Androgen Abnormalities in the Adolescent Girl
Amy D. DiVasta
S. Jean Emans
Signs of androgen excess, such as hirsutism and acne, can be troubling to the adolescent girl. Oligomenorrhea or irregular menstrual bleeding in association with signs of androgen excess should suggest the possibility of polycystic ovary syndrome (PCOS), the most common cause of androgen excess in adolescence. Among women with androgen excess, PCOS accounts for 72% to 85%, idiopathic hirsutism for 5% to 7%, late-onset congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency for 1% to 4.5%, and adrenal and ovarian tumors for <0.5% (1,2).
Polycystic Ovary Syndrome
PCOS, also called functional ovarian hyperandrogenism or androgen excess syndrome, is the most common endocrinopathy in premenopausal women, affecting 6% to 8% of reproductive-aged women (3). Despite this high prevalence, the diagnostic criteria, pathophysiology, and treatment of PCOS are still debated. PCOS is a spectrum of clinical disorders associated with increased androgen production from the ovaries, often associated with insulin resistance. Clinical symptoms may include hirsutism, acne, obesity, irregular menses (oligomenorrhea, amenorrhea, menorrhagia), and infertility. For most patients, symptoms of androgen excess begin from the time of menarche.
Diagnosis
As of 2011, there have been three sets of criteria proposed to diagnose PCOS in adult women: the 1990 National Institutes of Health (NIH) conference (4), the Rotterdam consensus conference (5), and the Androgen Excess Society (now the Androgen Excess and PCOS Society) guideline (6) (Table 11-1). The 1990 NIH criteria, formed by consensus, defined PCOS as a combination of hyperandrogenism (clinical or biochemical) and oligoanovulation, with exclusion of other etiologies such as Cushing syndrome, hyperprolactinemia, and late-onset CAH (4). Probable criteria included insulin resistance, perimenarchal onset, elevated luteinizing hormone (LH)–to–follicle-stimulating hormone (FSH) ratio, and polycystic ovary (PCO) morphology by ultrasound. The 2003 Rotterdam conference expanded the diagnostic criteria, requiring that two of the following three criteria were met: (a) oligomenorrhea and/or anovulation, (b) clinical and/or biochemical signs of hyperandrogenism, and (c) PCO by transvaginal ultrasound (5). The Rotterdam definition resulted in the possibility of four discrete PCOS phenotypes: (1) irregular menstrual pattern plus PCO morphology plus hyperandrogenism, (2) irregular menses plus hyperandrogenism, (3) hyperandrogenism plus PCO morphology, and (4) irregular menses plus PCO morphology (7). Whether this last category of patients (phenotype 4) should be diagnosed under the broad rubric of PCOS remains a matter of dispute. While some patients with irregular menses and PCO morphology have similar metabolic findings to women with PCOS, labeling these adolescents with a PCOS diagnosis may be premature.
In response to a need for further clarification, the Androgen Excess Society published their guidelines in 2006, underscoring the need to define PCOS as a disorder with primary hyperandrogenism and narrowing the definition. The Androgen Excess Society criteria for PCOS include (a) hyperandrogenism (hirsutism and/or hyperandrogenemia) and (b) ovarian dysfunction (oligoanovulation and/or PCO on ultrasound) (6). Thus, irregular menses and PCO morphology alone (phenotype 4 above) were not sufficient for the diagnosis.
The diagnosis of PCOS remains challenging in younger adolescents. Transient oligomenorrhea and mild hyperandrogenemia are common in the first few years after menarche. Over time, some girls will establish normal ovulatory menses (8), whereas others may have persistent anovulatory cycles and classic PCOS. Distinguishing between these two groups can be difficult. However, persistence of anovulation for more than 2 years is a strong predictor of continued irregularity (9). In our opinion, the Androgen Excess Society criteria are the most useful for the diagnosis of PCOS during adolescence.
