Abnormal Uterine Bleeding
 |
Abnormal uterine bleeding is the single most common complaint that reproductive age women bring to their clinicians. All clinicians who provide primary care for women must therefore be familiar with its causes and have an organized, logical approach to the evaluation and treatment of the problem.
Anovulatory or dysfunctional uterine bleeding describes the spectrum of abnormal menstrual bleeding patterns that can occur in anovulatory women who have no medical illness or pelvic pathology. The mechanisms involved in anovulatory bleeding vary, but each reflects an abnormal pattern of steroid hormone stimulation that deviates from the sequence characterizing the normal ovulatory menstrual cycle. The key to successful clinical management of dysfunctional bleeding is to recognize or identify which mechanism is operating or responsible. Anovulatory bleeding can be effectively and confidently managed with medical treatment regimens based on sound physiologic concepts. The treatment regimens described in this chapter are time-tested and designed to achieve two specific but interrelated goals. The first is to reverse the abnormalities of endometrial growth and development that result from chronic anovulation and predispose to excessive and prolonged menstrual flow. The second is to induce or restore cyclic predictable menses of normal volume and duration.
Bleeding related to a wide assortment of pathology inside and outside of the reproductive tract can masquerade as anovulatory bleeding. A careful menstrual history and physical examination usually provide most of the information needed to distinguish anovulation from other causes of abnormal bleeding. When pathology is strongly suspected or treatment for presumed anovulatory bleeding fails, additional evaluation is indicated but is also straightforward.
Terminology
Clinicians use a wide variety of terms to describe abnormal patterns of menstrual bleeding that do not always mean or communicate the same thing to others. Traditional terms having Greek or Latin roots still are used widely to describe different abnormalities relating to the frequency, regularity, duration and volume of menses.
Traditional Terms Describing Abnormalities of Menstrual Bleeding
• | Amenorrhea | absent menses |
• | Oligomenorrhea | infrequent menses, occurring at intervals > 35 days |
• | Polymenorrhea | frequent menses, occurring at intervals < 24 days |
• | Metrorrhagia | menses occurring at irregular intervals |
• | Menorrhagia or | abnormally long or heavy menses, lasting > 7 days |
Hypermenorrhea | or involving blood loss > 80 mL |
Although the definitions above are reasonably well established, the terms are not always used or understood accurately.1, 2 For example, in the United States, the term abnormal uterine bleeding generally describes all abnormal patterns of bleeding that may result from a wide variety of causes, including anovulation, pregnancy, uterine pathology, and coagulopathies.3 The term dysfunctional uterine bleeding is synonymous with anovulatory bleeding, in the absence of pregnancy or any demonstrable pathology (a diagnosis of exclusion), and the term menorrhagia describes regular, heavy or prolonged bleeding. However, in other countries, dysfunctional uterine bleeding and menorrhagia often are used to describe both ovulatory (regular) or anovulatory (irregular) bleeding that is heavy or prolonged.1 The confusion surrounding the exact meaning of the traditional terms has spurred a call to abandon them, in favor of simple terms that can be understood by patients and translated easily into languages other than English, with the ultimate goal of improving communication among health care providers, investigators, and patients. To that end, recommendations arising from an international consensus conference proposed terms to describe the most important features of menstrual bleeding during the reproductive years, as follows1:
Characteristic | Descriptive Terms | Normal Limits |
Frequency of menses | Frequent | <24 days |
Normal | 24-38 days | |
Infrequent | >38 days | |
Regularity (cycle to cycle variation) | Absent | _ |
Regular | ± 2-20 days | |
Irregular | > 20 days | |
Duration of flow | Prolonged | > 8 days |
Normal | 4-8 days | |
Shortened | < 4 days | |
Volume of monthly blood loss | Heavy | >80 mL |
Normal | 5-80 mL | |
Light | < 5 mL |
The suggested normal limits for frequency, regularity, and duration of menstrual flow were based on the 5th and 95th percentiles for data drawn from population studies.4,5 and 6 As such, they are influenced by the prevalence of common anovulatory disorders, such as the polycystic ovary syndrome, in a given population. Consequently, the population-based norms are
wider than the generally accepted norms for menstrual frequency (24-35 days), regularity (± 5 days variation), and duration (2-7 days) among ovulatory women. The normal limits for the volume of menstrual blood loss were based primarily on measurements of hemoglobin loss in a Swedish community.4 The expectation is that a structured menstrual history can clarify the details needed to categorize a patient’s complaint in clear and simple terms (e.g., irregular, heavy menstrual bleeding).7, 8
wider than the generally accepted norms for menstrual frequency (24-35 days), regularity (± 5 days variation), and duration (2-7 days) among ovulatory women. The normal limits for the volume of menstrual blood loss were based primarily on measurements of hemoglobin loss in a Swedish community.4 The expectation is that a structured menstrual history can clarify the details needed to categorize a patient’s complaint in clear and simple terms (e.g., irregular, heavy menstrual bleeding).7, 8
Although the effort to simplify and standardize the terminology used to describe menstrual abnormalities is reasoned and laudable, the adoption of a new nomenclature likely will be slow because the traditional terms, however confused, are firmly entrenched.
