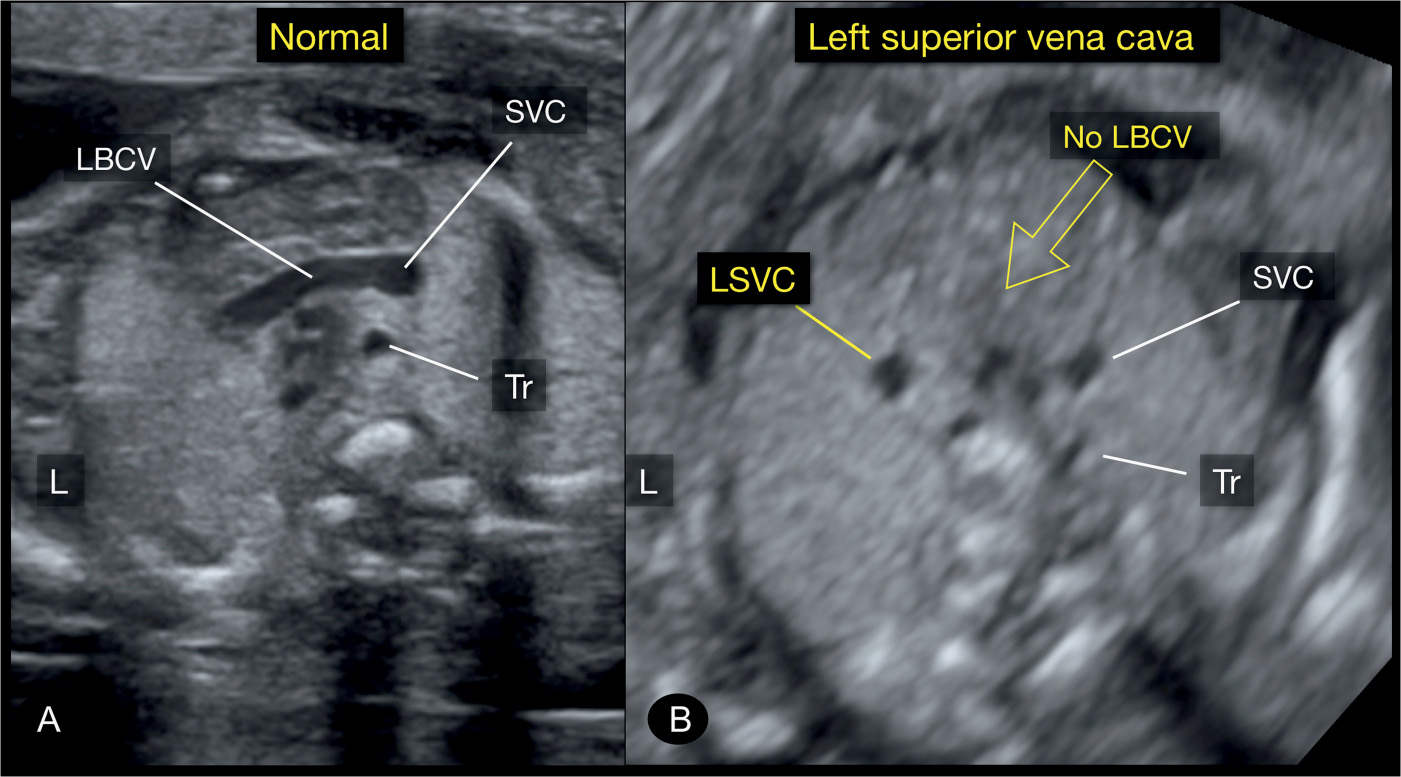
Figure 31.8: Axial plane of the upper mediastinum, corresponding to plane 4 in Figure 31.2, in a normal fetus (A) and in a fetus with a left superior vena cava (LSVC) (B). Note in A the left brachiocephalic vein (LBCV) crossing from the left jugular vein to the right superior vena cava (SVC). Note in B the absence of the LBCV. Tr, trachea; L, left.
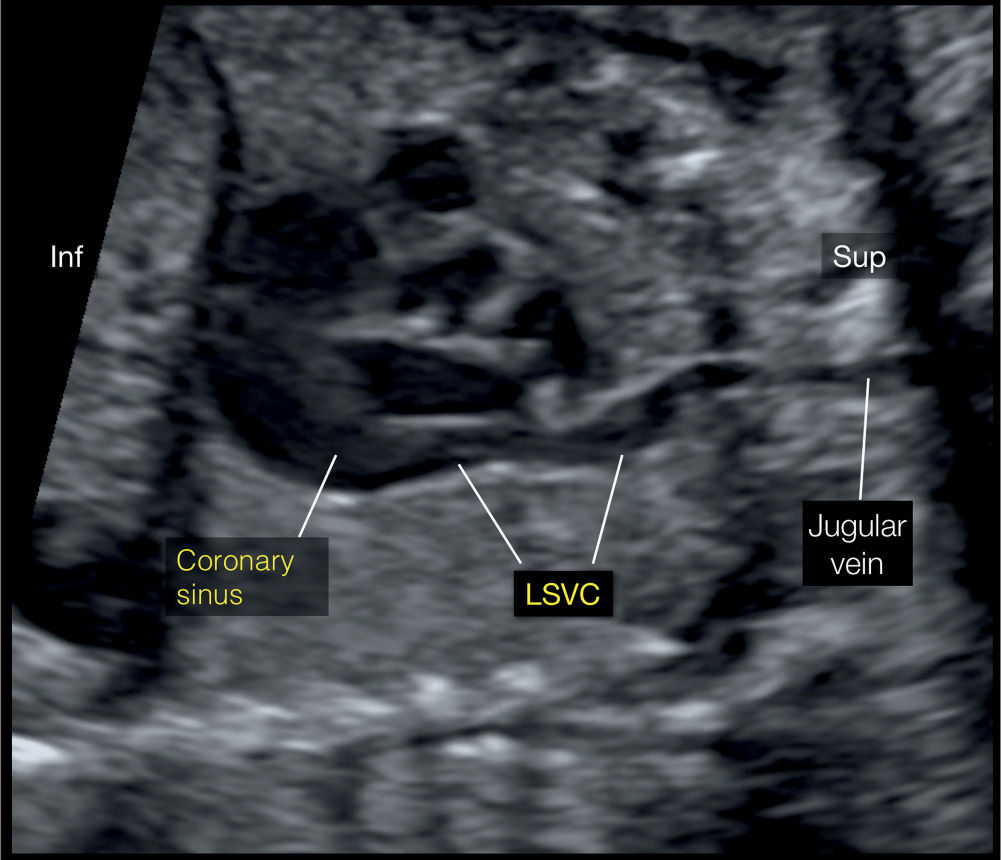
Figure 31.9: Left parasagittal plane, corresponding to plane 5 in Figure 31.2. Note the course of the left superior vena cava (LSVC) and its drainage into the coronary sinus. Inf, inferior; Sup, superior.