Who is at risk? Prepubertal risk factors are being identified. Premature pubarche, as well as fetal growth restriction, low birth weight (<2500 g), and excessive postnatal catch-up growth, may precede clinical androgen excess, insulin resistance, and the metabolic syndrome (a constellation of cardiovascular risk factors: glucose intolerance, dyslipidemia, hypertension, and central obesity) (10,11,12,13,14). Having a mother with PCOS is also an independent risk factor for later development of PCOS (14,15). Other risk factors that predict that a young adolescent will go on to develop a stable PCOS phenotype include an LH/FSH ratio >2, body mass index (BMI) >30 kg/m2, increased serum androstenedione or testosterone, acanthosis nigricans, premature adrenarche, and metabolic syndrome (16,17).
Physiology: Gonadotropins, Estrogens, and Androgens
PCOS begins in the perimenarchal period and may be considered an abnormal and exaggerated transition to puberty in four key physiologic processes: (a) an increase in LH secretion, (b) an increase in adrenal androgen production, (c) an increase in body mass, and (d) the onset of adult patterns of insulin resistance (IR).
The abnormal patterns of gonadotropin secretion seen in many patients with PCOS are apparent very early in adolescence (18,19). Exaggerated pulsatile release of LH with increased pulse frequency and amplitude (20) may be a primary defect
or secondary to ovarian enzyme dysregulation (19). LH levels are often tonically elevated, and the LH/FSH ratio is frequently elevated (>3:1) (21). BMI inversely correlates with LH levels, but not frequency (22), so that obese patients may not have an elevated LH/FSH ratio (23). High LH levels stimulate the ovary to secrete increased androgens (androstenedione and testosterone) from the stromal tissue; these androgens are converted peripherally to estrone and estradiol. These estrogens have been hypothesized to augment pituitary sensitivity to gonadotropin-releasing hormone (GnRH) (20,21).
or secondary to ovarian enzyme dysregulation (19). LH levels are often tonically elevated, and the LH/FSH ratio is frequently elevated (>3:1) (21). BMI inversely correlates with LH levels, but not frequency (22), so that obese patients may not have an elevated LH/FSH ratio (23). High LH levels stimulate the ovary to secrete increased androgens (androstenedione and testosterone) from the stromal tissue; these androgens are converted peripherally to estrone and estradiol. These estrogens have been hypothesized to augment pituitary sensitivity to gonadotropin-releasing hormone (GnRH) (20,21).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In contrast to the high LH concentrations, FSH levels are low to normal. The low FSH concentrations may result from (a) the inhibitory feedback of estrogen on FSH, (b) the relative insensitivity of FSH secretion to GnRH, and (c) the production of inhibin from the polycystic ovaries that may stimulate androgen production and inhibit FSH release. In the ovary, a relative aromatase defect (due to FSH deficiency and high intraovarian androgen levels) impairs follicular maturation and induces follicular atresia.
Additionally, androgens decrease sex hormone–binding globulin (SHBG), which is produced in the liver. Reductions in SHBG increase free testosterone and augment testosterone’s effects. In contrast, estrogen increases SHBG. In hirsute women, low SHBG concentrations facilitate the rapid uptake of free androgens and their peripheral conversion to estrogen in muscle and adipose tissue. Since SHBG concentrations are low, PCOS patients have free estrogen levels that are higher than in normal women in the midfollicular phase of the cycle (20). The constant, acyclic levels of estradiol result in unopposed stimulation of the endometrium, placing the adult woman with PCOS at a higher risk of developing endometrial cancer (24,25).
Physiology: Production of Androgens
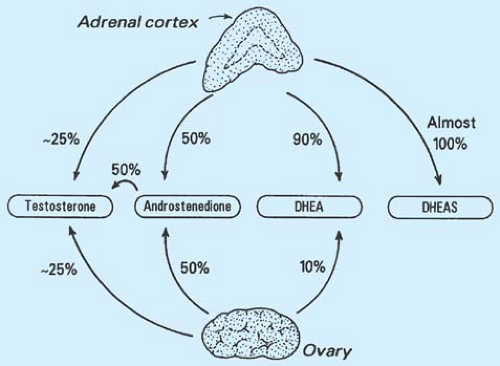
Multiple organs contribute to androgen production, each with diurnal and menstrual variation (Fig. 11-1). The major adrenal androgens are dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), androstenedione, and testosterone. The diurnal variation of DHEA is similar to that of cortisol, with peak levels in the early morning. DHEAS has a long half-life, with less fluctuation in concentrations; thus, DHEAS levels are more easily interpreted in the evaluation of hirsute patients. Androstenedione is secreted equally by the adrenal glands and the ovaries and has about 10% of the androgenic potency of testosterone.