Normal Menstrual Bleeding
It is ovulation or, more specifically, the organized sequence of endocrine signals that characterizes the ovulatory cycle, that gives menses regularity, predictability, and consistency. The endocrinology of the normal menstrual cycle is discussed in detail in Chapter 6. Only the most basic concepts and characteristics are summarized here, with a focus on the major events and mechanisms that control the endometrial cycle and the volume and duration of menstrual flow.
During the follicular phase of the normal ovarian cycle (corresponding to the proliferative phase of the endometrial cycle), estrogen levels rise, slowly at first and then more rapidly, as the dominant ovarian follicle emerges, grows, and matures. In response to that estrogen, the functional layer of the endometrium regrows, after having been shed during the preceding menses. After ovulation, the corpus luteum derived from the ovulatory follicle continues to produce estrogen, but now and more importantly, also progesterone. During the luteal phase of the ovarian cycle (corresponding to the secretory phase of the endometrial cycle), estrogen and progesterone levels rise together as the corpus luteum grows to maturity. In response to the combined actions of estrogen and progesterone, the endometrium transforms and organizes in preparation for the anticipated arrival and implantation of a conceptus. If pregnancy and rapidly rising levels of human chorionic gonadotropin (hCG) do not come to its “rescue,” the corpus luteum regresses spontaneously in a form of preprogrammed cell death. As it does, estrogen and progesterone levels fall steadily, eventually withdrawing the functional support for the endometrium. Menses begin, marking the end of one endometrial cycle and the beginning of another.
From the endometrial perspective, the endocrine features of the ovarian cycle are quite simple; the quantities of hormones produced are not nearly as important as the sequence in which they appear: estrogen, followed by estrogen and progesterone, followed by withdrawal of both hormones. Of all the different hormone effects on the endometrium, estrogen-progesterone stimulation produces the most stable endometrium, and their combined withdrawal yields the most consistent menstrual characteristics. The sequence is so controlling that most ovulatory women have a pattern, volume, and duration of menstrual flow they recognize as their own and come to expect, very often accompanied by an equally consistent and predictable pattern of premenstrual molimina (bloating, breast tenderness, mood swings). Even slight deviations from the usual pattern in the timing, amount, or length of flow can cause concern. Careful attention to the finer details of the menstrual history can be very helpful in distinguishing anovulatory bleeding from other causes.
Variations in menstrual flow and cycle length are common at the extremes of reproductive age, during the early teenage years and those preceding the menopause. Menstrual cycles often are irregular for the first 12-18 months after menarche, due to immaturity
of the hypothalamic-pituitary-ovarian axis.9, 10 In a study conducted by the World Health Organization, the median length of the first cycle after menarche was 34 days; almost 40% of cycles were longer than 40 days and fewer than 10% were less than 20 days.11 Cycles remain relatively long for the first 5-7 years after menarche, thereafter decreasing gradually in length and becoming more regular.11 The prevalence of anovulatory cycles is higher in women under age 20 and over age 40.12, 13 Menstrual cycle characteristics generally do not change appreciably during the reproductive years,6 although overall cycle length and variability slowly decrease. On average, mean cycle length and range reach their lows at about age 40-42.6, 14 Over the subsequent 8-10 years before the menopause, the trend is reversed; both average cycle length and variability increase steadily as ovulation becomes less regular and frequent.5, 14,15 and 16 Mean cycle length is greater in women at the extremes of body mass and composition; both high and low body mass index (BMI), body fat mass, and body lean mass are associated with an increased mean cycle length.17, 18
of the hypothalamic-pituitary-ovarian axis.9, 10 In a study conducted by the World Health Organization, the median length of the first cycle after menarche was 34 days; almost 40% of cycles were longer than 40 days and fewer than 10% were less than 20 days.11 Cycles remain relatively long for the first 5-7 years after menarche, thereafter decreasing gradually in length and becoming more regular.11 The prevalence of anovulatory cycles is higher in women under age 20 and over age 40.12, 13 Menstrual cycle characteristics generally do not change appreciably during the reproductive years,6 although overall cycle length and variability slowly decrease. On average, mean cycle length and range reach their lows at about age 40-42.6, 14 Over the subsequent 8-10 years before the menopause, the trend is reversed; both average cycle length and variability increase steadily as ovulation becomes less regular and frequent.5, 14,15 and 16 Mean cycle length is greater in women at the extremes of body mass and composition; both high and low body mass index (BMI), body fat mass, and body lean mass are associated with an increased mean cycle length.17, 18
