Background
The incidence of endometrial cancer increases with age and is associated with medical comorbidities such as obesity and diabetes. Although a few cohort studies of <500 patients showed an association between comorbidity and survival in patients with endometrial cancer, the degree of association must be better described. The Adult Comorbidity Evaluation 27 is a validated comorbidity instrument that provides a score of 0–3 based on the number of and severity of medical comorbidities.
Objective
This study was performed to explore the association between medical comorbidities and survival of patients with endometrial cancer.
Study Design
Patients who were diagnosed with endometrial cancer from 2000–2012 were identified from the prospectively maintained Siteman Cancer Center tumor registry. Patients who underwent primary surgical treatment for endometrioid, serous, and clear cell endometrial carcinoma were included. Patients who primarily were treated with radiation, chemotherapy, or hormone therapy were excluded. Patients with uterine sarcomas or neuroendocrine tumors were excluded. Patients with missing Adult Comorbidity Evaluation 27 scores were also excluded from analysis. Information that included patient demographics, Adult Comorbidity Evaluation 27 score, tumor characteristics, adjuvant treatment, and survival data were extracted from the database. The association of Adult Comorbidity Evaluation 27 and overall and recurrence-free survival was explored in a multivariable Cox regression analysis after being controlled for variables that have been found to be associated significantly with survival in univariable analysis.
Results
A total of 2073 patients with a median age of 61 years (range, 20–94 years) at diagnosis were identified. The Adult Comorbidity Evaluation 27 score was 0, 1, 2, and 3 in 22%, 38%, 28%, and 12% of patients, respectively. Stage distribution was I (73%), II (5%), III (15%), and IV (7%), and grade distribution was 1 (52%), 2 (23%), and 3 (25%). Most patients had endometrioid histologic condition (87%) followed by serous (11%) and clear cell (3%) endometrial carcinoma. The median overall survival time for the entire cohort was 54 months (95% confidence interval, 3–154 months), and the median recurrence-free survival was 50 months (95% confidence interval, 2–154 months). On univariable analysis, age, race, marital status, stage, grade, histologic condition, and treatment type were associated significantly with overall survival and recurrence-free survival. After adjustment for these covariates, patients with an Adult Comorbidity Evaluation 27 score of 2 had a 52% higher risk of death (95% confidence interval, 1.16–2.00); patients with an Adult Comorbidity Evaluation 27 score of 3 had a 2.35-fold increased risk of death (95% confidence interval, 1.73–3.21) compared with patients with an Adult Comorbidity Evaluation 27 score of 0. Similarly, patients with an Adult Comorbidity Evaluation 27 score of 2 had a 38% higher risk of recurrence (95% confidence interval, 1.07–1.78); patients with Adult Comorbidity Evaluation 27 score of 3 had a 2.05-fold increased risk of recurrence (95% confidence interval, 1.53–2.75) compared with patients with an Adult Comorbidity Evaluation 27 score of 0. We found no interaction between Adult Comorbidity Evaluation 27 score and age, stage, or treatment type.
Conclusion
Our findings demonstrate the importance of comorbidities in the estimation of the prognosis of patients with endometrial cancer, even after adjustment for age and known tumor-specific prognostic factors such as stage, grade, histologic condition, and adjuvant treatment.
The probability of the development of endometrial cancer (EC) increases with age, and its incidence has increased in all age groups over the last decade. Advancing age is also associated with a higher risk of recurrence and poor survival. As a result, age is used in risk-stratification algorithms of early-stage EC trials. EC is also associated with comorbid conditions that include obesity and diabetes mellitus, and >50% of patients with early stage EC die of intercurrent illnesses rather than cancer. Therefore, it is important to study the effect of comorbidities on the survival of these patients.