Early Gestation
The detection of LSVC is difficult before 20 weeks’ gestation. If LSVC is suspected, the three-vessel-trachea view or the demonstration of a dilated coronary sinus provides the best option for diagnosis in early gestation. Increased nuchal translucency has been shown in 29% of fetuses with LSVC irrespective of its association with other cardiac malformations or heterotaxy syndrome (4).
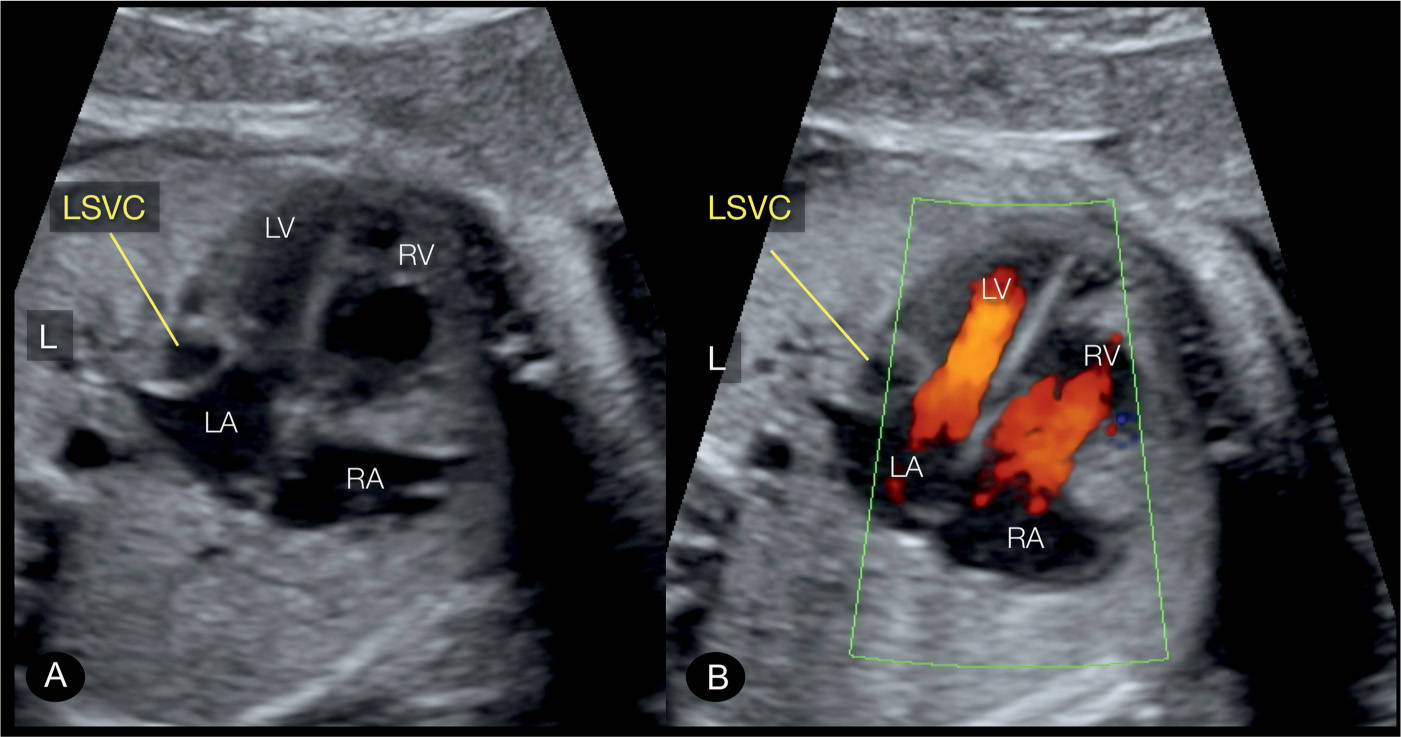
Figure 31.10: Apical four-chamber view in a fetus with persistent left superior vena cava (LSVC) in gray scale (A) and color Doppler (B). Note that the LSVC bulges into the left atrium (LA) in A and B. Color Doppler shows that the bulge of the LSVC into the LA somewhat hinders the diastolic filling of the left ventricle (LV). RV, right ventricle; RA, right atrium; L, left.