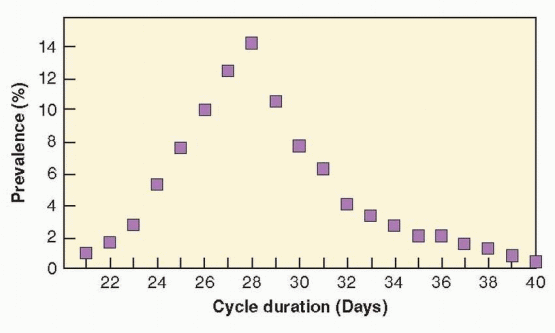
In general, variations in cycle length reflect differences in the length of the follicular phase of the ovarian cycle. Women who have a 25-day cycle ovulate on or about cycle day 10-12 and those with a 35-day cycle ovulate approximately 10 days later. Within a few years after menarche, the luteal phase becomes extremely consistent (13-15 days in duration) and remains so until the perimenopause.5, 14 At age 25, over 40% of cycles are between 25 and 28 days in length, and between age 25 and 35, over 60% are. Although 28 days is the most commonly reported intermenstrual interval, only approximately 15% of cycles among reproductive aged women actually are 28 days in length. Less than 1% of women have a regular cycle lasting less than 24 days or more than 35 days.19 Most women have cycles that last from 24 to 35 days, but at least 20% of women experience irregular cycles.6
 |
The usual duration of menstrual flow is 4-6 days, but for some women (approximately 3%) menses may last as few as 2 days or as many as 7 days.20 The average volume of menstrual blood loss is approximately 30 mL;4 greater than 80 mL is abnormal. Flow can be excessive without being abnormally long because most of menstrual blood loss occurs during the first 3 days.21, 22
Women who menstruate more often than every 24 days or less often than every 35 days deserve evaluation,5, 6 as do those who consistently flow for more than 7 days and women with monthly menstrual blood loss exceeding 80 mL. Any of these abnormal patterns can result in anemia that also requires treatment.23, 24 The intermenstrual interval and duration of menses are relatively easy to determine, but the volume of menstrual blood loss is difficult to measure. The correlation between perceived and actual blood loss is relatively poor.25 In population-based studies, one-fourth to one-third of women with normal periods considered their menstrual blood loss excessive, and 40% of those with documented menorrhagia (blood loss > 80 mL) described their menses as light or moderate.4, 26 Complaints of heavy menstrual bleeding appear to relate more to perceived interference with daily function than to actual blood loss,27 and evidence indicates that psychosocial factors may
have significant influence on those perceptions; the incidence of depression and anxiety is increased among women with complaints of heavy menstrual bleeding.28,29 and 30
have significant influence on those perceptions; the incidence of depression and anxiety is increased among women with complaints of heavy menstrual bleeding.28,29 and 30
Mechanisms Controlling Onset and Cessation of Normal Menstruation
A conceptual understanding of the mechanisms involved in the onset and cessation of normal menstrual bleeding provides both the foundation and the context for understanding the pathophysiology of anovulatory bleeding.
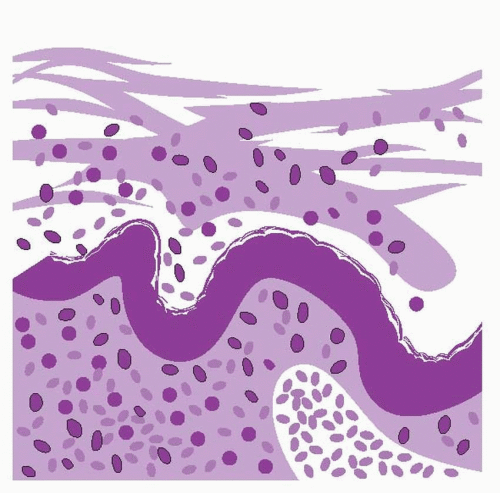
The classic concepts of normal menstruation derived primarily from direct observations of the cyclic changes in endometrium transplanted from the uterus to the anterior chamber of the eye in nonhuman primates; vascular events played the key role in the explanation for how menses both began and ended.31, 32 Basically, menstruation was envisioned as ischemic necrosis of the endometrium caused by vasoconstriction of the spiral arterioles in the basal layer, triggered by withdrawal of estrogen and progesterone. Similarly, the end of menses was explained by longer and more intense waves of vasoconstriction, combined with coagulation mechanisms activated by vascular stasis and endometrial collapse, aided by rapid reepithelialization mediated by estrogen derived from the emerging new follicular cohort.
The results of more contemporary investigations do not support the classic hypoxia theory of menstruation. Perfusion studies in women have failed to demonstrate reduced endometrial blood flow just before menses.33 Hypoxia-inducible factor (HIF)-1, a nuclear protein that activates gene transcription in response to reduced cellular oxygen (the earliest known marker of response to hypoxia), is barely detectable and not widely distributed in human premenstrual endometrium cultured under hypoxic conditions.34 Histologically, early menstrual endometrium exhibits focal necrosis, inflammation, and coagulation rather than the diffuse hyalinization or coagulation necrosis that would be expected to result from vasoconstriction and hypoxia.35 Slowly but surely over the last decade or so, the operational paradigm for menstruation has shifted. Instead of vascular events, the central theme of the new model of the initiation of menstruation is an enzymatic autodigestion of the functional layer of the endometrium and its subsurface capillary plexus, possibly extending to the spiral arteriolar system in the basal layer.35 The classic concept of the mechanisms that end normal menstruation is essentially unchanged; coagulation mechanisms, local vasoconstriction, and re-epithelialization all contribute to hemostasis in the menstrual endometrium with vascular events playing the key role.