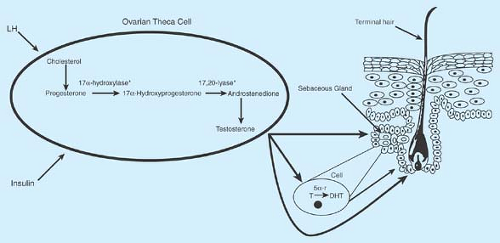
Testosterone sources vary: 0% to 30% comes from the adrenal glands, 5% to 25% comes from the ovaries, and 50% to 60% is produced by the peripheral conversion of precursors such as DHEA and androstenedione. In hirsute women with PCOS, the ovaries are responsible for a much greater percentage of the testosterone production. Ovarian theca cells from PCOS patients convert androgen precursors to testosterone more efficiently than cells from normal women (26). Virtually all dihydrotestosterone (DHT) comes from peripheral conversion of testosterone through the enzymatic action of 5α-reductase; DHT is the biologically active and potent androgen acting on the hair follicle (Fig. 11-2). Increased activity of cytochrome P450c17α may be responsible for increased ovarian and adrenal steroid production (3). Prenatal androgen exposure may disrupt the hypothalamic–pituitary–adrenal axis and lead to this enzyme dysregulation (27).
Stepwise increases in testosterone production correspond to increasing hirsutism and clitoromegaly (28). Free (or unbound) testosterone concentrations correlate better with clinical signs of hirsutism than total testosterone levels. All but a small fraction of serum testosterone is bound tightly to SHBG, and more weakly bound to albumin. Free and albumin-bound testosterone are the biologically active forms. Hirsute women with elevated androgens have SHBG concentrations lower than those of normal women.
Physiology: Insulin Resistance
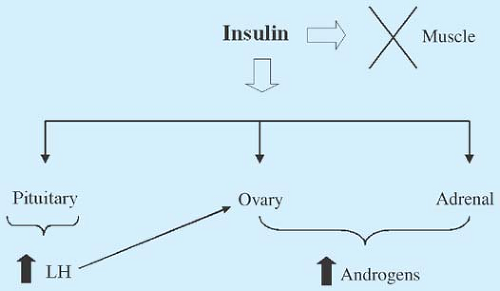
Despite known changes in gonadotropins and ovarian steroidogenesis, insulin also plays a role in the pathogenesis of PCOS (Figs. 11-2 and 11-3). The majority of women with PCOS have insulin resistance (IR) and compensatory hyperinsulinemia
(29); these abnormalities occur independent of BMI (30,31). Obese women with PCOS have an additional component of insulin resistance related to their overweight. Defects in insulin receptor signaling and reduced adipocyte GLUT-4 expression appear to cause the IR in PCOS (32,33). IR is also an early marker of PCOS in adolescents; teens with PCOS have a 50% reduction in insulin sensitivity compared to obese controls (34). Also supporting insulin’s role in the pathogenesis of PCOS is the finding that women with type 2 diabetes mellitus have an increased risk of having PCOS (35,36,37).
(29); these abnormalities occur independent of BMI (30,31). Obese women with PCOS have an additional component of insulin resistance related to their overweight. Defects in insulin receptor signaling and reduced adipocyte GLUT-4 expression appear to cause the IR in PCOS (32,33). IR is also an early marker of PCOS in adolescents; teens with PCOS have a 50% reduction in insulin sensitivity compared to obese controls (34). Also supporting insulin’s role in the pathogenesis of PCOS is the finding that women with type 2 diabetes mellitus have an increased risk of having PCOS (35,36,37).