Medical comorbidities can affect cancer patient survival and life-expectancy strongly, independent of cancer stage and disease status. Comorbidity is an independent prognostic factor that affects survival in other gynecologic cancers that include cervical and ovarian cancer. Nicholas et al showed that a diagnosis of diabetes mellitus and/or hypertension in patients with EC resulted in a decreased survival, even after adjustment for age, stage, and grade. Truong et al studied the effect of age, Karnofsky performance status, and Charlson comorbidity score on the treatment, recurrence, and survival of patients with EC. They found that advanced age was associated with a decline in prescribed postoperative radiation therapy. Age and performance status were independent predictors of overall survival (OS) but not recurrence-free survival (RFS). A Charlson comorbidity score of 0–1 vs ≥2 was not associated significantly with the adjuvant treatment prescribed, OS, or RFS. However, there was a trend towards the prescription of less radiation therapy in patients with a score of ≥2 when compared with patients with a score of 0-1.
The Charlson comorbidity index was developed to predict the probability of death in patients with medical comorbidities. A score of ≥3 is associated with an almost 60% 1-year mortality rate. The score is based on age and 16 medical conditions, but the severity of the condition is accounted for only in diabetes mellitus and cancer and liver disease. Furthermore, it does not take into account morbidity from obesity, which is a well-established risk factor for EC. In comparison, the Adult Comorbidity Evaluation 27 (ACE-27) is a 26-item comorbidity index that accounts for the presence and severity of individual medical illnesses by grading them into 1–4 comorbidity classes: none, mild, moderate, or severe ( Table 1 ). Comorbid conditions that are controlled with or without medications, that do not cause symptoms limiting activities of daily living, and that have not required hospitalization are considered mild . Comorbid conditions that require active treatment modifications, that include residual disability that limits activities of daily living, or that require hospitalization or surgery to treat comorbid are considered moderate . Comorbid conditions that have caused major complications, irreversible end-organ damage, uncontrolled symptoms, and disability that requires full support in activities of daily living are considered severe . A body mass index <38 kg/m 2 received a score of zero, and all others received a score of 2. The overall ACE-27 score can be none (0), mild (1), moderate (2), or severe (3) and is defined according to the highest scored ailment. In cases with ≥2 grade 1 ailments, a final score of 1 is assigned. In cases with ≥2 grade 2 ailments in different organ systems, a final score of 3 is assigned. ACE-27 has been validated to provide prognostic information about patients with cancer in large prospectively maintained tumor registries.
| Cardiovascular system |
| Myocardial infarct |
| Angina/coronary artery disease |
| Congestive heart failure |
| Arrhythmias |
| Hypertension |
| Venous disease |
| Peripheral arterial disease |
| Respiratory system |
| Gastrointestinal system |
| Hepatic disease |
| Stomach or intestinal disease |
| Pancreatic disease |
| Renal system: end-stage renal disease |
| Endocrine system: diabetes mellitus |
| Neurologic system |
| Stroke |
| Dementia |
| Paralysis |
| Neuromuscular |
| Psychiatric system |
| Rheumatologic system |
| Immunologic system: AIDS |
| Malignancy |
| Solid tumors |
| Leukemia and myeloma |
| Lymphoma |
| Substance abuse |
| Alcohol |
| Illicit drugs |
| Obesity |
Read et al reviewed the prognostic impact of ACE-27 score in different cancer types and found that comorbidity had the most prognostic impact in patients with high cancer survival rates. In the United States, the 5-year survival rate from EC is >80%. Therefore, it is important to describe the relationship between comorbidity and survival in these patients. The objective of this study was to assess the association between ACE-27 score and survival outcomes that include OS and RFS in patients with EC.
Methods
Study design and setting
We performed a retrospective cohort study of women who had been diagnosed with EC from 2000–2012. After Institutional Review Board approval was obtained, patients were identified from the prospectively maintained Siteman Cancer Center Tumor Registry. The analysis included patients with stages I-IV disease who had been treated surgically. Only patients with endometrioid, serous, and clear cell adenocarcinoma were included in the analysis. Thus, we excluded patients with uterine sarcomas. Patients who were treated primarily with hormones, radiation, or chemotherapy were excluded. Patients with missing ACE-27 data were excluded.