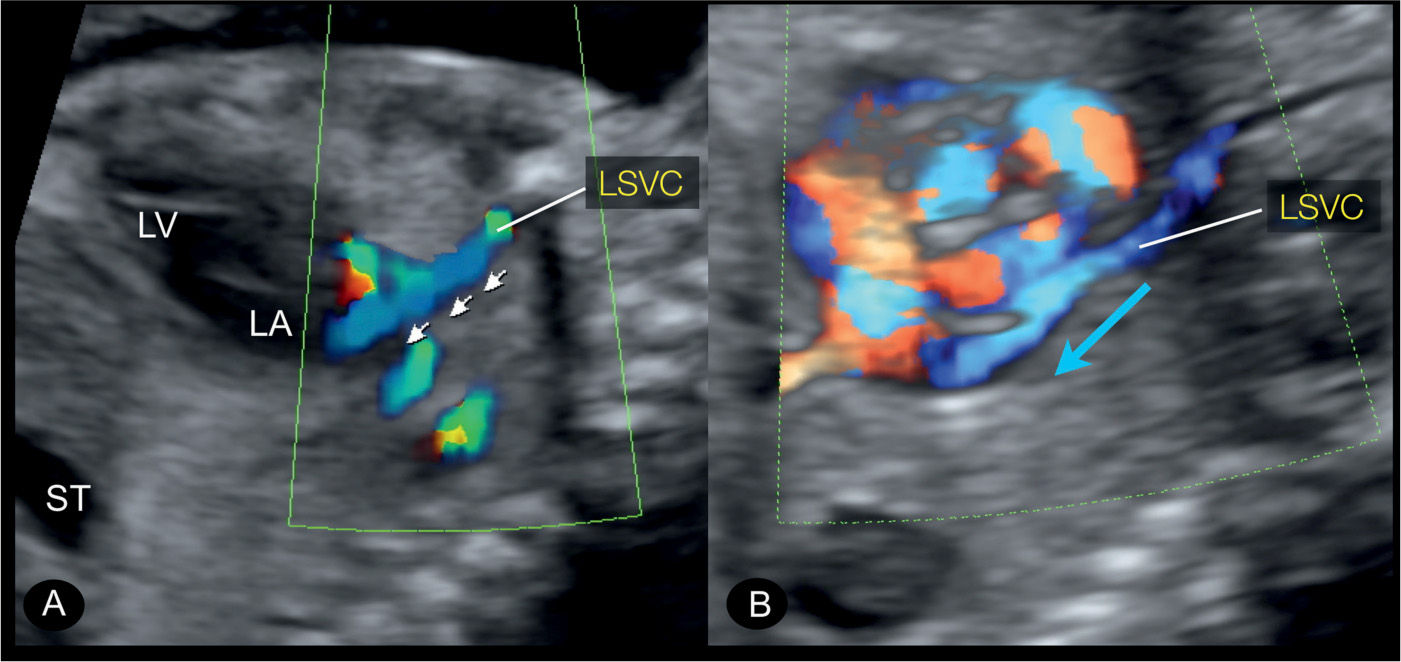
Figure 31.11: Left parasagittal plane in color Doppler in two fetuses with persistent left superior vena cava (LSVC). This plane corresponds to plane 5 in Figure 31.2. The course of the LSVC is highlighted on color Doppler with the direction of blood flow from the head and left arm toward the heart in a downstream flow pattern (small arrows in A and blue arrow in B). ST, stomach; LA, left atrium; LV, left ventricle.
Three-Dimensional Ultrasound
Because LSVC can be demonstrated in different transverse planes, tomographic imaging of three-dimensional (3D) volumes can display the LSVC in multiple levels. The use of the novel 3D biplane mode with the simultaneous demonstration of the dilated coronary sinus, and in the corresponding orthogonal plane the LSVC draining into it, is promising (Fig. 31.12). Surface mode in the four-chamber plane or in the three-vessel view can demonstrate the LSVC as an additional vessel, and color Doppler with glass-body mode or inversion mode can confirm its anatomic location. Figure 31.13 shows a novel use of color Doppler glass-body mode, which allows for the visualizing of the SVC and the LSVC in a frontal view (see also Chapter 15).
Associated Cardiac and Extracardiac Findings
Associated cardiac malformations are common and primarily include heterotaxy syndrome, left ventricular tract obstructive defects, and conotruncal anomalies (3, 4, 17). In two reported fetal series totaling 136 cases of LSVC diagnosed on fetal echocardiography in tertiary centers, absence of associated congenital heart disease was noted in 17 of 136 (12.5%) (3, 4). Heterotaxy, which accounted for the most common associated cardiac malformation, occurred in 55 of 136 (40%) of total LSVC cases and in 55 of 119 (46%) of LSVC with associated congenital heart disease (3, 4). Atrioventricular septal defects were the most common associated cardiac malformation in the heterotaxy group, and ventricular septal defects and coarctation of the aorta were among the most common cardiac malformations in the nonheterotaxy group (3, 4). In our experience, LSVC can also be present in fetuses with mesocardia or dextrocardia without heterotaxy. The association of LSVC with partial or total anomalous pulmonary venous connection (TAPVC) is often difficult to diagnose (21). On occasions, the right SVC is absent and the LSVC is the only vein draining systemic venous blood from the upper body (18, 19).