The enzymatic degradation of the endometrium triggered by estrogen-progesterone withdrawal involves a number of different but interrelated mechanisms including the release of intracellular lysosomal enzymes, proteases from infiltrating inflammatory cells, and the actions of matrix metalloproteinases. In the first half of the secretory phase, acid phosphatase and other potent lytic enzymes are confined to intracellular lysosomes, their release inhibited by progesterone via stabilization of lysosomal membranes. As estrogen and progesterone levels fall in the days preceding menses, lysosomal membranes destabilize and the enzymes within are released into the cytoplasm of epithelial, stromal, and endothelial cells, and eventually, into the intercellular space. These proteolytic enzymes digest their cellular constraints as well as surface membranes and desmosomes (intercellular bridges). In the vascular endothelium, their actions result in platelet deposition, prostaglandin release, vascular thrombosis, extravasation of red blood cells, and tissue necrosis.35, 36
Progesterone withdrawal also stimulates an inflammatory response in the endometrium. Just before menstruation, the total number of leukocytes in the endometrium increases
markedly to as much as 40% of the stroma.37, 38 The inflammatory infiltrate (including neutrophils, eosinophils, and macrophages or monocytes) is drawn by chemo-attractive molecules (chemokines) synthesized by endometrial cells, some of which are downregulated by progesterone (interleukin 8; IL-8).37 When activated, the leukocytes produce a wide assortment of regulatory molecules including cytokines, chemokines, and a range of enzymes that contribute to degradation of the extracellular matrix, directly, or indirectly, via activation of other proteases.
markedly to as much as 40% of the stroma.37, 38 The inflammatory infiltrate (including neutrophils, eosinophils, and macrophages or monocytes) is drawn by chemo-attractive molecules (chemokines) synthesized by endometrial cells, some of which are downregulated by progesterone (interleukin 8; IL-8).37 When activated, the leukocytes produce a wide assortment of regulatory molecules including cytokines, chemokines, and a range of enzymes that contribute to degradation of the extracellular matrix, directly, or indirectly, via activation of other proteases.
Matrix metalloproteinases are a family a proteolytic enzymes that degrade components of the extracellular matrix and basement membrane.39 The metalloproteinases include collagenases that degrade interstitial and basement membrane collagens, gelatinases that further digest collagens, and stromelysins that attack fibronectin, laminin, and glycoproteins. Each member of the family is substrate specific and secreted as an inactive zymogen that requires activation by plasmin, leukocyte proteases, or other metalloproteinases. The expression, secretion, and activation of endometrial matrix metalloproteinases is cycle dependent and increases markedly in the late secretory phase just before menstruation.40, 41 Overall, progesterone inhibits endometrial metalloproteinase expression, an action mediated by transforming growth factor (TFG)-b.42 Progesterone withdrawal has the opposite effect—increased metalloproteinase secretion and activation, followed by dissolution of the extracellular matrix.43 Local modulators (predominantly cytokines), derived from endometrial epithelial, stromal, and endothelial cells, and natural tissue inhibitors of matrix metalloproteinases that bind the active form of the enzymes also play an important role in their regulation.44 In cycles of conception wherein elevated progesterone levels are sustained, matrix metalloproteinase activity remains effectively suppressed. In the normal menstrual cycle, metalloproteinase expression is suppressed again after menses, presumably by increasing estrogen levels.
Progressive enzymatic degradation of the endometrium eventually disrupts the subsurface capillary and venous vascular system, causing interstitial hemorrhage; dissolution of the surface membrane allows blood to escape into the endometrial cavity. Ultimately, degeneration extends to the deepest extent of the functional layer where rupture of the basal arterioles contributes to bleeding. A natural cleavage plane develops at the junction of the loose, vascular, edematous stroma with the basal layer. Desquamation begins in the fundus and gradually extends toward the isthmus. The end result is the typical deflated and shallow but dense menstrual endometrium.45, 46
The menstrual fluid is comprised of an autolysed endometrium rich in inflammatory exudates, red blood cells, and proteolytic enzymes.35, 46 One of those enzymes, plasmin, formed by activation of its inactive precursor, plasminogen, has potent fibrinolytic actions that help to prevent clotting of menstrual fluid and to facilitate the expulsion of degenerated tissue. Plasminogen activators that mediate the conversion of plasminogen to plasmin are found in late secretory and menstrual endometrium and are released from degenerated endometrial vascular endothelium.35, 46 The volume of menstrual bleeding is controlled, at least to some extent, by the local balance between fibrinolysis and clotting. Endometrial stromal cell tissue factor and plasminogen activator inhibitor (PAI)-1 promote clotting and help to balance fibrinolytic processes.47,48 and 49 Early in menstruation, intravascular platelet plugs, and later, thrombi form at the shedding surface, helping to limit blood loss. Their importance to hemostasis in the menstrual endometrium can be inferred from the increased volumes of menstrual blood loss observed in women with thrombocytopenia and von Willebrand disease. Ultimately, however, the cessation of menstrual bleeding depends on vasoconstriction in the denuded spiral arterioles in the basal layer of the endometrium, and also possibly in the radial arteries of the superficial myometrium. Endothelins are potent long-acting vasoconstrictors of vascular smooth muscle produced by endometrial glandular, stromal, and endothelial cells. Menstrual endometrium contains high concentrations of endothelins and prostaglandins, which together cause intense vasoconstriction in the spiral arterioles.35
The myometrial contractions associated with menstrual events very likely reflect the actions of prostaglandin F2a, but in contrast to postpartum bleeding, myometrial contractions are not important for control of menstrual bleeding.
The myometrial contractions associated with menstrual events very likely reflect the actions of prostaglandin F2a, but in contrast to postpartum bleeding, myometrial contractions are not important for control of menstrual bleeding.