Fasting insulin levels are positively correlated with circulating testosterone and androstenedione concentrations (38). Even though peripheral tissues are resistant to insulin action, insulin and insulin-like growth factor-1 (IGF-1) work with LH to directly increase androgen production from the ovaries, even in nonobese women. Insulin stimulates androgen accumulation in ovarian stroma obtained from hyperandrogenic women (39) and can regulate thecal and stromal responsiveness to gonadotropins (40). High insulin concentrations increase IGF-1, decrease the binding protein IGF-BP1, and stimulate activity of the ovarian enzyme P450c17α, which is pivotal to the production of androgens by the ovary (Fig. 11-2). Additionally, nutritional factors and insulin play important roles in SHBG production. Insulin decreases hepatic SHBG production, which increases free testosterone levels (41,42,43) and may contribute to the higher free testosterone concentrations seen in overweight girls than normal-weighted girls early in adolescence (17).
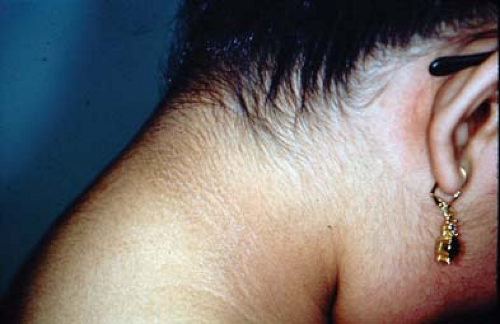
Identifying IR in adolescents with PCOS is of particular importance because of the elevated risk of non-insulin-dependent diabetes mellitus (NIDDM) among adult women with PCOS. The prevalence of NIDDM in young women with PCOS is 10 times higher than that of normal women (44). Acanthosis nigricans (velvety, thickened, hyperpigmented skin in the nape of the neck, in the axillae, under the breasts, in the vulva, in the inguinal area, and in other body folds) (Fig. 11-4) is common among adolescents and adults with PCOS and suggests IR (45,46). The so-called HAIR-AN syndrome (hyperandrogenism, insulin resistance, and acanthosis nigricans) is used to describe the severe end of the spectrum (approximately 3% of patients with androgen excess).
In studies of PCOS, 20% to 35% of women had impaired glucose tolerance (IGT), while 7.5% to 17.7% had NIDDM diagnosed by a 2-hour glucose tolerance test (GTT) (47,48,49). In nonobese women with PCOS, the incidence was lower but still significant, with 10.3% having IGT and 1.5% diabetes (47). At greatest risk were obese women with a first-degree relative with NIDDM. Fasting glucose was a poor predictor of the 2-hour glucose level; fasting glucose levels alone would have identified a much lower prevalence of diabetes mellitus (DM) (48,49). Similar results have been found in adolescents with PCOS; 9.7% to 30% had IGT and 4% had NIDDM (50,51). Fasting glucose measures would only have identified 25% of patients with IGT, while abnormal glucose/insulin ratios (<4.5, associated with IR in adults) would have had a high false-positive rate.
How does glucose tolerance change over time? Adult data suggest significant deterioration of glucose tolerance over time even in those with initially normal GTT results. In a longitudinal study of women with PCOS and normal baseline GTT, 9% developed IGT and 8% developed DM (52). Of those with IGT at baseline, 54% developed DM. Baseline BMI was a significant predictor of adverse glucose tolerance at follow-up. Based on these data, testing for impaired glucose tolerance by glucose tolerance testing or hemoglobin A1C levels is an important part of the follow-up of adolescents with PCOS (see Laboratory Studies) (53).
Other Pathways to Polycystic Ovary Syndrome
The role of the adrenal glands in the pathogenesis of PCOS is controversial. Although 25% to 35% of patients with PCOS have mildly to moderately elevated DHEAS levels (300 to 600 μg/dL) and evidence of hyperresponsive adrenal glands after adrenocorticotropic hormone (ACTH) testing on adrenal scintiscans (54), most adolescents with androgen excess do not have specific adrenal enzyme deficiencies (see section on late-onset CAH, p. 175). Although some patients may have an “exaggerated adrenarche” (55), the adrenal hyperandrogenism is due to dysregulation of adrenal steroidogenesis, possibly due to increased activity of cytochrome P450c17α CYP 17 (17α-hydroxylase/C17,20-lyase) in the ovary. Adrenal hyperandrogenism may also be an inherited risk factor for PCOS (32). Emotional stress at puberty could perhaps also cause increased ACTH production, adrenal sensitivity, or both (56,57). The relative hyperestrogenic state of PCOS may be an important factor in enhancing adrenal sensitivity. Insulin may also increase adrenal androgen production.