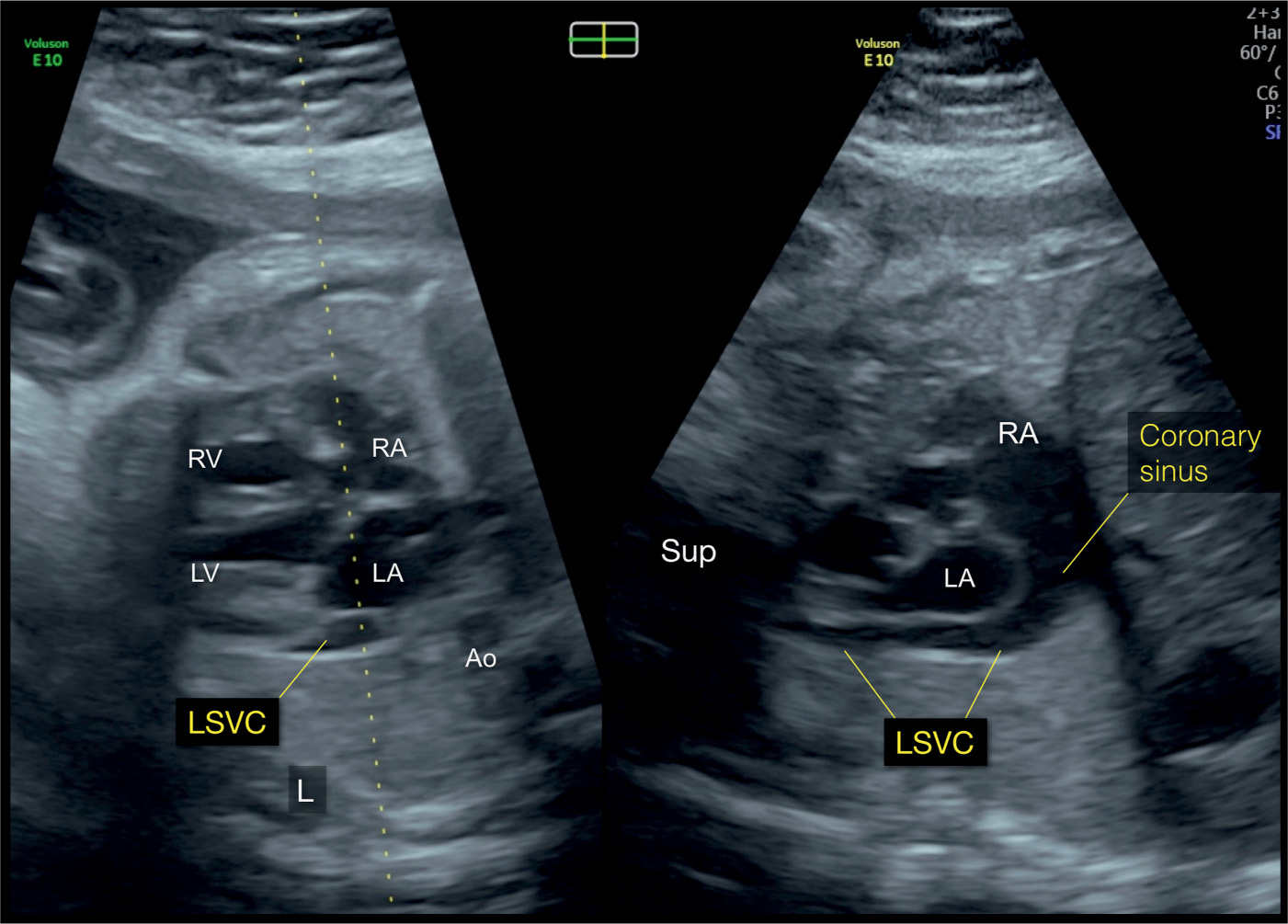
Figure 31.12: Biplane mode display from an axial view of the four-chamber view in a fetus with a left superior vena cava (LSVC). The dotted line of the biplane mode is placed along the atria and the LSVC (left panel). The orthogonal plane (right panel) shows the LSVC in its longitudinal course, inferior to the left atrium (LA), and draining into the dilated coronary sinus. The coronary sinus drains into the right atrium (RA). The left and right panels correspond to planes 2 and 5, respectively, in Figure 31.2. Ao, aorta; LV, left ventricle; RV, right ventricle; Sup, superior; L, left.
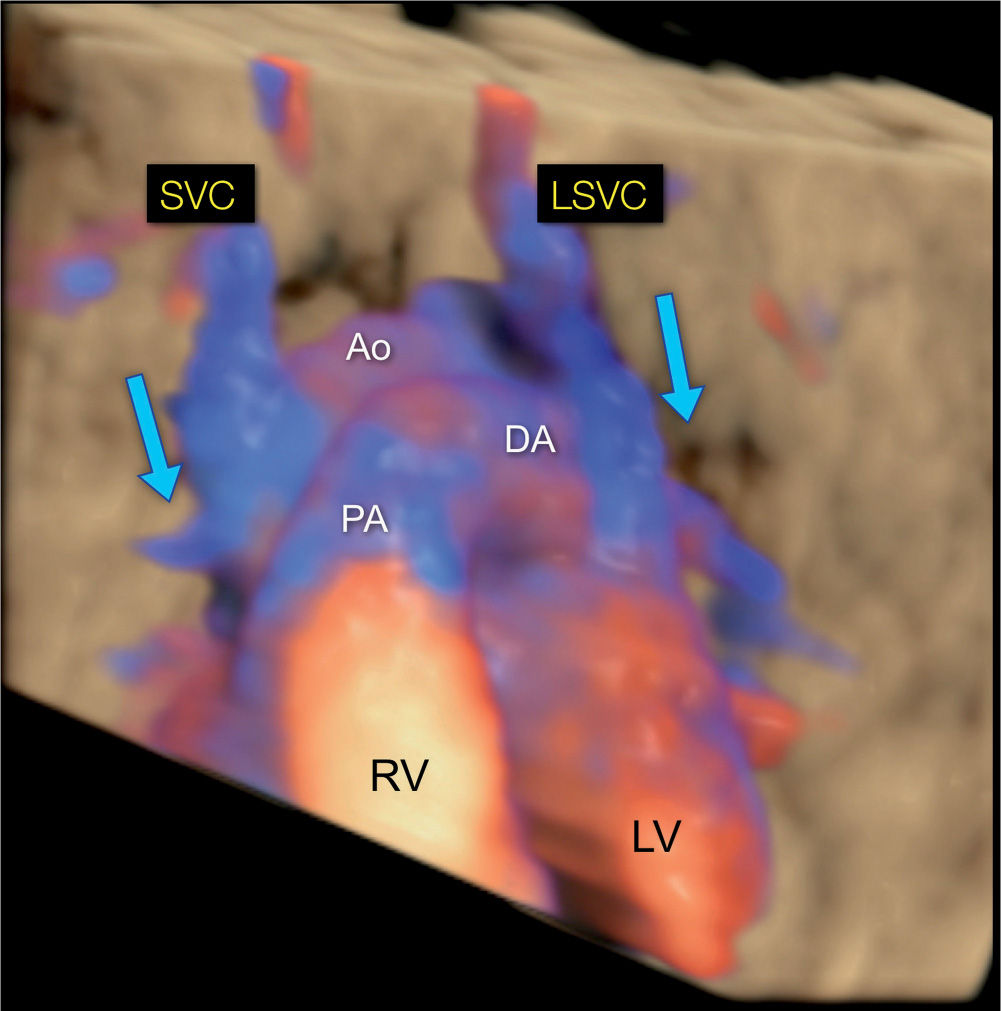
Figure 31.13: 3D rendering in color Doppler and in glass-body mode of a fetus with a left (LSVC) and a right (SVC) superior vena cava. The view is from the anterior aspect of the chest, similar to the scheme in Figure 31.1. Note the direction of blood flow in both LSVC and SVC draining from the head and arms toward the heart (blue arrows). Ao, Aorta; LV, left ventricle; RV, right ventricle; PA, pulmonary artery; DA, ductus arteriosus.
