Androgen Excess Disorders
Richard S. Legro
Ricardo Azziz
Androgen excess, or hyperandrogenism, is a common, albeit heterogeneous, endocrine disorder of women. The clinical presentation of androgen excess may range from mild hirsutism, acne, or subtle ovulatory dysfunction to the rare patient with frank virilization and masculinization. Androgen excess disorders include the polycystic ovary syndrome (PCOS), nonclassic adrenal hyperplasia (NCAH), the hyperandrogenic insulin-resistant acanthosis nigricans (HAIR-AN) syndrome, and androgen-secreting neoplasms. Although the most recognizable clinical feature of androgen excess is hirsutism, it should be noted that not all patients with hirsutism have evidence of androgen excess, as in the patient with idiopathic hirsutism (IH). Likewise, not all patients with an androgen excess disorder have clinically evident hirsutism, to wit the Asian patient with PCOS. However, because of its frequent association with PCOS, clinically evident androgen excess (e.g., hirsutism) is a useful marker for the presence of metabolic abnormalities in these women, including insulin resistance and glucose intolerance resulting in an increased risk for type 2 diabetes mellitus (DM) and possibly also cardiovascular disease (CVD). With these new insights, the burden of care for the treating physician has increased beyond that of solely treating the presenting complaint to include the detection and, if possible, prevention of these metabolic consequences.
Normal Androgen Production and Metabolism
Androgen Production and Action
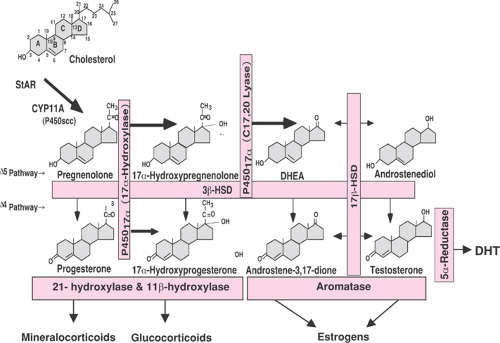
Androgens are C19 steroids (that is they contain 19 carbons) produced from circulating low-density lipoprotein (LDL) cholesterol (a C27 molecule). As in most metabolic pathways, the first step is rate limiting, that is, the conversion of cholesterol to the weak progestogen pregnenolone (a C21 steroid) by cytochrome P450scc (Fig. 39.1). The zona reticularis of the adrenal cortex, the theca, and possibly stroma of the ovaries secrete androgens produced through de novo synthesis from cholesterol. In addition, circulating steroid precursors can be metabolized further in these organs or in peripheral tissues, including the liver, adipose tissue stroma, and the pilosebaceous unit (PSU) in skin to more potent androgens (e.g., the conversion of testosterone to dihydrotestosterone [DHT]) and to estrogens via the action of aromatase, or they can be inactivated and readied for excretion (Fig. 39.2). The origins of the principal circulating plasma androgens in pre- and postmenopausal women are summarized in Table 39.1. Androstenedione is the most important precursor of testosterone and DHT, while dehydroepiandrosterone (DHEA) accounts for only 5% and 13% of circulating testosterone in normal women. Overall, DHEA has little androgenic activity and is best viewed as a pro-androgen.
Androgens, either directly or through metabolites, can act systemically in a classic endocrine fashion or locally in a paracrine and intracrine fashion (e.g., in the PSU or in the ovarian follicle). Androgens exert their genomic effects through interaction with the androgen receptor, a member of the nuclear receptor superfamily. The unbound androgen receptor is a cytoplasmic protein, and on ligand binding, it translocates into the nucleus. In the nucleus, it influences the transcription of target genes through a complex process that includes interactions with other transcription factors and coactivators. As noted, androgens also can exert their effects indirectly, though metabolites such as the estrogens. Androgens, like other sex steroids, also exert
nongenomic effects through binding to membrane-bound steroid receptors as well as nonreceptor-mediated actions at the plasma membrane.
nongenomic effects through binding to membrane-bound steroid receptors as well as nonreceptor-mediated actions at the plasma membrane.