Recent interest has also focused on the possible relationship between premature pubarche and the later development of PCOS, especially if insulin resistance is involved (13,58,59,60,61,62). An association between premature pubarche, hyperinsulinemia, and low birth weight has suggested that PCOS is a part of the metabolic syndrome (12). Patients with premature pubarche may have adrenal hyperresponsiveness, elevated insulin and IGF-1 levels, and decreased concentrations of SHBG and IGF-BP1.
Given the familial pattern of PCOS, potential genetic linkages are being explored (63,64). In a study of siblings, 22% of sisters of probands with PCOS fulfilled criteria for PCOS, and another 24% had hyperandrogenemia with regular menses (65). The probands, sisters with PCOS, and sisters with hyperandrogenemia had elevated serum LH levels compared to controls.
Similarly, brothers of women with PCOS have higher DHEAS levels than control men (66).
Similarly, brothers of women with PCOS have higher DHEAS levels than control men (66).
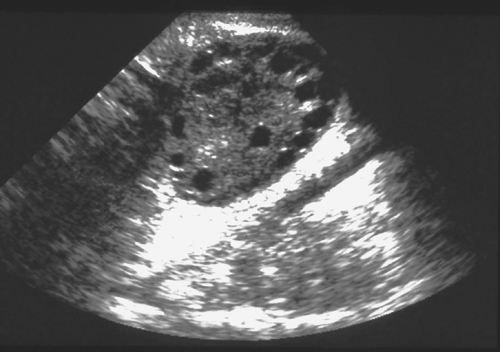 Figure 11-5. Polycystic ovary in ultrasound. Note the “string of pearls” small subcortical follicles. |
Genetic and endocrinologic factors also play a role in whether signs of hyperandrogenism are clinically evident. Patients have variable end-organ sensitivity of the pilosebaceous unit to the same level of hormones. Women of some ethnic backgrounds (i.e., Asians) are less susceptible to hirsutism and acne. In a study of women with PCOS from the United States, Italy, and Japan, similar concentrations of LH, testosterone, and estradiol were found, but levels of 3α-androstanediol (a reflection of utilization of androgens by target tissues) were high in women from Italy and the United States and normal in Japanese women, who had much less hirsutism (67).
Ultrasound Findings
The clinician needs to be aware of a number of caveats about the use of ultrasound (US) in adolescents and adult women with PCOS including the diagnostic criteria for PCOS, the variability of studies related to particular ultrasound findings such as peripheral distribution of follicles, the predominant use of transabdominal rather than transvaginal US in adolescents, and the overlap of PCO morphology with findings in normal women. Ovarian morphology by US in PCOS is variable (30,68,69,70,71,72); the ovaries may appear normal or enlarged, with multiple tiny cysts. The Rotterdam US criteria define PCO as the presence of ≥12 follicles measuring 2 to 9 mm in diameter OR increased ovarian volume >10 mL in at least one ovary as measured by transvaginal US. The classic, peripheral pattern of follicles underneath the cortex of the ovary is called a “string of pearls” configuration. The presence of ≥10 follicles and peripheral follicle distribution pattern have been noted to be a sensitive feature for diagnosing PCOS in adults (72). Even using less sensitive transabdominal US, one study reported multiple peripheral follicles in 80% of adolescents with PCOS (73). However, the peripheral pattern is not necessary for diagnosis of PCOS and not included in the Rotterdam criteria (74,75) (Fig. 11-5). Multicystic ovaries with the small cysts distributed throughout the ovary without increased stroma are more typical of early pubertal or anovulatory adolescents. However, the distinction can be difficult and may represent a spectrum (76). Since most adolescents have transabdominal, not transvaginal, US, counting the number of follicles is challenging; thus, volume alone is used as the most common diagnostic criteria in adolescents (see also Chapter 23). Ovarian stromal hyperechogenicity and increased cross-sectional area are common, but not uniformly found. A stroma/total area ratio >0.34 measured by transvaginal US has been noted to be sensitive in diagnosing PCOS; similarly, stromal brightness (increased stroma to total area) was felt by one group to be most specific (72). Measurement of serum antimüllerian hormone (AMH), secreted by the granulosa cells of developing follicles, may become a surrogate for US in the future. AMH values correlate well with antral follicle count, and may aid in the diagnosis in cases where US is unavailable (77). However, more research is needed before this becomes available for clinical practice.