Data collection
We extracted demographic data from the database that included age of diagnosis, race, and marital status. Medical information regarding alcohol use, tobacco use, ACE-27 score, surgical procedure, cancer stage, grade, histologic condition, and adjuvant treatment was also collected. Age was divided into the 3 risk categories described by Keys et al. ACE-27 scores were entered into the database by trained certified tumor registrars after a comprehensive review of patient medical records. Tumor stage was based on the 2009 International Federation of Gynecology and Obstetrics guidelines. Histologic condition was coded as serous or clear cell if >10% of the tumor consisted of that histologic condition. The dates of death and recurrence were extracted from the database. Death and recurrence information was updated in the database semiannually when patients, their family, and their physicians were contacted. The National and Social Security Death Index was queried for the patients lost to follow up.
The primary outcome was OS, and the secondary outcome was RFS. OS was calculated from the date of diagnosis to date of death or last follow-up evaluation. Patients who were alive at last follow-up evaluated were censored for OS analysis. RFS was calculated from the date of diagnosis to date of recurrence (after remission) or progression (despite primary treatment) or death. Recurrence and progression were determined by computed tomography imaging or biopsy.
Statistical analysis
Standard descriptive statistics were used to describe the study cohort. Median, range, and 95% confidence interval (CI) were used to describe continuous variables such as age of diagnosis, follow-up time, OS, and RFS. Categoric variables were described as proportions of the entire cohort. Kaplan-Meier survival analysis with log-rank test was used to determine the statistical significance of difference in survival between patients with increasing ACE-27 scores. Univariable Cox proportional hazards regression was used to identify factors that were associated with OS and PFS. Variables that were identified as significantly associated with OS at alpha level .05 were included in the multivariable model. If a covariate did not meet statistical significance on univariate analysis but was deemed clinically important, it was included in the multivariable model. Alcohol use was excluded in the multivariable model because the ACE-27 includes this data point. Unadjusted and adjusted hazard ratios (HR adj ) for death and recurrence were calculated with 95% CI. The proportional hazards assumptions was tested by log minus log plots.
Results
Description of cohort
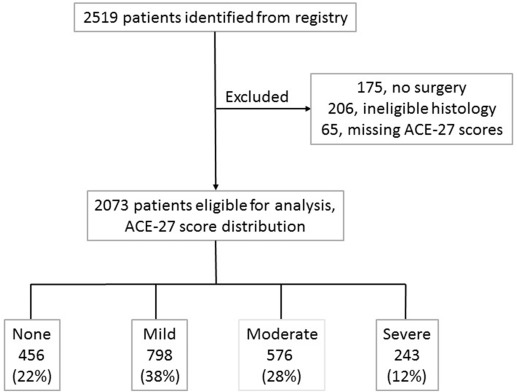
We identified 2519 patients with EC from the Siteman Cancer Center Tumor Registry. We excluded 175 patients who did not undergo surgery, 206 patients with ineligible histologic condition, and 65 patients with missing ACE-27 scores ( Figure 1 ). The distribution of variables was not different in the 65 patients with missing ACE-27 scores. Most patients had a mild comorbidity score followed by moderate, none, then severe. The distribution of variables is presented in Table 2 . Most patients were white and married or in a partnership and had never used alcohol or tobacco. Most patients had stage I and grade 1 cancers with endometrioid histologic condition. The median age of the entire cohort and the patients with recurrence was 61 years (range, 20-94 years) and 65 years (range, 34-92 years), respectively. Of the 2073 evaluable patients, 310 (15%) experienced recurrence; 515 (25%) died, and 242 (12%) experienced recurrence and died. Most patients were alive without disease (72%), with a median follow-up time of 63 months (95% CI, 5–158). The median OS and PFS was 54 (95% CI, 3–155) and 51 months (95% CI, 2–155), respectively.