Androgen Clearance
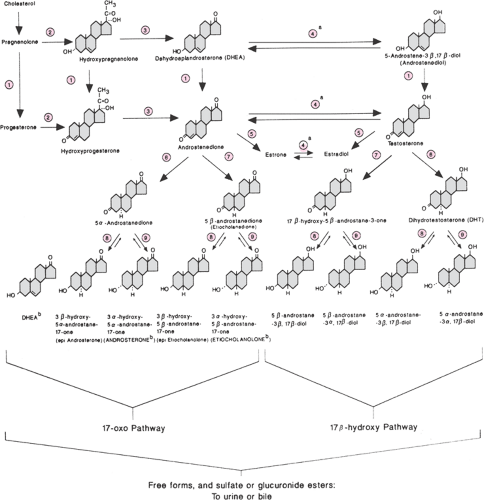
The clearance of androgens is accomplished by hepatic extraction and peripheral metabolism, which are highly dependent on the unbound portion of circulating steroid. Approximately 10% of testosterone and 50% of androstenedione are metabolized peripherally in women. Clearance of androgens by the hepatic splanchnic circulation involves mainly catabolism via 5α- and 5β-reductase. Androgen metabolites are further conjugated by the liver (95% glucuronic and 5% sulfuric), facilitating their urinary excretion (Fig. 39.2). Some 15% of androgen sulfates are excreted in bile, of which 80% are reabsorbed in the gut. Peripheral metabolism of androgens also occurs in the various target tissues, including skin, muscle, brain, and adipose tissue. A-ring aromatization (via aromatase activity), 17β-hydroxysteroid dehydrogenization (also known as 17β-ketosteroid reductase), 3α- and 3β-oxo-reduction, and 5α- and 5β-A-ring reduction (via 5α- and 5β-reductase) give rise to estrogens; to weaker metabolites such as 5α-androstane-3α,17β-diol, androsterone, and etiocholanolone; or to more potent androgens such as DHT. Androgen metabolites are subsequently conjugated by the liver and excreted in the urine and bile. Measurement of these metabolites (e.g., 3α-androstanediol glucuronide) was proposed as a circulating marker of peripheral androgen excess, but its clinical use currently is negligible.
Bioavailability of Androgens
Androgens circulate in the body bound by a variety of proteins, including albumin, cortisol-binding globulin, acid α2-glycoprotein, and most importantly, sex hormone–binding globulin (SHBG, formerly known as testosterone or testosterone-estradiol–binding globulin [TeBG]). Because of its much higher concentration and total amount, albumin has a much greater overall binding capacity for androgens than SHBG. However, the affinity of androgens for SHBG is several orders of magnitude higher than that of albumin, and thus SHBG binds the largest portion of
circulating testosterone. Androgens bound to SHBG are essentially not bioavailable; alternatively, those androgens complexed to albumin are more readily available for tissue interaction due to the much lower affinity of this protein for these steroids relative to their affinity for the androgen receptor, representing a potentially functional androgen pool or reservoir. The liver produces SHBG, and production is stimulated by estrogen, particularly oral forms, and inhibited by androgens, and most importantly, insulin. These factors lead to lower levels of SHBG and higher
bioavailable androgens in males and patients with androgen excess disorders compared with those found in healthy women.
circulating testosterone. Androgens bound to SHBG are essentially not bioavailable; alternatively, those androgens complexed to albumin are more readily available for tissue interaction due to the much lower affinity of this protein for these steroids relative to their affinity for the androgen receptor, representing a potentially functional androgen pool or reservoir. The liver produces SHBG, and production is stimulated by estrogen, particularly oral forms, and inhibited by androgens, and most importantly, insulin. These factors lead to lower levels of SHBG and higher
bioavailable androgens in males and patients with androgen excess disorders compared with those found in healthy women.
TABLE 39.1 Percent Origin of Androgens in Women | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
As noted below, the production, metabolism, and circulating levels of androgens are affected, among other factors, by age, menopausal status, the presence of obesity, and medications that affect hepatic clearance.