In addition, pelvic US can be used to measure the endometrial thickness and endometrial homogeneity and evaluate for endometrial hyperplasia, which occurs in one-third of adult women with PCOS who are not regularly menstruating and not receiving oral contraceptives or regular progestin withdrawal (78). Less than four menses per year and an endometrial thickness >7 mm are risk factors for endometrial hyperplasia in adults with PCOS (79).
Women with hirsutism and regular menses (idiopathic hirsutism) frequently have PCO demonstrated by US (80). “PCO-like” ovaries can occur in patients who have other sources of androgen excess, such as CAH, adrenal adenomas or carcinomas, or conditions associated with chronic anovulation (including Cushing disease, acromegaly, hypothyroidism, and hyperprolactinemia). Ultrasound can help to define the likely pathogenesis in some patients and to exclude other ovarian
lesions, but it cannot be used to definitively establish or exclude the diagnosis of PCOS. In one small study, 100% of nonobese adolescents with PCOS had PCO, compared to 75% of obese PCOS patients and 31% of obese controls (30). Up to 25% to 30% of young adult women may have PCO by US (71). Among women presenting only with PCO on US, 20% have PCOS. The degree of total ovarian enlargement or stromal volume does not appear to be correlated with the testosterone levels (81). Pelvic US may be less helpful in suggesting a diagnosis of PCOS in adolescents than in adults (82), but can provide supporting evidence and can exclude other abnormalities.
lesions, but it cannot be used to definitively establish or exclude the diagnosis of PCOS. In one small study, 100% of nonobese adolescents with PCOS had PCO, compared to 75% of obese PCOS patients and 31% of obese controls (30). Up to 25% to 30% of young adult women may have PCO by US (71). Among women presenting only with PCO on US, 20% have PCOS. The degree of total ovarian enlargement or stromal volume does not appear to be correlated with the testosterone levels (81). Pelvic US may be less helpful in suggesting a diagnosis of PCOS in adolescents than in adults (82), but can provide supporting evidence and can exclude other abnormalities.
Quality of Life
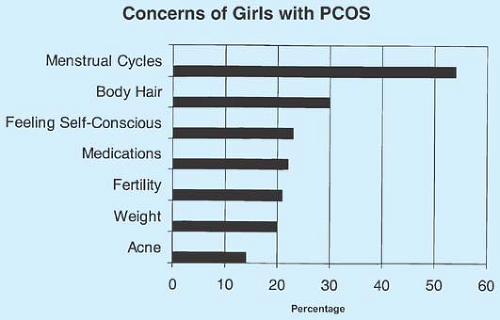
PCOS has a profound impact on quality of life. Adolescents with PCOS report significant disruption in general health perceptions, physical functioning, and family activities (83). The patient’s perceived severity of PCOS, not the clinical severity assessed by the health care provider, was associated with reported quality of life. Girls with PCOS report more body image distress (84) and higher levels of depression (85). In addition, adolescents with PCOS are concerned about their fertility (86), similar to adult women (Fig. 11-6).
Androgen Excess, Hirsutism, and Androgenic Alopecia
In most cases, excess androgen production from the ovaries and/or adrenal glands is responsible for the clinical problem of hirsutism. Whatever the cause, the self-image of the maturing adolescent may be negatively impacted by excess hair growth, and a careful explanation of the etiology, diagnosis, and therapeutic options is warranted.