| Characteristic | Patients (N=2073), n (%) | Unadjusted hazard ratio for death | 95% Confidence interval | Unadjusted hazard ratio for recurrence | 95% Confidence interval |
|---|---|---|---|---|---|
| Age, y | |||||
| <50 | 258 (12) | Reference | Reference | ||
| 50–69 | 1328 (64) | 1.41 | 0.98–2.03 | 1.54 | 1.10–2.17 |
| ≥70 | 487 (24) | 4.36 | 3.03–6.28 | 4.15 | 2.94–5.86 |
| Race | |||||
| White | 1807 (87) | Reference | Reference | ||
| Black | 243 (12) | 1.42 | 1.12–1.80 | 1.42 | 1.14–1.78 |
| Other | 23 (1) | 0.51 | 0.16–1.59 | 0.44 | 0.14–1.37 |
| Marital status | |||||
| Married/partner | 1081 (52) | Reference | Reference | ||
| Divorced/separated | 255 (12) | 1.31 | 0.97–1.75 | 1.23 | 0.94–1.62 |
| Widowed | 388 (19) | 2.70 | 2.20–3.31 | 2.55 | 2.10–3.09 |
| Single | 338 (16) | 1.37 | 1.05–1.78 | 1.35 | 1.06–1.72 |
| Unknown | 11 (1) | 2.30 | 0.95–5.58 | 1.99 | 0.82–4.81 |
| Alcohol use | |||||
| Never | 1314 (63) | Reference | Reference | ||
| Current | 427 (21) | 0.77 | 0.60–0.99 | 0.84 | 0.67–1.05 |
| Former | 34 (2) | 1.45 | 0.80–2.65 | 1.63 | 0.96–2.78 |
| Unknown | 298 (14) | 1.06 | 0.85–1.32 | 0.98 | 0.79–1.22 |
| Tobacco use | |||||
| Never | 1267 (61) | Reference | Reference | ||
| Current | 207 (10) | 1.18 | 0.88–1.57 | 1.08 | 0.82–1.41 |
| Former | 329 (16) | 1.21 | 0.95–1.55 | 1.16 | 0.93–1.46 |
| Unknown | 270 (13) | 1.09 | 0.86–1.38 | 0.98 | 0.78–1.24 |
| Adult Comorbidity Evaluation 27 | |||||
| None | 456 (22) | Reference | Reference | ||
| Mild | 798 (38) | 1.33 | 1.05–1.69 | 1.30 | 1.04–1.62 |
| Moderate | 576 (28) | 1.20 | 0.92–1.56 | 1.11 | 0.87–1.43 |
| Severe | 243 (12) | 2.01 | 1.49–2.72 | 1.74 | 1.31–2.31 |
| Stage | |||||
| 1 | 1524 (73) | Reference | Reference | ||
| 2 | 97 (5) | 1.93 | 1.29–2.87 | 1.71 | 1.16–2.517 |
| 3 | 314 (15) | 3.54 | 2.88–4.37 | 3.53 | 2.91–4.293 |