Androgens, Age, and Menopause
The metabolism and circulating levels of androgens can be altered significantly by age. Most notably, adrenal androgen production clearly decreases with age, beginning in the premenopausal years. Serum dehydroepiandrosterone sulfate (DHEAS) concentrations decrease linearly with age and begin at about 20 years, independent of menopause. Decreased secretion of 17-hydroxyprogesterone, 17-hydroxypregnenolone, DHEA, and androstenedione also has been observed in postmenopausal women following acute adrenal stimulation. In normal postmenopausal women, plasma concentrations of androstenedione are about half that observed in premenopausal females, although there is no observable difference in the clearance rate of this steroid between premenopausal and postmenopausal women. After menopause, androstenedione levels continue to gradually decrease with age. Testosterone levels are less affected by age and menopause, and it is clear that the ovary continues to produce a significant amount of testosterone in the postmenopause. Most of the decrease in circulating testosterone with age and menopause is explainable by the lower androstenedione levels.
Androgens and Obesity
In eumenorrheic obesity, the production rate (PR) and metabolic clearance rate (MCR) of ovarian- and adrenal-secreted androgens are increased, while circulating levels change only minimally. The increased MCR may be due to an obesity-related decrease in the plasma concentration of the carrier protein SHBG. Steroid sequestration by fat also may increase steroid clearance, leading to an extremely large pool of sex hormones in obese individuals. Adipose tissue metabolism of steroids, including aromatization and 17β-hydroxysteroid dehydrogenation, also contributes to the increased MCR of androgens, whereas alterations in hepatic conjugation and extraction also can be a contributing factor. The increased PR of androgens in obesity may simply be due to the operation of a servo-control mechanism compensating for the increased MCR. Alternatively, the increased ovarian and adrenal production noted in obesity may reflect changes in the intraglandular and/or circulating concentration of androgens themselves, or of estrogens, prolactin, growth hormone, and other growth factors, and most importantly, insulin. In addition, the accelerated turnover of androgens in obesity may increase tissue exposure to these steroids, potentially enhancing the effect of androgens in this condition. Although weight reduction improves the abnormalities noted in steroid levels, it is not known whether the elevated PR and MCR of androgens also normalize following weight reduction.
The increased production (or intake) of androgens, which can be altered by age and degree of obesity, is likely the most important determinant of clinically evident androgenicity. However, other factors such as the potency of the androgen produced (DHT > testosterone > androstenedione > DHEA), the amount of androgen that is free or weakly bound in serum (i.e., the amount bioavailable), the degree and type of central and peripheral metabolism (e.g., local or hepatic amounts of 5α-reductase, 17-hydroxysteroid dehydrogenase, or aromatase activities), or tissue sensitivity (e.g., local androgen receptor concentration) undoubtedly also play a role in determining and modifying the phenotype of androgen excess.
Signs and Symptoms of Androgen Excess
Androgen excess can result in various clinical signs and symptoms, including abnormalities of the PSU, such as hirsutism, acne, and androgenic alopecia, or dysfunction of the hypothalamic–pituitary–ovarian (i.e., ovulatory and menstrual dysfunction) or of the hypothalamic–pituitary–adrenal axes (adrenal androgen excess). If the androgen excess is very severe, virilization and/or masculinization also can be apparent. Changes in mood or sense of well-being with androgen excess may be related to neuroendocrine changes stemming from abnormalities in the endocrine and metabolic axes and to the social and psychologic stigmata of androgen excess, such as hirsutism and infertility, and associated obesity.
Androgen Excess and the Pilosebaceous Unit
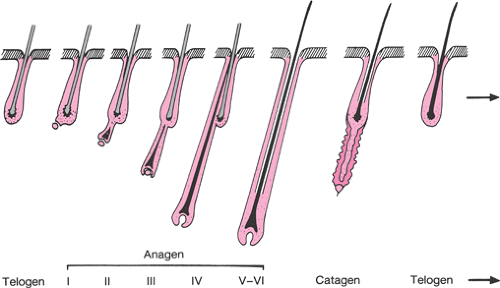
The PSU is the common skin structure that gives rise to both hair follicles and sebaceous glands, which are found everywhere on the body except the palms, soles, and lips. The density is greatest on the face and scalp (400 to 800 glands/cm2) and lowest on the extremities (50 glands/cm2). The number of PSUs does not generally increase after birth (about 5 million), but they can become more prominent through activation and differentiation. Generally, three phases of the hair growth cycle can be considered. The period of active growth is termed anagen, after which the hair follicle enters a resting or catagen phase, of varying lengths of time (Fig. 39.3). During this transition, the hair shaft separates from the dermal papillae at the base. The separated hair is then shed during the telogen phase. The period of anagen varies from 3 years on the scalp to 4 months on the face. For corresponding parts of the skin, men have longer anagen phases than women do, which may be partially due to their higher circulating androgen levels.
