Objective
We sought to provide evidence-based guidelines regarding the diagnosis and management of amniotic fluid embolism.
Study Design
A systematic literature review was performed using MEDLINE, PubMed, EMBASE, and the Cochrane Library. The search was restricted to English-language articles published from 1966 through March 2015. Priority was given to articles reporting original research, in particular randomized controlled trials, although review articles and commentaries were consulted. Abstracts of research presented at symposia and scientific conferences were not considered adequate for inclusion. Evidence reports and published guidelines were also reviewed, and additional studies were located by reviewing bibliographies of identified articles. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used for defining the strength of recommendations and rating quality of the evidence. Consistent with US Preventive Task Force guidelines, references were evaluated for quality based on the highest level of evidence.
Results and Recommendations
We recommend the following: (1) we recommend consideration of amniotic fluid embolism in the differential diagnosis of sudden cardiorespiratory collapse in the laboring or recently delivered woman (GRADE 1C); (2) we do not recommend the use of any specific diagnostic laboratory test to either confirm or refute the diagnosis of amniotic fluid embolism; at the present time, amniotic fluid embolism remains a clinical diagnosis (GRADE 1C); (3) we recommend the provision of immediate high-quality cardiopulmonary resuscitation with standard basic cardiac life support and advanced cardiac life support protocols in patients who develop cardiac arrest associated with amniotic fluid embolism (GRADE 1C); (4) we recommend that a multidisciplinary team including anesthesia, respiratory therapy, critical care, and maternal-fetal medicine should be involved in the ongoing care of women with AFE (Best Practice); (5) following cardiac arrest with amniotic fluid embolism, we recommend immediate delivery in the presence of a fetus ≥23 weeks of gestation (GRADE 2C); (6) we recommend the provision of adequate oxygenation and ventilation and, when indicated by hemodynamic status, the use of vasopressors and inotropic agents in the initial management of amniotic fluid embolism. Excessive fluid administration should be avoided (GRADE 1C); and (7) because coagulopathy may follow cardiovascular collapse with amniotic fluid embolism, we recommend the early assessment of clotting status and early aggressive management of clinical bleeding with standard massive transfusion protocols (GRADE 1C).
The practice of medicine continues to evolve, and individual circumstances will vary. This publication reflects information available at the time of its submission for publication and is neither designed nor intended to establish an exclusive standard of perinatal care. This publication is not expected to reflect the opinions of all members of the Society for Maternal-Fetal Medicine.
Amniotic fluid embolism is a rare but potentially lethal condition. Because of a lack of international consensus regarding diagnostic criteria, estimates of both incidence and mortality rates associated with amniotic fluid embolism vary widely. These issues have recently been reviewed in detail and are not the focus of this manuscript.
Rather we emphasize that despite its low incidence in the general population of pregnant women, both maternal and perinatal morbidity and mortality are significant with amniotic fluid embolism, even in cases ideally managed. Because of the rarity of this condition, most physicians and institutions have limited experience with the management of amniotic fluid embolism.
The purpose of this document is to provide clinicians with information that may improve the ability to make a timely diagnosis and establish appropriate supportive treatment to patients suffering from amniotic fluid embolism to improve maternal and perinatal outcomes.
What is amniotic fluid embolism and what are its clinical features?
A detailed review of the pathophysiology of amniotic fluid embolism is beyond the scope of this document but may be found elsewhere and is summarized in Figures 1 and 2 . It appears to involve a complex sequence of events triggered in certain women by entrance into the maternal circulation of material from the fetal compartment, resulting in an abnormal activation of proinflammatory mediator systems similar to the systemic inflammatory response syndrome.