Associated extracardiac malformations are common and primarily include abnormalities of the spleen and bowel in fetuses with heterotaxy syndrome (3). Other common extracardiac abnormalities include single umbilical artery and abnormalities of the umbilical venous system (3, 4). Chromosomal anomalies, such as trisomies 21 and 18 and others, were reported in 9% of LSVC in one study (3).
Differential Diagnosis
LSVC is commonly missed in the majority of cases, especially when isolated. Misdiagnosing a dilated coronary sinus in association with LSVC as an atrial septal defect (22), atrioventricular septal defect (23), or anomalous pulmonary venous connection (24) is a typical pitfall. Differential diagnosis of LSVC includes the vertical vein in supracardiac pulmonary venous connection (see section “Total and Partial Anomalous Pulmonary Venous Connections”). Color Doppler can help differentiate LSVC from the vertical vein as the blood flow is toward the heart in LSVC and in opposite direction in the vertical vein (7).
Prognosis and Outcome
Pregnancy course of a fetus with LSVC depends on the underlying associated cardiac abnormalities, if any. In “isolated” LSVC, attention should be given to the growth of the left ventricle and the aortic isthmus, as aortic coarctation can develop in utero in a fetus with an apparently isolated LSVC. Isolated LSVC does not seem to be associated with clinical problems postnatally. Neonatal echocardiography is, however, recommended to rule out additional findings. When faced with an apparently isolated LSVC prenatally, we inform the parents about the good prognosis and recommend an echocardiogram postnatally.
KEY POINTS  Persistent Left Superior Vena Cava
Persistent Left Superior Vena Cava
 Persistent LSVC is thought to result from failure of the left anterior and common cardinal veins to involute.
Persistent LSVC is thought to result from failure of the left anterior and common cardinal veins to involute.
 LSVC joins the coronary sinus and drains into the right atrium in 92% of cases or into the left atrium in the remainder of cases.
LSVC joins the coronary sinus and drains into the right atrium in 92% of cases or into the left atrium in the remainder of cases.
 Identifying LSVC can be achieved in four transverse planes and in one longitudinal plane; the four-chamber view, a plane just posterior to the four-chamber view, the three-vessel-trachea view, the left brachiocephalic view, and a left parasagittal view.
Identifying LSVC can be achieved in four transverse planes and in one longitudinal plane; the four-chamber view, a plane just posterior to the four-chamber view, the three-vessel-trachea view, the left brachiocephalic view, and a left parasagittal view.
 Increased nuchal translucency has been shown in 29% of fetuses with LSVC.
Increased nuchal translucency has been shown in 29% of fetuses with LSVC.
 Heterotaxy accounts for the most common associated cardiac malformation with LSVC.
Heterotaxy accounts for the most common associated cardiac malformation with LSVC.
 Ventricular septal defects and coarctation of the aorta were among the most common associated cardiac malformations in the nonheterotaxy group with LSVC.
Ventricular septal defects and coarctation of the aorta were among the most common associated cardiac malformations in the nonheterotaxy group with LSVC.
 Chromosomal anomalies were reported in 9% of LSVC in one study.
Chromosomal anomalies were reported in 9% of LSVC in one study.
 Isolated LSVC does not seem to be associated with clinical problems postnatally.
Isolated LSVC does not seem to be associated with clinical problems postnatally.
● INFERIOR VENA CAVA INTERRUPTION WITH AZYGOS CONTINUATION
Definition, Spectrum of Disease, and Incidence
The azygos vein is formed in the retroperitoneal abdominal space by the joining of several small veins, including lumbar and right intercostal veins (see also Chapter 10). The normal azygos vein courses along the right side of the spine, passes through the posterior diaphragm, right and posterior to the heart in the chest, and drains into the SVC at the level of its connection with the right atrium (25). The hemiazygos vein courses along the left side of the body, in similar course to the inferior portion of the azygos vein. At the thoracic level, the hemiazygos vein drains into the azygos vein. Despite its small caliber, the azygos vein can be visualized from early gestation when high-definition color Doppler is used. With grayscale ultrasound, the normal azygos vein can only be demonstrated in the late second and third trimesters of gestation (Fig. 10.10).
One of the most common abnormalities of the IVC is its interruption at the intrahepatic level (Fig. 31.14). The interruption is typically suprarenal in anatomic location. The interrupted IVC typically connects with the azygos vein (or hemiazygos vein) in order to return systemic blood to the right atrium. This condition is called “IVC interruption with azygos continuation.” Blood from the lower body is thus drained via the azygos venous system to the SVC, which drains into the right atrium (see Fig. 31.14). This results in dilation of the azygos vein (Figs. 31.15 and 31.16). In rare conditions, the interrupted IVC drains into a dilated hemiazygos vein, with the latter draining into a persistent LSVC (Fig. 31.16). Although the IVC interruption with azygos continuation can present as an isolated finding, it is more commonly associated with left atrial isomerism (see Chapter 30). The presence of interrupted IVC with azygos continuation is noted in about 80% to 90% of left isomerism cases (26, 27). Although the incidence of isolated cases of IVC interruption is currently unknown, its combination with left isomerism is found in between 2.2% and 4.2% of infants with congenital heart disease (28) (see also Chapter 30). Occasionally, other vein(s), such as the ductus venosus or others, can drain into the azygos vein, which leads to its dilation, despite the presence of a normal (uninterrupted) IVC (29).