Surface re-epithelialization also contributes to hemostasis in the menstrual endometrium. The process occurs very rapidly, beginning at the mouth of the basal portions of residual glands in areas otherwise completely denuded, and spreading outward. The peripheral regions of the cavity at the isthmus and near the tubal ostia (which do not shed during menses) also contribute to its resurfacing.38, 46 Generally, by cycle day 5, these scattered areas of epithelial proliferation converge and fuse; bleeding stops completely only when the new epithelial surface is complete. The mechanisms that govern this initial phase of tissue repair and the role that estrogen has, if any, are uncertain. In the first few days of the new cycle, circulating estrogen levels and endometrial estrogen and progesterone receptor concentrations are low and unchanged from premenstrual levels.50, 51 Moreover, even after oophorectomy and vigorous endometrial denudation, the endometrium heals, suggesting that the initial phase of tissue repair is largely independent of estrogen.38, 46
The stroma regenerates from stem cells located in the basal layer of the endometrium, but only after a confluent surface epithelium has been restored. Damaged endometrial vessels are quickly repaired. New vessel growth and mitotic activity in all parts of the regenerated human endometrium coincide with increasing serum estrogen levels and rising endometrial estrogen and progesterone receptor concentrations.38, 46 Matrix metalloproteinases present in the menstrual endometrium and other proteases may be important mediators of the release and activation of growth factors needed for endometrial repair. Vascular endothelial growth factor is an important promoter of endometrial mitosis and can be induced by tumor necrosis factor (TNF)-a, TGF-b, and insulin-like growth factor-1.35, 52, 53 Experimental evidence derived from model systems suggests that activins and other members of the TGF-b superfamily also may play a role.38, 54
There are two basic reasons why normal menstrual bleeding is self-limited.
In response to a simultaneous estrogen-progesterone withdrawal, endometrial shedding is universal. Because the onset and end of menses relate to organized cyclic hormonal events, menstrual changes occur uniformly, throughout the endometrial cavity. Shedding of the functional layer and exposure of the basal regenerative layer of the endometrium stimulates coagulation, vasoconstriction, and epithelial reconstruction mechanisms that effectively limit the volume and duration of bleeding.
In response to cyclic sequential estrogen-progesterone stimulation, growth and development of the endometrial epithelium, stroma, and microvasculature is structurally stable and random breakdown is avoided. The sequence of events leading to the enzymatic disintegration of the endometrium proceeds in an orderly and synchronous fashion. The endometrium is not just repaired, but is completely remodeled, at regular intervals.
Endometrial Responses to Steroid Hormones: Physiologic and Pharmacologic
The normal menstrual bleeding that occurs at the end of an ovulatory cycle results from estrogen-progesterone withdrawal. The same mechanism operates when the corpus luteum
is removed or when its gonadotropin support is suddenly interrupted during the luteal phase, such as by treatment with a gonadotropin-releasing hormone (GnRH) antagonist. Other examples include the bleeding that follows discontinuation of both estrogen and progesterone in women receiving cyclic postmenopausal hormone therapy and the bleeding that comes at the end of a standard cycle of treatment with an estrogen-progestin contraceptive. The bleeding that follows estrogen-progesterone withdrawal generally is regular, predictable, and consistent in volume and duration. However, estrogen-progesterone withdrawal is not the only pattern of steroid hormone signals that can provoke endometrial bleeding. Bleeding also can result from estrogen withdrawal, estrogen breakthrough, progestogen withdrawal, and progestogen breakthrough.
is removed or when its gonadotropin support is suddenly interrupted during the luteal phase, such as by treatment with a gonadotropin-releasing hormone (GnRH) antagonist. Other examples include the bleeding that follows discontinuation of both estrogen and progesterone in women receiving cyclic postmenopausal hormone therapy and the bleeding that comes at the end of a standard cycle of treatment with an estrogen-progestin contraceptive. The bleeding that follows estrogen-progesterone withdrawal generally is regular, predictable, and consistent in volume and duration. However, estrogen-progesterone withdrawal is not the only pattern of steroid hormone signals that can provoke endometrial bleeding. Bleeding also can result from estrogen withdrawal, estrogen breakthrough, progestogen withdrawal, and progestogen breakthrough.
Estrogen Withdrawal Bleeding
One clinical example of estrogen withdrawal bleeding is that which may follow bilateral oophorectomy during the follicular phase of the cycle. The bleeding that occurs after removal of the ovaries can be delayed by exogenous estrogen therapy, but will occur when treatment stops. Other examples include cyclic estrogen-only hormone therapy in castrate or postmenopausal women and the midcycle bleeding that can accompany the transient but abrupt fall in estrogen levels immediately preceding ovulation.
Estrogen Breakthrough Bleeding
The best clinical examples of estrogen breakthrough bleeding are the different patterns of bleeding observed in women with chronic anovulation. The amount and duration of estrogen breakthrough bleeding can vary widely, depending on the amount and duration of unopposed estrogen stimulation that the endometrium has received. Relatively low levels of chronic estrogen exposure typically result in intermittent spotting or staining that is generally light in volume but may be prolonged. In contrast, sustained high level estrogen stimulation commonly results in long intervals of amenorrhea punctuated by acute episodes of often profuse bleeding that vary in duration.