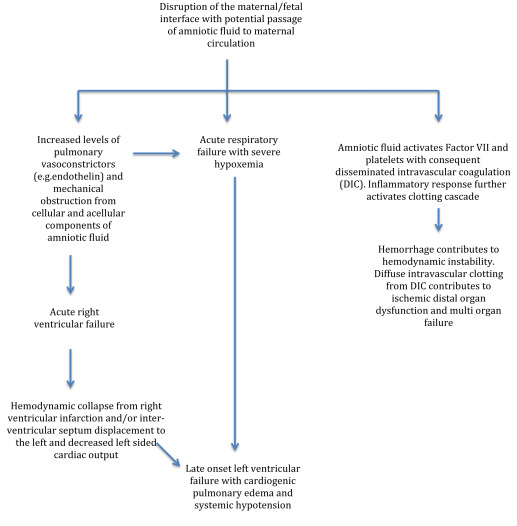
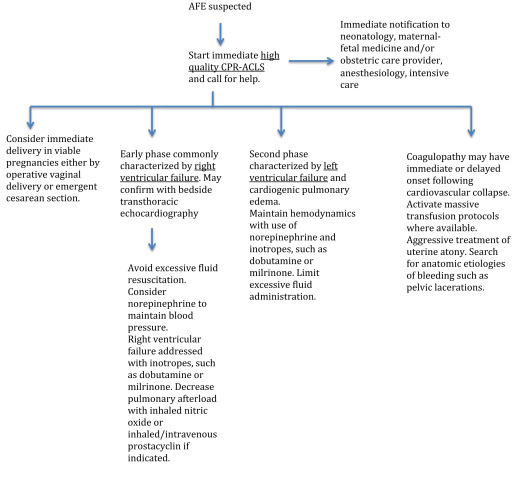
The typical presentation of amniotic fluid embolism includes a triad of sudden hypoxia and hypotension, followed in many cases by coagulopathy, all occurring in relation to labor and delivery. The diagnosis of amniotic fluid embolism is clinical, based on the presence of these elements and the exclusion of other likely causes. Amniotic fluid embolism should be considered in the differential diagnosis in any pregnant or immediately postpartum woman who suffers sudden cardiovascular collapse or cardiac arrest, seizures, severe respiratory difficulty, or hypoxia, particularly if such events are followed by a coagulopathy that cannot be otherwise explained.
The analysis of the national registry reveals that 70% of cases of amniotic fluid embolism occur during labor, 11% after a vaginal delivery, and 19% during a cesarean delivery. These figures suggest that the mode of delivery may alter the timing of amniotic fluid embolism but not its occurrence. In rare instances, amniotic fluid embolism may occur during the first or second trimesters of pregnancy, at the time of pregnancy termination, or amniocentesis.
The clinical presentation of amniotic fluid embolism is, in its classic form, dramatic. A period of anxiety, change in mental status, agitation, and a sensation of doom may precede the event. Patients may progress rapidly to cardiac arrest, with pulseless electrical activity, asystole, ventricular fibrillation, or pulseless ventricular tachycardia. In cases occurring prior to delivery, electronic fetal monitoring will demonstrate decelerations, loss of variability, and terminal bradycardia as oxygenated blood is shunted away from the uterus, and catecholamine-induced uterine hypertonus causes a further decline in uterine perfusion.
Dissemninated intravascular coagulation is present in up to 83% of cases. The coagulopathy of amniotic fluid embolism may occur in conjunction with the cardiopulmonary manifestations, be manifest only after initial cardiopulmonary resuscitation has been completed, or in very rare cases may be the only finding in women without cardiorespiratory compromise.
Dissemninated intravascular coagulation is commonly manifested by hemorrhagic complications including bleeding from venipunctures or surgical sites, hematuria, gastrointestinal hemorrhage, and vaginal bleeding. As with any condition involving diminished uterine perfusion, coexistence with uterine atony is not uncommon. However, bleeding from incompletely controlled atony followed by hypovolemic shock and either a consumptive or dilutional coagulopathy cannot be attributed to amniotic fluid embolism, nor does amniotic fluid embolism occur as a mild coagulopathy followed hours later by sudden cardiovascular collapse in the absence of interval hemorrhage and hypovolemia.
Reported risk factors for amniotic fluid embolism include situations in which the exchange of fluids between the maternal and fetal compartments is more likely, such as operative delivery (cesarean or vaginal), placenta previa, placenta accreta, and abruption. An association between induction of labor and amniotic fluid embolism is inconsistently reported. Abnormalities of uterine tone (hypo- or hypertonous) described commonly in cases of amniotic fluid embolism may be the consequence of uterine hypoperfusion secondary to profound maternal shock and hypoxia with massive catecholamine release, rather than the cause.
Other putative risk factors include cervical lacerations, uterine rupture, eclampsia, polyhydramnios, and multiple gestations; as outlined in the previous text, a tendency to overdiagnose amniotic fluid embolism in cases actually involving other causes of primary hemorrhage may contribute to these reports. Sociodemographic risk factors such as maternal age and race/ethnicity are also reported in some series. However, given the rare and unpredictable nature of amniotic fluid embolism, there are no risk factors sufficiently established to justify any alteration in standard obstetric care.
How should you manage a patient with sudden cardiac arrest in whom amniotic fluid embolism is suspected?
Amniotic fluid embolism should be considered in the differential diagnosis of sudden cardiorespiratory compromise in any pregnant or recently postpartum patient (GRADE 1C). Initial resuscitation of cardiac arrest does not require a specific diagnosis of amniotic fluid embolism because initial maternal treatment (with basic cardiac life support and advanced cardiac life support protocols) is similar, regardless of the exact etiology.
We do not recommend the use of any specific diagnostic laboratory test to either confirm or refute the diagnosis of amniotic fluid embolism; at the present time, amniotic fluid embolism remains a clinical diagnosis (GRADE 1C).
We recommend the provision of immediate high-quality cardiopulmonary resuscitation with standard basic cardiac life support and advanced cardiac life support protocols in patients who develop cardiac arrest associated with amniotic fluid embolism (GRADE 1C). We recommend that a multidisciplinary team including anesthesia, respiratory therapy, critical care, and maternal-fetal medicine should be involved in the ongoing care of women with AFE (Best Practice) . The most critical immediate action is to start chest compressions before rescue breathing is administered.
Chest compressions should be performed similarly to nonpregnant individuals. The hands of the provider should be placed in the lower half of the sternum. Chest compressions should be performed hard and fast, achieving a depth of at least 2 inches and allowing complete chest recoil between compressions. Patients who are undelivered should be tilted to the left lateral decubitus position or, preferably, have the uterus displaced laterally by an assistant to prevent aortocaval compression by the gravid uterus.
The use of vasopressors, antiarrhythmic agents, and defibrillating doses is not different from those utilized in nonpregnant individuals. Although concerns that electric arcing may occur if fetal monitors are in place at the time of cardioversion or defibrillation are largely theoretical, it is reasonable to remove such monitors while cardiopulmonary resuscitation is in progress. However, the presence of such monitors should not delay defibrillation when indicated. The components of high-quality cardiopulmonary resuscitation are summarized in Table 1 .
| Components |
|---|
| Rapid chest compressions (100 × minute) Perform hard compressions, achieving a depth of at least 2 inches Assure adequate chest recoil between compressions Minimize interruptions of chest compressions Avoid prolonged pulse checks (no more than 5–10 seconds) Resume chest compressions immediately after defibrillating Switch provider of compressions every 2 minutes to avoid fatigue Lateral displacement of uterus during resuscitation |
If the patient is undelivered at the time of cardiac arrest, expeditious delivery is indicated if the fetus has reached an age of potential viability (≥23 weeks). Not only may this be life saving for the fetus but in theory may assist in maternal resuscitation by removing venacaval compression. An operative vaginal delivery (forceps or vacuum assisted) should be performed in laboring patients in whom obstetrical conditions support such an intervention. If a vaginal delivery is not an option, emergency cesarean delivery is generally indicated.
Classically the indication for a perimortem cesarean delivery has been a failure to obtain spontaneous circulation after 4 minutes of cardiopulmonary resuscitation to reduce the profound fetal hypoxia occurring during maternal cardiac arrest. This time frame is ideal but is rarely achievable when cardiac arrest is unexpected.
We recommend that preparations for emergent perimortem cesarean delivery be initiated simultaneously with the initiation of cardiopulmonary resuscitation, and if the cardiac arrest is still ongoing as the instruments become available, proceed with cesarean delivery. The dismal prognosis of adult cardiac arrest not amenable to, or unresponsive to, immediate direct current countershock suggests that maternal prognosis will not be significantly compromised by such an operation.
Some authors recommend moving this threshold to 20 weeks to improve maternal perfusion, but no evidence exists that such previable cesarean delivery improves the outcome in cases of amniotic fluid embolism–related maternal cardiac arrest.
Following cardiac arrest with amniotic fluid embolism, we recommend immediate delivery in the presence of a fetus ≥23 weeks of gestation (GRADE 2C). In cases of maternal hemodynamic instability that does not involve one of the lethal dysrhythmias, cases must be individualized based on the fetal age and degree of compromise, maternal condition, and the availability of anesthetic support. No data exist to guide delivery decisions under these circumstances.
The literature contains innumerable case reports in which various novel therapeutic modalities have been used in women with presumptive amniotic fluid embolism, and the patient did not die. Unfortunately, evidence of a causal, as opposed to an anecdotal, connection between most of these and survival from amniotic fluid embolism is lacking. We focus here only on the better-supported ancillary treatment options.
The use of venoarterial extracorporeal membrane oxygenation has been described in cases of amniotic fluid embolism refractory to conventional resuscitation maneuvers. However, the use of anticoagulation during extracorporeal membrane oxygenation may worsen bleeding in the profoundly coagulopathic patient with active hemorrhage. Because of these concerns, as well as lack of adequate evidence of benefit, extracorporeal membrane oxygenation is controversial and not routinely recommended in the management of amniotic fluid embolism.
After successful resuscitation, postcardiac arrest management is of paramount importance. Hemodynamic instability is common, and patients may require fluids, vasopressors, and inotropes. The goal is to maintain a mean arterial blood pressure of 65 mm Hg. Fever may worsen ischemia-reperfusion injury to the brain and should be aggressively treated. Hyperoxia will also worsen ischemia-reperfusion injury, and administration of 100% oxygen after the survival of cardiac arrest should be avoided. The inspired fraction of oxygen should be weaned to maintain a pulse oxymetry value of 94-98%. Serum glucose should be maintained between 140 mg/dL and 180 mg/dL with the use of intravenous insulin infusions if needed.
Mild therapeutic hypothermia, defined as cooling the patient to a temperature between 32°C and 34°C for 12–24 hours, has been recommended by the American Heart Association to increase the rate of a favorable neurological outcome and reduce mortality following cardiac arrest. A recent study, however, found no differences in outcomes between targeted temperatures of 33°C vs 36°C among survivors of cardiac arrest who were treated with mild therapeutic hypothermia. Current guidelines recommend targeted temperature management of cardiac arrest victims aiming at temperatures between 32° and 36°C.
The data on therapeutic hypothermia during pregnancy is limited to case reports. Most survivors of amniotic fluid embolism will have been delivered during the course of successful resuscitation. The main concern limiting the use of therapeutic hypothermia in these patients is a concern that this may increase the risk of hemorrhage. In patients not demonstrating significant disseminated intravascular coagulation and bleeding, therapeutic hypothermia should be considered. Targeting a temperature of 36°C (as opposed to lower temperatures with a concomitant increased risk of hemorrhage) is an option. Such decisions must be made in conjunction with the available medical critical care team.
How should you manage a patient with sudden cardiac arrest in whom amniotic fluid embolism is suspected?
Amniotic fluid embolism should be considered in the differential diagnosis of sudden cardiorespiratory compromise in any pregnant or recently postpartum patient (GRADE 1C). Initial resuscitation of cardiac arrest does not require a specific diagnosis of amniotic fluid embolism because initial maternal treatment (with basic cardiac life support and advanced cardiac life support protocols) is similar, regardless of the exact etiology.
We do not recommend the use of any specific diagnostic laboratory test to either confirm or refute the diagnosis of amniotic fluid embolism; at the present time, amniotic fluid embolism remains a clinical diagnosis (GRADE 1C).
We recommend the provision of immediate high-quality cardiopulmonary resuscitation with standard basic cardiac life support and advanced cardiac life support protocols in patients who develop cardiac arrest associated with amniotic fluid embolism (GRADE 1C). We recommend that a multidisciplinary team including anesthesia, respiratory therapy, critical care, and maternal-fetal medicine should be involved in the ongoing care of women with AFE (Best Practice) . The most critical immediate action is to start chest compressions before rescue breathing is administered.
Chest compressions should be performed similarly to nonpregnant individuals. The hands of the provider should be placed in the lower half of the sternum. Chest compressions should be performed hard and fast, achieving a depth of at least 2 inches and allowing complete chest recoil between compressions. Patients who are undelivered should be tilted to the left lateral decubitus position or, preferably, have the uterus displaced laterally by an assistant to prevent aortocaval compression by the gravid uterus.
The use of vasopressors, antiarrhythmic agents, and defibrillating doses is not different from those utilized in nonpregnant individuals. Although concerns that electric arcing may occur if fetal monitors are in place at the time of cardioversion or defibrillation are largely theoretical, it is reasonable to remove such monitors while cardiopulmonary resuscitation is in progress. However, the presence of such monitors should not delay defibrillation when indicated. The components of high-quality cardiopulmonary resuscitation are summarized in Table 1 .