The effects of androgens are most visible on the PSU. Androgens stimulate the transformation of fine, unpigmented vellus hairs to coarse, pigmented, thickened terminal hairs that generally measure >5 mm in length, a process called terminalization, in skin areas sensitive to the effects of androgens. The peripheral effects of androgens are determined primarily by the intracellular actions of the enzymes 17β-hydroxysteroid dehydrogenase (converting androstenedione to testosterone) and 5α-reductase (converting testosterone to the more potent androgen DHT) and the androgen receptor content. Before puberty, body hair is primarily composed of fine, short, unpigmented vellus hairs. The increase in androgen production observed with pubertal development transforms some of these, mainly in androgen-sensitive areas of skin such as the axilla and the genital triangle, into the coarser, longer, pigmented terminal hairs observed in adults. It should be noted that not all skin areas are androgen sensitive. For example, the development of terminal hairs in body areas such as the eyebrows, eyelashes, and the temporal and occipital scalp is relatively androgen independent.
Excess androgen-dependent hair growth present in women can result in clinically evident hirsutism (see below). Paradoxically, androgens can exert opposite effects on the hair follicles of the scalp, causing conversion of terminal follicles to velluslike follicles, a process called miniaturization. This effect may lead to the development of androgenic alopecia in women (see below) or male-pattern baldness characterized by frontal and sagittal scalp hair loss. Androgens also can cause increased sebum production and abnormal keratinization of the PSU, contributing to the development of seborrhea and acne evident at puberty and in women with androgen excess. There are ethnic and genetic differences that can modify the effects of
androgens on skin, as demonstrated by the lesser degree of hirsutism present in Asian women with PCOS (Fig. 39.4).
androgens on skin, as demonstrated by the lesser degree of hirsutism present in Asian women with PCOS (Fig. 39.4).
Hirsutism
Hirsutism is the presence of terminal (coarse) hairs in females in a malelike pattern. Excessive growth of coarse hairs of the lower forearms and lower legs alone does not constitute hirsutism, although women suffering from hirsutism may note an increase in the pigmentation and growth rate of hairs on these body areas. Hirsutism should be viewed much as polycystic ovaries, as a sign rather than a diagnosis. Most commonly, hirsutism is associated with androgen excess. Although the term idiopathic hirsutism was coined to identify the presence of hirsutism without other identifiable cause or abnormality, this may actually reflect our limited ability to assess androgen action in the peripheral compartment or even in the circulation (see below).
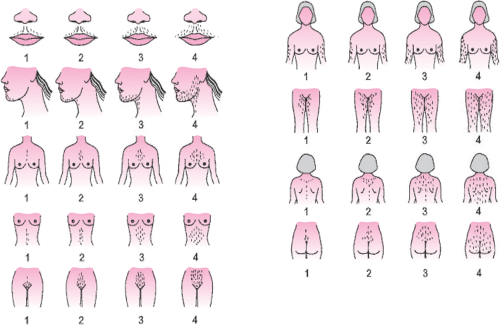
The definition of hirsutism is somewhat variable. The most commonly used method of estimating the amount of terminal hair present in a malelike distribution in women is the visual scale first described by Ferriman and Gallwey in 1961. While these investigators originally assessed 11 body areas, today we use a modification of this scale (Fig. 39.5), which assesses 9 body areas (the modified Ferriman-Gallwey or mFG score). The cutoff value for defining hirsutism varies, with Ferriman using a score 5, Hatch and colleagues proposing a score of 8, and Knochenhauer and colleagues suggesting a score of 6.
In a study of 633 unselected women seen for a pre-employment physical, we determined that a value of 8 defined the upper 95th percentile of the distribution of the mFG scores. However, principal component and univariate analyses denoted two nearly distinct clusters that occurred above and below an mFG value of 2, with the bulk of the scores below. Overall, an mFG score of at least 3 was observed in 22.1% of all subjects (i.e., the upper quartile), and of these subjects, 69% complained of being hirsute, similar to the proportion of women with an mFG score of at least 8 who considered themselves to be hirsute (70%). This proportion was much greater than that of women with an mFG score below this value (16%). There were no significant differences between black and white women. Furthermore, in a study 228 women complaining of unwanted hair growth but who demonstrated minimal increases in mFG (≥5), approximately 50% demonstrated androgen excess. These data suggest the likelihood that even minimal increases in the mFG may denote an underlying endocrine abnormality.