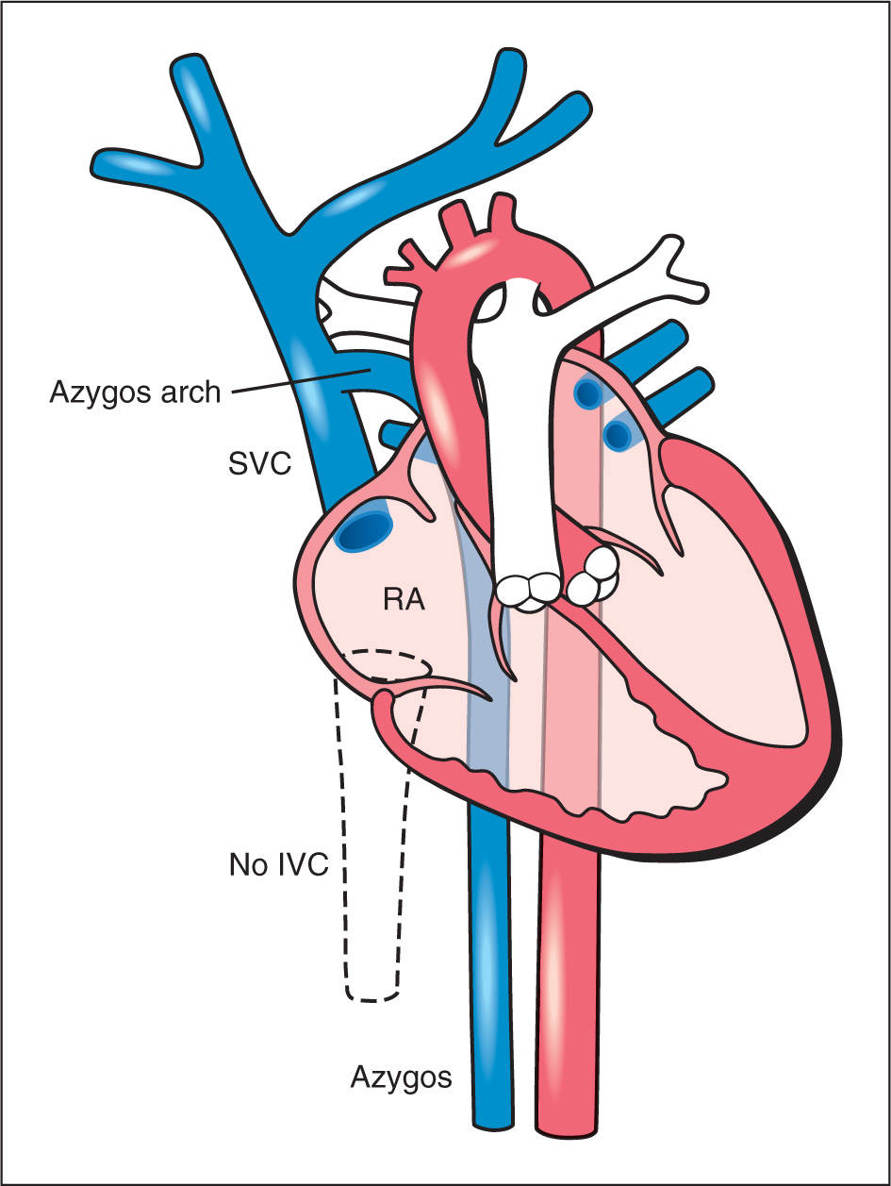
Figure 31.14: Schematic drawing of an interrupted inferior vena cava (IVC) (dashed lines) with an azygos vein continuation. The intrahepatic IVC is not formed and the venous blood from the lower body is drained back to the heart through a dilated azygos vein. The azygos vein and the aorta appear of similar size in a side-by-side orientation. The azygos vein drains into the superior vena cava (SVC) across the azygos arch. RA, right atrium.
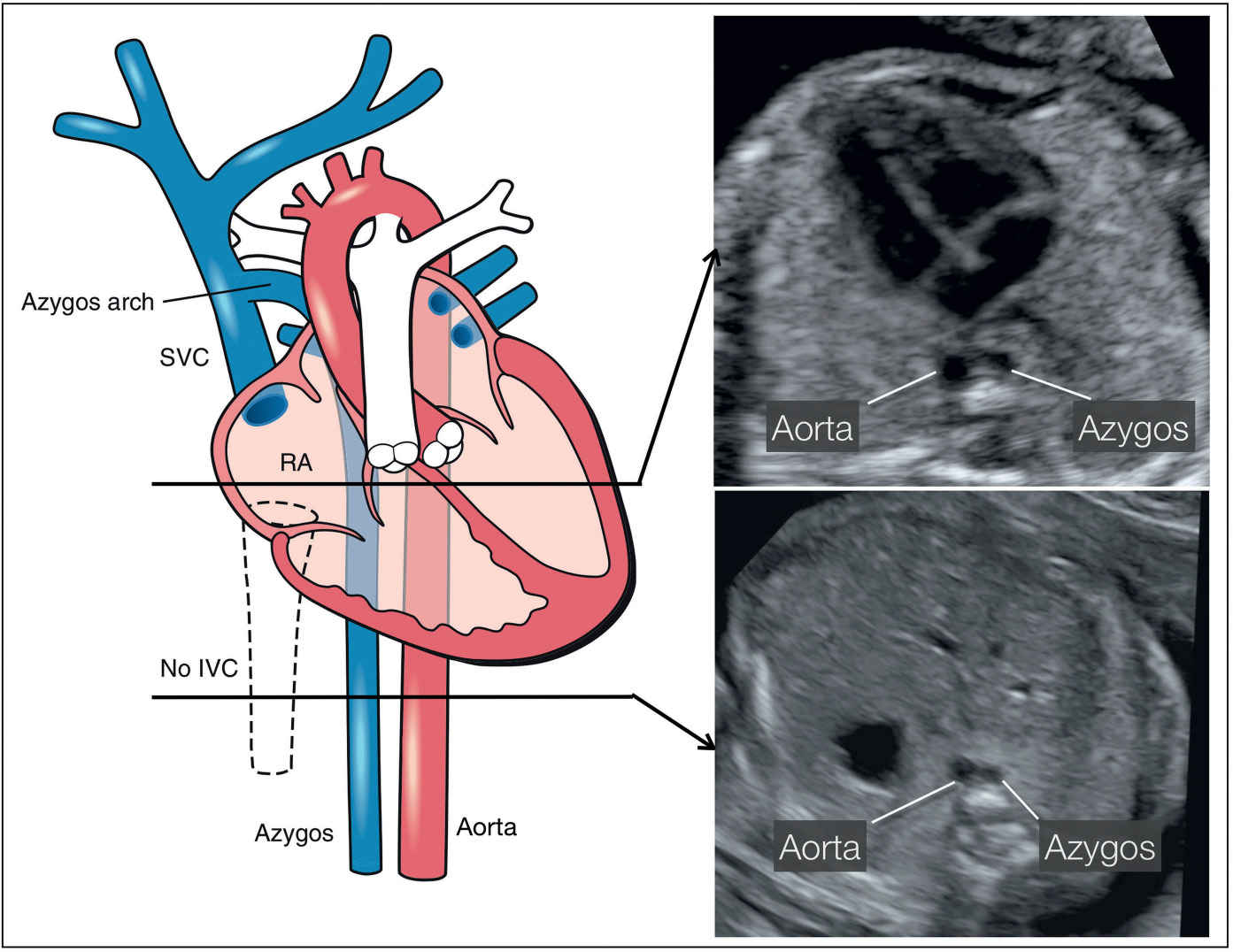
Figure 31.15: Schematic drawing of an interrupted inferior vena cava (IVC) (dashed lines) with an azygos vein continuation and two corresponding ultrasound axial planes at the level of the four-chamber view (right upper) and the upper abdomen (right lower). Note the side-by-side course of the azygos and descending aorta in the four-chamber plane (right upper) and in the upper abdominal plane (right lower) (see text for more details). SVC, superior vena cava; RA, right atrium.
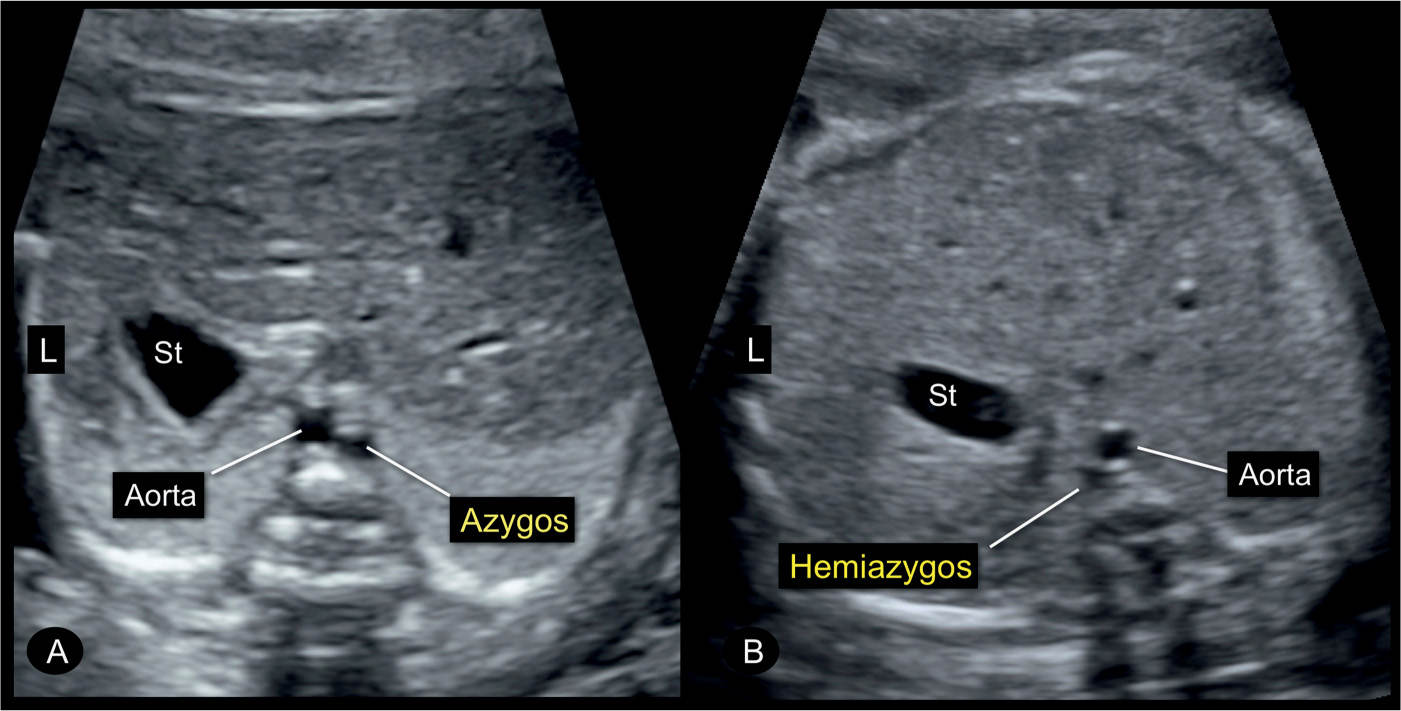
Figure 31.16: Transverse planes of the fetal abdomen in two fetuses (A and B) with an interruption of the inferior vena cava and azygos vein continuation. Note the presence of the typical double-vessel sign in front of the spine with the azygos vein in A to the right of the aorta and the hemiazygos vein in B to the left of the aorta. Interruption of the inferior vena cava is occasionally an isolated finding but also could be associated with left atrial isomerism. St, stomach; L, left.