| 4 | 138 (7) | 11.45 | 9.09–14.43 | 12.40 | 9.94–15.46 |
| Grade | |||||
| 1 | 1080 (52) | Reference | Reference | ||
| 2 | 480 (23) | 1.63 | 1.28–2.07 | 1.82 | 1.45–2.27 |
| 3 | 513 (25) | 4.52 | –5.53 | 4.67 | 3.86–5.65 |
| Histologic condition | |||||
| Endometrioid | 1797 (87) | Reference | Reference | ||
| Serous | 223 (11) | 3.79 | 3.09–4.64 | 3.78 | 3.12–4.59 |
| Clear cell | 53 (2) | 3.70 | 2.52–5.44 | 3.96 | 2.76–5.68 |
| Adjuvant therapy | |||||
| None | 1349 (65) | Reference | Reference | ||
| Radiation | 232 (11) | 1.87 | 1.44–2.44 | 1.80 | 1.40–2.32 |
| Chemotherapy | 268 (13) | 4.64 | 3.76–5.73 | 5.00 | 4.10–6.09 |
| Chemoradiation | 224 (11) | 1.94 | 1.48–2.55 | 2.24 | 1.75–2.87 |
Kaplan-Meier survival by ACE-27 score
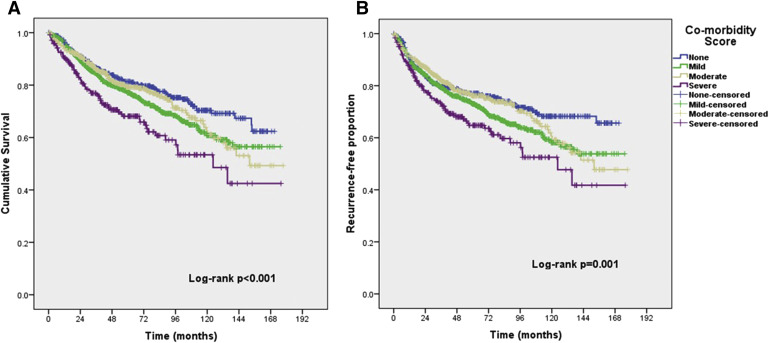
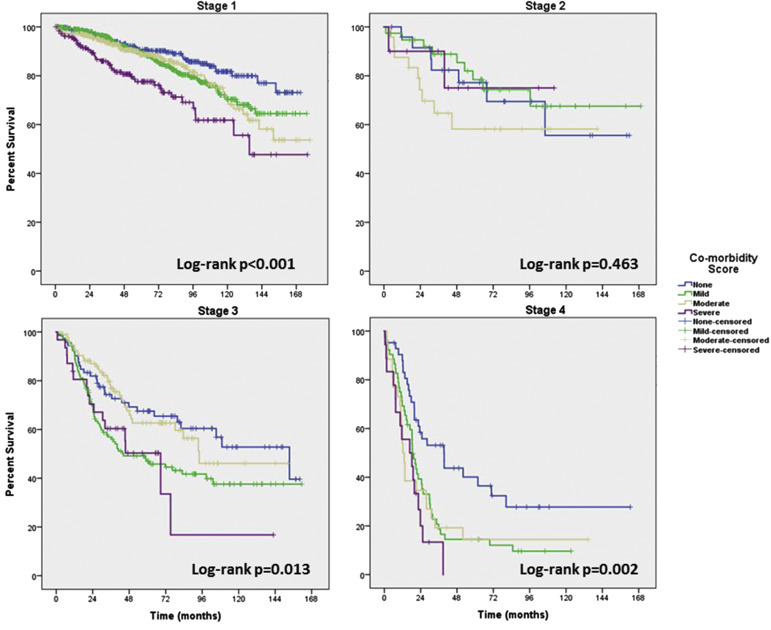
Kaplan-Meier survival plots of OS and PFS for the 4 different ACE-27 scores are shown in Figure 2 . The overall log-rank test shows that there is a significant difference in OS ( P <.001) and PFS (chi-square, 16.9; P =.001) between the different ACE-27 scores. Pairwise comparisons between the difference ACE-27 scores show that the OS and PFS for patients with a severe ACE-27 score is worse than a score of none, mild, or moderate. The proportions of death were 100 of 456 patients (21.9%), 214 of 798 patients (26.8), 124 of 576 patients (21.5), and 77 of 243 patients (31.7%) for none, mild, moderate, and severe score, respectively. The median time to death was 12.7 years for patients with moderate comorbidity and 10.4 years (95% CI, 7.2–13.5) for patients with severe comorbidity. Median survival was not reached for patients with none or mild comorbidity. After stratification by stage, a higher grade of comorbidity was still associated with worse survival for stages 1, 3, and 4 ( Figure 3 ).