Acne
The PSU, in addition to the hair follicle, also contains a sebaceous gland that produces an oily protective secretion known as sebum. The excessive production of sebum in response to androgen action may lead to oily skin, clogged hair follicles, folliculitis, and the development of acne. Elevations in serum androgen levels have been noted in patients with acne, particularly in those with concurrent hirsutism, although not all investigators agree. Although the persistence or appearance of acne in adulthood has been suggested to be more frequently associated with androgen excess, we observed evidence of hyperandrogenemia in the majority of 30 consecutive nonhirsute acneic patients regardless of age. Some investigators have observed that while serum androgen levels may be relatively normal in acneic patients, the conversion of androgens to DHT via 5α-reductase was increased in the affected skin. These data
suggest that androgen suppression may be useful in treating acne in many of these patients, and it is clear that acne frequently improves following treatment with antiandrogens, oral contraceptives, or glucocorticoid suppression. Thus, empiric treatment with oral contraceptives, or even with judicious use of glucocorticoids, may be justified in acneic patients without other evidence of overt androgen excess.
suggest that androgen suppression may be useful in treating acne in many of these patients, and it is clear that acne frequently improves following treatment with antiandrogens, oral contraceptives, or glucocorticoid suppression. Thus, empiric treatment with oral contraceptives, or even with judicious use of glucocorticoids, may be justified in acneic patients without other evidence of overt androgen excess.
Androgenic Alopecia
Scalp hair loss as a consequence of androgen excess can take two forms. In severe cases, where massive androgen excess and virilization/masculinization is present, patients can demonstrate the typical pattern of balding found in men (i.e., premature male-pattern balding). More common, however, is the so-called androgenic (also termed androgenetic, as an inherited etiology often is suspected) alopecia of women (i.e., female-pattern balding). In female androgenic alopecia, a diffuse thinning of hair throughout the sagittal scalp is primarily noted, and approximately 40% of women with androgenic alopecia have some form of hyperandrogenemia. However, if only nonhirsute women with androgenic alopecia are considered, then only approximately 20% of these patients are found to be hyperandrogenemic.
Androgens and Hypothalamic–Pituitary–Ovarian Axis Dysfunction
Androgens, indirectly (e.g., possibly through conversion to estrogens) and directly, may alter the secretion of gonadotropins in women. For example, women with PCOS appear to have reduced hypothalamic sensitivity to progesterone that may be mediated by elevated androgens, since normal sensitivity can be restored with the androgen receptor blocker flutamide. Alternatively, other data suggests that in the absence of severe androgens excess, the direct effect of androgens on the hypothalamic–pituitary axis appears limited. For example, the infusion of the nonaromatizable androgen DHT to five women with PCOS did not alter the mean levels, pulsatile patterns, or sensitivity to gonadotropin-releasing hormone (GnRH) of luteinizing hormone (LH) or follicle-stimulating hormone (FSH). Overall, the mechanisms underlying the hypothalamic–pituitary dysfunction of PCOS and the potential role that androgens play in the same remains unclear. Finally, excessive androgens also may directly inhibit follicular development at the ovary, which may result in disrupted folliculogenesis and the accumulation of multiple small cysts within the ovarian cortex, the so-called “polycystic” ovary.
Androgens and Hypothalamic–Pituitary–Adrenal Axis Dysfunction
Adrenal androgen excess (i.e., elevated levels of DHEA and DHEAS and of the adrenal fraction of androstenedione) is a concomitant finding in many women with androgen excess. It should be noted that the age-associated decline in DHEAS levels is observable and similar in both control and PCOS women. Hence, estimates of the prevalence of adrenal androgen excess require consideration of age. In a study of 213 (27 black and 186 white) women with PCOS and 182 (88 black and 94 white) age-matched healthy eumenorrheic nonhirsute women, the authors observed that the prevalence of DHEAS excess was approximately 20% among white and 30% among black PCOS patients when using age and race-adjusted normative values.