Both terminal hair (>0.5 cm long, coarse, pigmented) and vellus or lanugo hair (downy, fine, light colored) are found on the human body. Terminal hairs undergo a growing phase (anagen), an involutional phase (catagen), and a resting phase (telogen). The initiation of anagen is influenced by hormonal factors. The degree of hirsutism may be related to familial, racial, or ethnic factors that determine the capacity of the hair follicle to respond to androgens. Similarly, the distribution and density of the pilosebaceous units (sebaceous gland and hair follicle) are largely determined by genetic and racial/ethnic factors, but are also influenced by endocrinologic factors such as the rate/amount of androgen secretion, SHBG concentrations, the peripheral conversion of weak androgens to potent androgens, and the sensitivity of the pilosebaceous unit to androgens (87).
An increase in the distribution and quantity of terminal hair may lead to complaints of hirsutism. Tolerance for varying amounts of hirsutism is subjective, and often related to other female family members’ appearance. Hirsutism may also be noted by the physician during a routine physical examination or during an evaluation of irregular menses. Excessive downy hair (lanugo) is not related to androgen excess, and may occur in conditions of malnutrition such as anorexia nervosa.
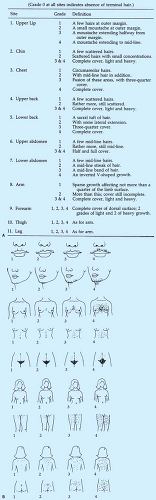
It can be difficult to establish whether hair growth is excessive since the spectrum of “normal” is ill-defined. Terminal hair on the lower arms/legs, face, abdomen, and back is common (88). Hirsutism is usually defined as the presence of sexual hair in a male pattern (terminal hair on the face, back, chest, abdomen, inner thighs). The Ferriman-Gallwey scoring system (89) has been used by many clinicians to objectively measure hirsutism (Fig. 11-7), with a score ≥6 indicating hirsutism (2); a score of 6 to 8 is the 95th percentile for black and white women (90).
Testosterone induces the production of enzymes in the hair follicle, so that once a terminal hair begins to grow, less androgen is required to stimulate continued growth. This mechanism may lead to a less than optimal response on hair growth by hormone suppression therapy, which by biochemical parameters (lowering of free testosterone) should be successful.
Etiology of Hirsutism
Most cases of significant hirsutism result from androgen overproduction by the ovaries or adrenal glands, with PCOS the most common diagnosis (72% to 85%) (2); late-onset 21-hydroxylase deficiency accounts for 1% to 4% (see section below on adrenal
enzyme deficiencies), “idiopathic hirsutism” for 5% to 7%, and tumors and Cushing disease for <1% (Table 11-2). The prevalence of idiopathic hirsutism has decreased over time as more careful evaluations of hirsute women with “normal menses” (i.e., drawing a progesterone level on days 22 to 24 of the cycle) have led to diagnoses of anovulation and PCOS (91). Increased free testosterone concentrations are detectable in 80% to 85% of hirsute women. Women with true idiopathic hirsutism should have regular menses, premenstrual molimina, normal androgen levels, and ovulatory serum progesterone levels. Idiopathic hirsutism may be due to increased hair follicle sensitivity to even low levels of androgen, increased conversion of testosterone to DHT, or overabundant hair follicles. Even among 62 hirsute ovulatory women, 8 (13%) had normal androgen levels (idiopathic hirsutism), 24 (39%) had characteristic PCO on US and/or an exaggerated response of 17-hydroxyprogesterone (17-OHP) to leuprolide, and 30 (48%) had unspecified hyperandrogenism (2). Thus, overlap of these categories continues to be a challenge.
enzyme deficiencies), “idiopathic hirsutism” for 5% to 7%, and tumors and Cushing disease for <1% (Table 11-2). The prevalence of idiopathic hirsutism has decreased over time as more careful evaluations of hirsute women with “normal menses” (i.e., drawing a progesterone level on days 22 to 24 of the cycle) have led to diagnoses of anovulation and PCOS (91). Increased free testosterone concentrations are detectable in 80% to 85% of hirsute women. Women with true idiopathic hirsutism should have regular menses, premenstrual molimina, normal androgen levels, and ovulatory serum progesterone levels. Idiopathic hirsutism may be due to increased hair follicle sensitivity to even low levels of androgen, increased conversion of testosterone to DHT, or overabundant hair follicles. Even among 62 hirsute ovulatory women, 8 (13%) had normal androgen levels (idiopathic hirsutism), 24 (39%) had characteristic PCO on US and/or an exaggerated response of 17-hydroxyprogesterone (17-OHP) to leuprolide, and 30 (48%) had unspecified hyperandrogenism (2). Thus, overlap of these categories continues to be a challenge.