Progestogen Withdrawal Bleeding
Progestogen withdrawal bleeding is observed when treatment with exogenous progesterone or a synthetic progestin is discontinued. Progestogen withdrawal bleeding usually occurs only when the endometrium has first been primed with endogenous or exogenous estrogen. The amount and duration of bleeding can vary widely and generally correlates with the level and duration of previous estrogen-stimulated endometrial proliferation. In women with marginal to frankly low estrogen levels or short intervals of amenorrhea, bleeding is generally light to scant and may not occur at all. In those with sustained high estrogen levels or long intervals of amenorrhea, bleeding can be heavy and somewhat prolonged, but still is self-limited. Between the extremes, the amount and duration of bleeding induced by progestogen withdrawal is typically similar to that observed at the end of a normal ovulatory cycle. In women receiving cyclic hormone therapy with exogenous estrogen and progestin, bleeding follows withdrawal of progestin even if estrogen treatment continues; progestin withdrawal bleeding can be delayed, but only if estrogen levels are increased by 10-20 fold.55
Progestogen Breakthrough Bleeding
Progestogen breakthrough bleeding occurs when the ratio of progestogen to estrogen is unfavorably high. Unless there is sufficient estrogen to balance its action, continuous treatment with exogenous progesterone or synthetic progestins will result in intermittent bleeding of varying duration that is generally light, a pattern very similar to low level estrogen breakthrough bleeding described above. Clinical examples of progestogen breakthrough bleeding are the bleeding observed in women using the progestin-only contraceptive “minipill” or other long-acting progestin-only contraceptive methods (progestin implants, depot medroxyprogesterone acetate).56 The breakthrough bleeding observed in women using combination estrogen-progestin contraceptives also is a form of progestogen breakthrough bleeding. Although all estrogen-progestin contraceptive regimens contain pharmacologic quantities of both estrogen and progestin, the progestin component is always the dominant hormone and the net effect on the endometrium is profoundly progestational.
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding can result from estrogen withdrawal bleeding, reflecting the transient fall in estrogen levels that accompanies regression of a follicular cohort, or from estrogen breakthrough bleeding, due to focal break down of an overgrown and structurally fragile endometrium under continuous estrogen stimulation. The heaviest episodes of anovulatory bleeding tend to occur in women with sustained high levels of estrogen; women with polycystic ovary syndrome, obese women, postmenarcheal adolescents, and perimenopausal women are common clinical examples. The clinical presentation spans the spectrum from the pale frightened teenager who has bled for weeks to the older woman who is deeply concerned that she may have cancer.
In contrast to the organized predictable pattern of sequential estrogen-progesterone stimulation and withdrawal that characterizes the normal ovulatory menstrual cycle, the patterns of ovarian steroid hormone production and endometrial stimulation in anovulatory women are disorganized and unpredictable. By definition, the anovulatory woman is always in the follicular phase of the ovarian cycle and in the proliferative phase of the endometrial cycle. There is no luteal or secretory phase because there is no ovulation or cycle. The only ovarian steroid signal the endometrium receives is estrogen, levels of which constantly fluctuate, rising and falling as each new cohort of follicles begins to grow but ultimately loses its developmental momentum and, sooner or later, lapses into atresia. Although the amplitude of the signal may vary, the message, growth, stays the same.
Over a period of time, an unrelenting, uninterrupted estrogen growth stimulus can stimulate the endometrium to proliferate to abnormal heights where it becomes fragile. Without the growth limiting and organizing effects of progesterone, the endometrium lacks the stromal support structure to maintain stability. Focal areas breakdown and bleed and, as those areas heal under the influence of continued estrogen stimulation, others break down and bleed. Persistent proliferative and hyperplastic endometrium characteristically exhibits numerous discrete foci of stromal breakdown near the epithelial surface, associated with pools of extravasated red blood cells, capillary platelet/fibrin thrombi, and repair-related changes recognized as ball-like aggregates of tightly packed stromal cells beneath a cap of intact but hypertrophied epithelium.35 The cause for the focal breakdowns in persistent proliferative endometrium is not entirely clear. However, abnormal endometrial growth involves not only epithelial and stromal cells but also the microvasculature.