It is possible that extra-adrenal androgens (e.g., ovarian) may alter adrenocortical steroidogenesis and androgen secretion. For example, various investigators have observed a 20% to 25% decrease in mean DHEAS levels following long-acting GnRH-α suppression in PCOS women with elevated levels of this adrenal androgen, although elevated adrenal androgen levels in these women rarely normalize with GnRH-α suppression. Vermesh and colleagues reported that a 2-hour infusion of testosterone in seven healthy women resulted in subtle inhibition of 21- and/or 11β-hydroxylase activities but not in 17,20-lyase or 3β-hydroxysteroid dehydrogenase activities. In turn, Azziz and colleagues prospectively studied the effect of 3 weeks of parenteral exogenous testosterone in seven healthy oophorectomized women. A significant change in the adrenal response to adrenocorticotrophic hormone (ACTH) stimulation was not observed, although an increase in the metabolism of DHEA to DHEAS was apparent. Thus, it appears that the secretion of adrenal androgens can be increased by extra-adrenal hyperandrogenemia, although the clinical relevance of such an effect is unclear.
Virilization and Masculinization
Virilization includes the appearance of sagittal and frontal balding, clitoromegaly, and severe hirsutism. Furthermore, if androgen levels are extremely elevated for a substantial period of time, the features of virilization may be accompanied by masculinization of the body habitus, with atrophy of the breasts, an increase in muscle mass, a redistribution of body fat, and a deepening of the voice. Premenopausal patients with virilization of masculinization almost always are amenorrheic. In general, virilization or masculinization should raise the suspicion of an androgen-secreting neoplasms or classic (but not nonclassic) adrenal hyperplasia. Occasionally, girls suffering from a severe insulin resistance syndrome may exhibit a moderate degree of virilization. These situations will be discussed in the following section.
Polycystic Ovary Syndrome
By far, the most common cause of androgen excess is PCOS, accounting for the vast majority of these patients. When defined strictly, it appears to affect approximately 7% of unselected women in the developed world. There is no firm consensus as to the definition of PCOS, although criteria arising from a 1990 National Institutes of Health (NIH) conference on the subject have proven clinically and investigationally useful and identified PCOS as unexplained hyperandrogenic chronic anovulation (Table 39.2). Diagnostic criteria, sometimes called the Rotterdam criteria, were proposed by an expert panel in 2003 (Table 39.2) and modify the prior 1990 NIH criteria by incorporating ovarian size and morphology into the schema. It appears that the Rotterdam criteria increase the affected population by about 50% and include less severe forms of the syndrome. A more recent recommendation for defining PCOS proposed by a task force of the Androgen Excess Society attempts to bridge this difference (Table 39.2).
Clinical and Biochemical Features of Polycystic Ovary Syndrome
Approximately 70% to 80% of women with PCOS demonstrates frank elevations in circulating androgens, particularly free testosterone, and 25% to 50% will have elevated levels of the adrenal androgen metabolite DHEAS. Prolactin levels usually are normal, although they may be slightly elevated (generally <40 ng/mL) in a small fraction of patients. The LH/FSH ratio is >2 to 3:1 in approximately 60% of these patients. As noted previously, the ovaries of up to 90% of patients with PCOS usually contain
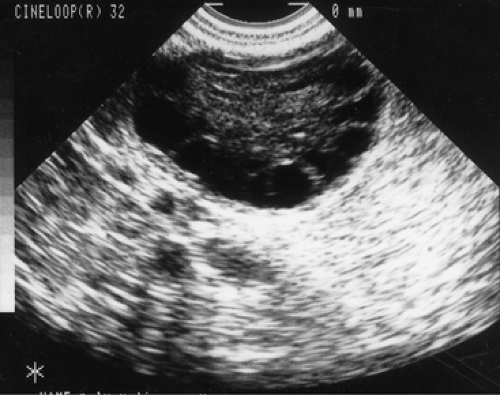
intermediate and atretic follicles measuring 2 to 5 mm in diameter, resulting in a polycystic appearance at sonography (Fig. 39.6). About 60% of PCOS patients are obese, although significant fractions are nonobese.
intermediate and atretic follicles measuring 2 to 5 mm in diameter, resulting in a polycystic appearance at sonography (Fig. 39.6). About 60% of PCOS patients are obese, although significant fractions are nonobese.