Drugs such as phenytoin, corticosteroids, danazol, diazoxide, minoxidil, anabolic steroids, and androgens cause hirsutism. Most, but not all, studies have implicated valproate in the development of PCOS (92,93). In one study, 80% of women treated with valproate before the age of 20 years had PCOS or hyperandrogenism (93). Thus, close observation of adolescents treated with valproate for signs of PCOS is indicated.
Pregnancy, hypothyroidism, anorexia nervosa, malnutrition, and chronic central nervous system disorders (e.g., mental and motor retardation) can also be accompanied by excess hair growth. Occasionally, stress may precipitate excess LH secretion and androgen production from the theca cells; this process reverses when the acute stress is over. Hyperprolactinemia may be accompanied by increased secretion of adrenal androgens, especially DHEAS, because of prolactin receptors in the adrenal glands. Hirsutism in this instance is mild, as prolactin blocks the conversion of testosterone to DHT, limiting the peripheral action of the androgens (87). A rare cause of hyperandrogenism is glucocorticoid resistance, caused by mutations in the receptor gene. Diagnostic criteria include elevated cortisol levels (in the absence of symptoms of Cushing syndrome), normal diurnal cortisol variation, lack of dexamethasone suppression, and elevation of adrenal androgens. Treatment is not usually needed (94).
Table 11-2 Causes of Hirsutism in Adolescents | |
|---|---|
|
Ovarian and adrenal tumors can present with rapid onset of hirsutism and virilization. 46,XY disorders of sex development (male pseudohermaphroditism) and mixed gonadal dysgenesis may present with virilization at puberty. A rapid progression of hirsutism or the presence of virilization (clitoromegaly, temporal hair recession, deepening of the voice, changes in muscle pattern) should prompt an immediate assessment of hormone status to exclude the possibility of an androgen-producing ovarian tumor or male gonad (see Chapters 3 and 21).
Androgenic Alopecia
Androgenic alopecia (AGA) is “male-pattern” hair loss, especially thinning over the vertex of the head and temporal recession, and can occur as a result of elevated serum androgens (95). Incidence data in females are lacking. AGA occurs as DHT acts on genetically susceptible hair follicles, eventually leading to finer and shorter hair growth. PCOS should be considered in all adolescents presenting with AGA, although only 10% of adults presenting with AGA alone have PCOS. Laboratory evaluation for this type of hair loss should include a complete blood count (CBC), thyroid panel, total and free testosterone concentrations, ferritin, and DHEAS level.
Adrenal Enzyme Deficiencies
Late-onset 21-hydroxylase (21-OH) deficiency, also termed nonclassic CAH, is found in 1% to 10% of adult women with
hirsutism (12,96,97,98,99,100,101,102) and in 2.3% to 19% of hirsute adolescents (12,98,103), depending in part on the race/ethnicity of the population and whether the participants are drawn from a referred or nonreferred sample. Late-onset CAH is more common in certain populations: 3% among Ashkenazi Jews and 1% to 2% among Hispanics (100,102). Thus, the utility of screening all girls with irregular menses and hirsutism for late-onset CAH depends on the ethnicity of the patients and the expected prevalence in the patient population.
hirsutism (12,96,97,98,99,100,101,102) and in 2.3% to 19% of hirsute adolescents (12,98,103), depending in part on the race/ethnicity of the population and whether the participants are drawn from a referred or nonreferred sample. Late-onset CAH is more common in certain populations: 3% among Ashkenazi Jews and 1% to 2% among Hispanics (100,102). Thus, the utility of screening all girls with irregular menses and hirsutism for late-onset CAH depends on the ethnicity of the patients and the expected prevalence in the patient population.
Nonclassic 21-OH CAH is an autosomal recessive disorder with a gene located on chromosome 6, in proximity to the HLA locus. More than 40 mutations of the CYP21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree