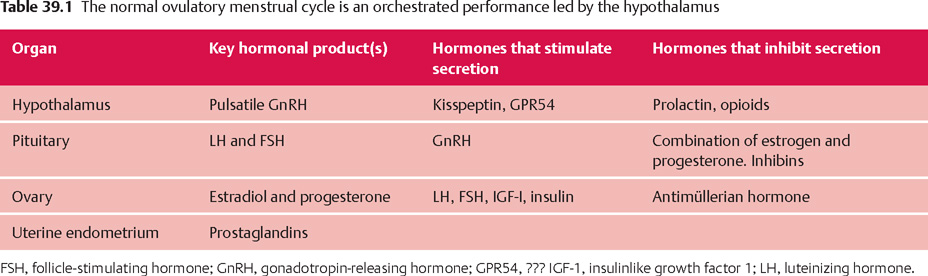
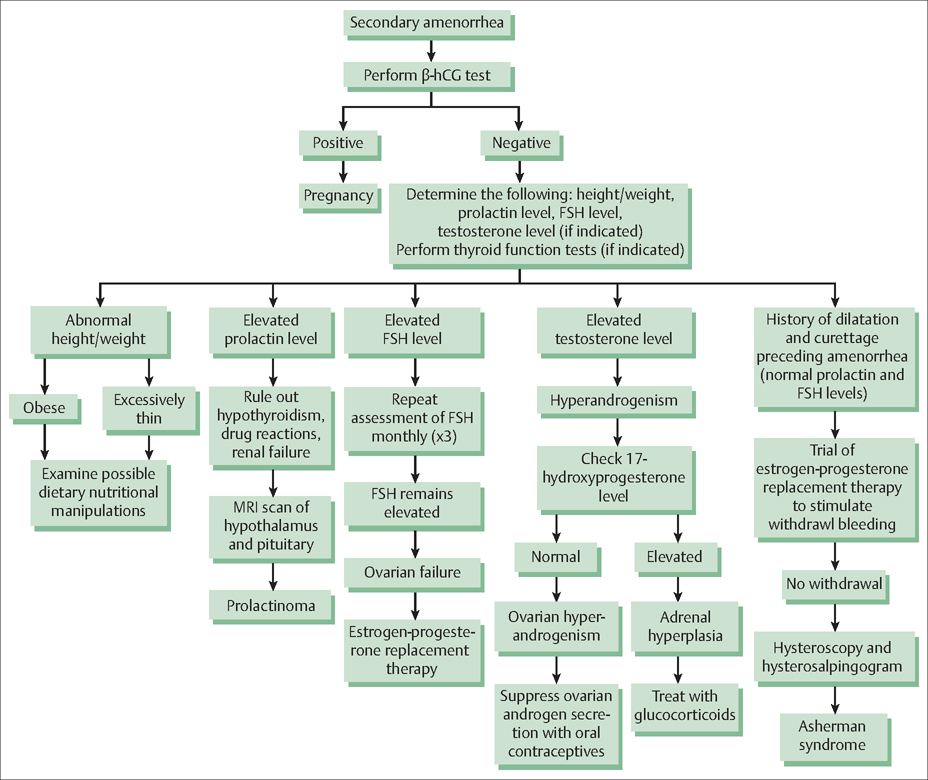
39 Amenorrhea and Dysfunctional Uterine Bleeding Robert L. Barbieri The menstrual cycle is orchestrated through the interaction of the hypothalamus, pituitary gland, ovaries, and uterus (Table 39.1). The conductor of the system is the hypothalamus that sets the beat by secreting gonadotropin-releasing hormone (GnRH) in a pulsatile fashion into the portal circulation. In turn, pulses of GnRH stimulate the pituitary to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which cause the development of an ovarian follicle. When the follicle reaches a size commensurate with the capacity to ovulate, the follicle secretes sufficient estradiol to trigger a surge of LH secretion from the pituitary. The LH surge causes the follicle to undergo rupture (ovulation) and the oocyte contained in the follicle matures and prepares for fertilization by proceeding through the final steps in meiosis I. If fertilization occurs, the embryo may implant in the endometrium about 6 days after ovulation. If no fertilization occurs, the corpus luteum is programmed to undergo apoptosis and cease secretion of estradiol and progesterone, causing the onset of menses and the beginning of a new cycle. At the endometrial level, in the uterus, the menstrual cycle has three key phases: 1) estradiol stimulates endometrial growth (from about 3 mm to 14 mm in depth); 2) progesterone induces differentiation of the endometrium in preparation for embryo implantation; and 3) the withdrawal of estradiol and progesterone results in sloughing of the endometrium and menstrual bleeding. Diseases and physiological processes that affect any of the four components of this orchestrated system can result in the absence of menstruation—amenorrhea. A girl may not start to menstruate when she reaches puberty (primary amenorrhea), or a woman who has been menstruating may have her cycles cease (secondary amenorrhea). Secondary amenorrhea, or adult-onset amenorrhea, is present when a woman who had been menstruating has no menses for longer than three of her previous cycles, or 6 months. The most common causes of secondary amenorrhea are: Fig. 39.1 Diagnosis and treatment of secondary amenorrhea. β-HCG, human chorionic gonadotropin; FSH, follicle-stimulating hormone. Secondary amenorrhea afects less than 1% of the adult female population, if pregnancy is excluded as a cause. Determining the cause of secondary amenorrhea begins with a focused history (past menstrual pattern, evidence for eating disorder, psychological stress, strenuous exercise), physical examination (calculated BMI, presence of hirsutism or virilization, galactorrhea), and measurement of serum human chorionic gonadotropin (HCG), prolactin, FSH, and testosterone. Based on this data, use of an algorithm can help direct diagnosis and care (Fig. 39.1). Low BMI, vigorous exercise, psychosocial stress, and nutritional abnormalities decrease GnRH production. This reduces LH and FSH secretion, stops ovarian follicle growth and secretion of estradiol and progesterone, and causes amenorrhea. Diagnosis: The patient typically has a history of regular vigorous exercise, psychosocial stress, reduced caloric intake and/or an eating disorder. On physical examination, the BMI may be less than 20 kg/m2. Serum FSH, prolactin and testosterone levels are usually in the normal range. Rarely, hypothalamic dysfunction caused by infltrative processes or tumors, such as sarcoidosis, histiocytosis, and hypothalamic cysts may cause amenorrhea. A progestin withdrawal test may help to assess the severity of the hypoestrogenism. In a patient with a hypothalamic cause of secondary amenorrhea, and no uterine bleeding after a progestin withdrawal test, the circulating estrogen levels are likely to be very low, and osteoporosis and vaginal atrophy may be present or develop. Women with nutritional causes of secondary amenorrhea may have triiodothyronine levels less than 70 ng/dL, reverse triiodothyronine levels more than 40 ng/dL, and elevated levels of urinary free cortisol (as observed in depressed women or women under significant stress).
Amenorrhea
Secondary Amenorrhea
Hypothalamic Dysfunction
| Organ | Cause | Relative frequency |
| Hypothalamus | Abnormalities of height/ weight and nutrition | 15% |
| Strenuous exercise | 10% | |
| Psychological stress | 10% | |
| Tumors of the hypothalamus including sarcoidosis, histiocytosis, craniopharyngioma) | <0.1% | |
| Pituitary | Prolactin-secreting pituitary tumor | 17% |
| Empty sella syndrome | 1% | |
| Sheehan syndrome | <1% | |
| ACTH-secreting tumor (Cushing disease) | <1% | |
| GH-secreting tumor (acromegaly) | <1% | |
| Ovary | Polycystic ovary syndrome | 30% |
| Primary ovarian failure | 10% | |
| Uterus | Asherman syndrome (intrauterine adhesions) | 5% |
| Other | Nonclassical adrenal hyperplasia | <1% |
| Thyroid disease | 1% | |
| Ovarian tumors | <1% |
ACTH, adenocorticotropin; GH, growth hormone
Treatment: Reversing the underlying cause of hypothalamic dysfunction often results in resumption of ovulatory menses. For example, for women with low BMI, nutritional counseling and supportive physician guidance may be associated with weight gain and resumption of ovulatory menses. Many women with amenorrhea and low GnRH secretion prefer to maintain the exercise and nutritional regimens that caused the amenorrhea. These women may be best treated with hormone therapy to prevent osteoporosis and vaginal atrophy. Cyclic or continuous hormone therapy may be used. Low doses of hormone therapy, such as that used to treat menopausal women; or standard doses of an estrogen—progestin contraceptive may be used for hormone therapy. Vitamin D (800 IU/day) and calcium (1200–1500 mg/day) should be advised to slow the rate of decline in bone mineral density associated with low estrogen levels.
Pituitary Dysfunction
The most common pituitary diseases that cause secondary amenorrhea are prolactin-secreting pituitary tumors (prolactinomas), the empty sella syndrome, Sheehan syndrome, and other pituitary tumors.
Prolactin-Secreting Pituitary Tumors
Most pituitary tumors are monoclonal, which indicates that they arise from a somatic mutation in a single progenitor cell. In general, pituitary tumors do not metasta-size and are slow growing.
Diagnosis: The most common causes of an elevation in the serum prolactin level are, in order of decreasing frequency:
- pregnancy
- the use of psychotropic medications that are dopamine agonists, such as haloperidol
- prolactin-secreting pituitary tumors
- hypothyroidism
- renal failure
If the prolactin is elevated and the HCG, thyroid-stimulating hormone and creatinine levels are normal, a magnetic resonance imaging (MRI) scan of the hypothalamus and pituitary should be performed to determine whether a pituitary tumor is detectable, and whether it is less than 10 mm in size (microadenoma) or greater than 10 mm (macroadenoma); this difference has clinical implications for treatment. If the MRI shows a pituitary tumor, it is important to measure serum insulinlike growth factor 1 (IGF-1) levels to assess if there is co-secretion of both prolactin and excessive quantities of growth hormone (GH).
Treatment: Microprolactinomas (<10 mm in diameter) have a generally benign course and can be managed by a primary care physician or endocrinologist. Observational studies indicate that over an interval of 10 years, 95% of microproalctinomas do not increase in size. The initial treatment of microprolactinomas should be a dopamine agonist such as bromocriptine or cabergoline. When treated with a dopamine agonist, most women with a prolactinoma will have normalization of their prolactin levels, shrinkage of their pituitary tumor, and resumption of ovulatory menses. If the patient prefers, she may be safely treated with an estrogen—progestin contraceptive, instead of a dopamine agonist. Estrogen—progestin treatment will prevent problems associated with hypoestrogenism, such as osteoporosis, but it will not lower the elevated levels of prolactin. In most cases, estrogen—progestin treatment does not cause the prolactinoma to grow.
Macroprolactinomas (>10 mm in diameter) may be associated with significant complications including headaches, visual field loss due to compression of the optic chiasm, or pituitary apoplexy. Management of macroprolactinomas should be led by an endocrinologist. The initial treatment of a macroprolactinoma is a dopamine agonist, but radiotherapy or surgery may be indicated in selected cases.
Women with a prolactinoma who wish to become pregnant should receive treatment with a dopamine agonist to induce ovulation. Dopamine agonists directly suppress prolactin production by the tumor, which allows for an increase in GnRH secretion, which stimulates, LH, FSH, estradiol, and progesterone secretion. Dopamine agonists normalize prolactin levels in about 80% of women with a prolactinoma. The main side effects with dopamine agonist treatment are nausea, vomiting, and orthostatic hypotension.
Empty Sella Syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