Venous capillaries in persistent proliferative and hyperplastic endometrium are increased, dilated, and often form abnormal irregular channels; ultrastructural studies have revealed a number of abnormal structural elements that predispose to fragility.57, 58 The abnormal microvasculature could be the result, but is more likely the proximate cause, of abnormal bleeding. The weight of available evidence from histologic and molecular studies indicates that anovulatory bleeding results from an increased density of abnormal vessels having a fragile structure prone to focal rupture, followed by release of lysosomal proteolytic enzymes from surrounding epithelial and stromal cells and migratory leukocytes and macrophages. Once initiated, the process is further aggravated by local release of prostaglandins, with greater sensitivity to those that vasodilate (PGE2) than to those that vasoconstrict (PGF2a).59 Other molecules (perforins) inhibit capillary plug formation and further degrade the capillary venous network. Vasoconstriction of basal endometrial and superficial myometrial vessels does not occur because tissue loss is only focal and superficial, and does not typically reach the basal layer where denudation triggers an intense vasoconstrictive response. The final mechanism that normally controls menstrual bleeding, surface epithelial reconstruction, operates in persistent proliferative endometrium, but not in a normal way. Epithelial repair is focal, in the areas of breakdown, not universal; the result is a constantly changing patchwork of small repairs instead of an organized and well structured remodeling.35
Differential Diagnosis of Abnormal Uterine Bleeding
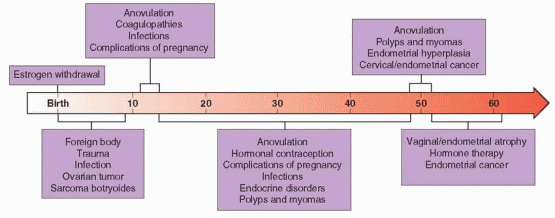
Anovulatory dysfunctional uterine bleeding is a diagnosis made by exclusion. The differential diagnosis includes problems relating to pregnancy, infection, vaginal and cervical abnormalities, benign and malignant uterine neoplasia, coagulopathies, endocrine disorders, trauma, foreign bodies, systemic disease, and bleeding relating to medications. The most common causes vary with age. In premenarcheal girls, foreign bodies, trauma, and infection are the most common. In postmenarcheal adolescents, anovulatory bleeding, coagulopathies, infections, and complications of pregnancy head the list. During the reproductive years, most abnormal bleeding results from anovulation, hormonal contraception, complications of pregnancy, infections, endocrine disorders, and polyps and myomas. In perimenopausal women, anovulation, benign uterine neoplasia, and endometrial hyperplasia cause the majority of problems, and in postmenopausal women, vaginal/endometrial atrophy and hormone therapy are the most common causes of abnormal bleeding; only about 10% of postmenopausal bleeding results from endometrial cancer.
 |
Complications of pregnancy always should be considered and excluded, particularly in adolescents who may be reluctant to reveal their sexual history. It is important to emphasize that the most common cause of a sudden departure from a well-established pattern of regular and predictable menses is a complication of pregnancy; threatened or spontaneous abortion and ectopic pregnancy are the most common, but possibilities also include retained products of conception and gestational trophoblastic disease.
Although abnormal bleeding is a relatively common problem in women using hormonal contraception or receiving physiologic combined continuous estrogen-progestin hormone therapy, the possibility of underlying pathology must not be forgotten. Infections such as cervicitis, endometritis, and salpingitis may be associated with abnormal bleeding. Bleeding relating to benign uterine neoplasia, chiefly cervical and endometrial polyps and uterine myomas, is confused frequently with anovulatory bleeding. Other pathology of the reproductive tract associated with abnormal bleeding includes adenomyosis and malignancies of the cervix and endometrium. Abnormal menstrual cycles occasionally are one of the earliest signs of a thyroid disorder (hypothyroidism or hyperthyroidism).60
The possibility of a coagulopathy also should be kept in mind, especially in adolescents whose menstrual history is short and not yet well defined. The most common cause of abnormal uterine bleeding in adolescents is anovulation, but up to a third may have a coagulation defect,61,62,63 and 64 including von Willebrand disease, Glanzmann thrombasthenia, idiopathic thrombocytopenic purpura, platelet dysfunction, and thrombocytopenia related to malignancy or treatment for malignancy. Bleeding disorders usually are associated with cyclic, regular, heavy or prolonged bleeding (menorrhagia). The same pattern may be observed in women receiving treatment with anticoagulants.65 Previous history of postpartum hemorrhage or excessive bleeding with surgery, dental procedures, or trauma should raise suspicion, but menorrhagia since menarche may be the only clue.66 Coagulation defects are not as rare as is generally perceived and may be found in 10-20% of women with unexplained menorrhagia.66,67,68 and 69
A variety of different medications can predispose to abnormal bleeding, by interfering with hemostasis (usually resulting in menorrhagia), by affecting the concentrations of endogenous or exogenous hormones (causing fluctuations in circulating levels), or by disrupting the hypothalamic-pituitary-ovarian axis. Drugs associated with abnormal menstrual bleeding include hormonal contraceptives, those used for postmenopausal hormone therapy, digitalis, anticonvulsants, anticoagulants, and psychopharmacologic medications. Some common herbs have estrogenic activity (e.g., ginseng) and may be associated with abnormal bleeding.70
Although uncommon, other diagnostic possibilities include systemic illnesses that predispose to anovulation or coagulation abnormalities; examples include diabetes mellitus, systemic lupus erythematosus, malignancy, and myelodysplasia. Chronic renal disease is associated with both ovulatory and platelet dysfunction. Liver disease can result in abnormal bleeding by adversely affecting estrogen metabolism (predisposing to anovulation) or the synthesis of clotting factors. In adolescents, genital trauma, sexual abuse, cervicitis relating to sexually-transmitted infections (Chlamydia trachomatis), and foreign bodies (e.g., retained tampons) merit specific consideration.