TABLE 39.2 Criteria for Defining Polycystic Ovary Syndrome | ||||
|---|---|---|---|---|
|
Metabolic Features of Polycystic Ovary Syndrome
While not part of the diagnostic criteria, many PCOS women appear to be uniquely insulin resistant and hyperinsulinemic. Approximately 50% to 70% of PCOS patients demonstrate profound insulin resistance and secondary hyperinsulinemia, independent of body weight. In PCOS, insulin resistance usually refers to the impaired action of insulin in stimulating glucose transport and in inhibiting lipolysis in adipocytes, and studies of the action of insulin in adipocytes, myocytes, and other tissues in this disorder appear to suggest the presence of an intracellular defect of insulin signaling.
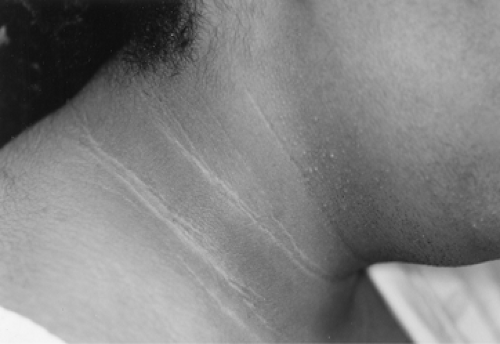
The compensatory hyperinsulinemia, resulting from the underlying insulin resistance, augments the stimulatory action of LH on the growth and androgen secretion of ovarian thecal cells while inhibiting the hepatic production of SHBG. In addition, treatment of PCOS patients with insulin sensitizers may lower circulating levels of LH, suggesting that insulin resistance, or more likely hyperinsulinemia, is in part responsible for the gonadotropic abnormalities observed in many women with PCOS, although not all agree. Since insulin also is a mitogenic hormone, the extremely elevated insulin levels may lead to hyperplasia of the basal layers of the epidermis, resulting in the development of acanthosis nigricans (a velvety, hyperpigmented change of the crease areas of the skin) (Fig. 39.7) and acrochordons (skin tags). Overall, insulin resistance and secondary hyperinsulinemia affects a large fraction of PCOS patients and may cause or augment the androgen excess of these patients.
Ovarian Morphology in Polycystic Ovary Syndrome
It should be noted that polycystic ovaries on sonography or at pathology are simply a sign of androgen excess and possibly PCOS. For example, this ovarian morphology frequently is seen in patients with adrenal hyperplasia (Table 39.3), and up to 25% of unselected women have polycystic ovaries on ultrasound, many of which are normoandrogenic regularly cycling. The classic polycystic ovary morphology on ultrasound has been described as containing multiple 2- to 8-mm subcapsular preantral follicles forming a “black pearl necklace” sign (Fig. 39.6). Hence, the appearance of polycystic ovaries, particularly on sonographic exam are considered to be a sign, albeit nondiagnostic, of androgen excess and PCOS. Finally, while polycystic ovaries are common in women with PCOS, their presence does not predict the metabolic or reproductive phenotype of these patients.
Polycystic Ovary Syndrome throughout the Life Span
Clinically evident PCOS tends to develop shortly after menarche and persists through most of the reproductive life. Nonetheless, some patients may present initially in the prepuberty with premature adrenarche, with affected girls displaying hyperinsulinemia, elevated DHEAS levels, and postmenarche oligomenorrhea. At the other end of the reproductive spectrum, both menstrual irregularity and hyperandrogenemia appear to normalize as PCOS women approach the perimenopause. However, mothers of women with PCOS have elevated testosterone levels compared with levels of controls, suggesting that mild elevations in androgens may persist into later life. Although the endocrine
and reproductive features of the disorder may improve with age, the associated metabolic abnormalities, particularly glucose intolerance, actually may worsen with age (see below).
and reproductive features of the disorder may improve with age, the associated metabolic abnormalities, particularly glucose intolerance, actually may worsen with age (see below).
TABLE 39.3 Syndromes or Disease Entities That Have Been Associated with Polycystic Ovaries | |
|---|---|
|