Ultrasound Findings
Gray Scale
The absence of the IVC with azygos continuation can be demonstrated in the axial planes between the upper abdomen and the upper mediastinum (Fig. 31.15). The suspicion is achieved on gray scale, typically after 20 weeks’ gestation, when the azygos vein is noted to be dilated, almost similar in size to the adjacent descending aorta. In an axial plane of the upper abdomen, typical findings include the absence of an IVC in the right abdomen and the demonstration of a dilated vein adjacent to the abdominal aorta (Figs. 31.15 and 31.16). This side-by-side view of the dilated azygos vein next to the descending aorta has been termed “double-vessel sign” (30). The double-vessel sign can also be seen in the four-chamber view plane, posterior to the left atrium (Fig. 31.17). At the level of the three-vessel-trachea view, the dilated azygos is easily recognized as it connects to the SVC (Fig. 31.18). A parasagittal view confirms the absence of an IVC entering the right atrium (Fig. 31.19). An adjacent parasagittal view of the abdomen and chest can demonstrate the azygos vein, parallel and posterior to the descending aorta (Figs. 31.20 to 31.22). The hepatic veins connect directly into the right atrium in the absence of IVC.
Color Doppler
Color Doppler is not an essential component of the diagnosis in the second half of gestation. Color Doppler, however, can demonstrate the opposite direction of flow between the dilated azygos vein and the descending aorta, thus confirming the diagnosis (Fig. 31.20). This can be noted in axial but better in parasagittal coronal (Fig. 31.20) views or simultaneously in both planes when scanning with the 4D biplane tool (Figs. 31.21 and 31.22). Furthermore, color Doppler in the three-vessel-trachea view can show the drainage of the dilated azygos or hemiazygos vein into the SVC or persistent LSVC, respectively (Fig. 31.18B). In early gestation, the use of color Doppler is quite helpful for the demonstration of the aorta and azygos vein in the parasagittal view (Fig. 31.23). Color Doppler can also be helpful in assessing for additional venous anomalies.
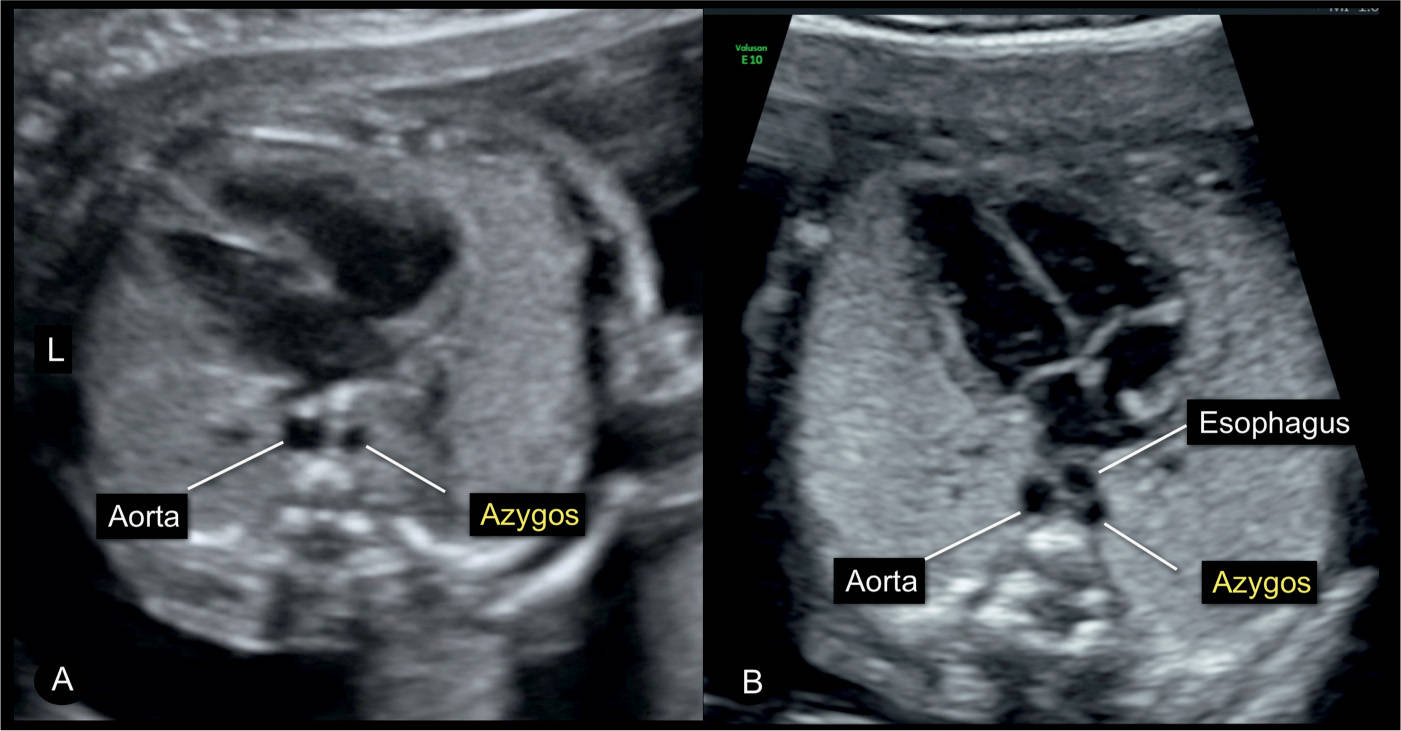
Figure 31.17: Four-chamber view in A of a fetus with normal cardiac anatomy and an interrupted inferior vena cava with azygos vein continuation. Note in A the presence of the typical double vessels in front of the spine, representing the dilated azygos vein and the descending aorta. The figure in B was captured during swallowing and the dilated esophagus appears as a third circular structure. Swallowing in a normal fetus can temporarily lead to the appearance of a double-vessel sign (compare with Fig. 7.16). L, left.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