The existence of a post-tubal ligation syndrome of menstrual abnormalities has been debated for decades. Numerous studies have addressed the question with conflicting results. Some have examined the prevalence of menstrual complaints before and after sterilization.71, 72 Others have compared the incidence of hospitalization or hysterectomy for abnormal uterine bleeding in women with and without a previous tubal sterilization procedure.73,74 and 75 The popular theory that extensive tubal electrocoagulation adversely affects ovarian blood supply and steroid hormone production was supported by data suggesting that the incidence of menstrual problems increased with time after sterilization by electrocautery but not in women sterilized with rings or clips.76,77 and 78 However, no correlation has been found
between poststerilization menstrual changes and the amount of tissue destroyed.76, 78 Analysis of data from the U.S. Collaborative Review of Sterilization, a multicenter prospective cohort study that followed almost 10,000 women for up to 5 years after a tubal sterilization procedure, revealed that sterilized women were no more likely than women with sterilized male partners to report persistent changes in intermenstrual bleeding or cycle length.79 Sterilized women were more likely to have decreased menstrual duration, volume, and pain, and among women with heavy bleeding at baseline, those sterilized were more likely to report decreased menstrual bleeding after the procedure.79 Another more recent study of menstrual patterns and ovarian function before and 3 months after bipolar electrocauterization of the fallopian tubes found no evidence for an adverse effect on menstrual characteristics or ovarian reserve (as assessed by basal FSH concentrations).80 These data suggest strongly that women who have a tubal sterilization procedure are no more likely than other women to have menstrual abnormalities.
between poststerilization menstrual changes and the amount of tissue destroyed.76, 78 Analysis of data from the U.S. Collaborative Review of Sterilization, a multicenter prospective cohort study that followed almost 10,000 women for up to 5 years after a tubal sterilization procedure, revealed that sterilized women were no more likely than women with sterilized male partners to report persistent changes in intermenstrual bleeding or cycle length.79 Sterilized women were more likely to have decreased menstrual duration, volume, and pain, and among women with heavy bleeding at baseline, those sterilized were more likely to report decreased menstrual bleeding after the procedure.79 Another more recent study of menstrual patterns and ovarian function before and 3 months after bipolar electrocauterization of the fallopian tubes found no evidence for an adverse effect on menstrual characteristics or ovarian reserve (as assessed by basal FSH concentrations).80 These data suggest strongly that women who have a tubal sterilization procedure are no more likely than other women to have menstrual abnormalities.
Diagnostic Evaluation of Abnormal Uterine Bleeding
A careful history and physical examination are the most useful tools for differentiating anovulatory bleeding from other causes. The details of the history and the physical findings narrow the number of possibilities meriting serious consideration and define the scope and content of the evaluation required to establish a diagnosis. The history should seek to define each of the following characteristics:
Intermenstrual interval (number of days, regularity)
Volume (heavy, light, or variable)
Duration (normal or prolonged, consistent or variable)
Onset of abnormal menses (perimenarcheal, sudden, gradual)
Temporal associations (postcoital, postpartum, post-pill, weight gain or loss)
Associated symptoms (premenstrual molimina, dysmenorrhea, dyspareunia, galactorrhea, hirsutism)
Underlying systemic illness (renal, hepatic, hematopoietic, thyroid)
Medications (hormonal, anticoagulants)
In the majority of women with true anovulatory bleeding, the menstrual history alone can establish the diagnosis with sufficient confidence that treatment can begin without additional laboratory evaluation or imaging. Infrequent, irregular, unpredictable menstrual bleeding that varies in amount, duration, and character and is not preceded by any recognizable or consistent pattern of premenstrual molimina or accompanied by any visible or palpable genital tract abnormality is not difficult to interpret. Conversely, regular monthly periods that are heavy or prolonged are more likely related to an anatomical lesion or a bleeding disorder than to anovulation.
Objective methods for measuring menstrual blood loss include the photometric alkaline hematin test (the gold standard for research purposes),81, 82 and menstrual pictograms (illustrations of blood stains of different size on feminine hygiene products),83 both of which provide an accurate means of quantifying menstrual blood loss.84 However, the most practical approach is the menstrual history. Although subjective, a history of changing pads or tampons more often than every 3 hours, use of more than 20 over a single menses, the need to change protection during the night, the passage of clots larger than an inch in diameter, menses lasting longer than 7 days, and diagnosis of anemia indicate abnormally heavy menstrual bleeding.26 Regardless of the actual amount of blood loss, menstrual bleeding that interferes with daily activities or causes anxiety and concern merits evaluation.
Midcycle bleeding may be an occasional consequence of the transient but abrupt fall in estrogen levels that occurs at the time of ovulation, but women who have recurrent episodes of intermenstrual bleeding often have intrauterine pathology and deserve evaluation.
Physical examination should aim first at establishing the source of bleeding when that is uncertain. Although most abnormal genital bleeding comes from the uterine corpus, other sources should be excluded, particularly in women whose bleeding is unrelated to the menstrual cycle. Extrauterine sources for abnormal bleeding include the urethra (urethritis), bladder (urinary tract infections, cancers), the vagina (vaginitis and ulcerative lesions), the cervix (ectropion, cervicitis, polyps, focal lesions), the vulva (trauma, skin lesions), and the anus and rectum (anal fissures, hemorrhoids, inflammatory bowel disease, cancers). Examination also should define uterine size (normal or enlarged), contour (smooth and symmetrical or irregular), consistency (firm or soft), and tenderness.
Laboratory Evaluation
Laboratory tests can be very helpful but are not always necessary. A sensitive urine or serum pregnancy test can quickly exclude the possibility that abnormal bleeding relates to a complication of pregnancy; a positive test leaves only a few diagnostic possibilities, which generally are not difficult to distinguish. A complete blood count to exclude anemia and thrombocytopenia is prudent in all women with complaints of abnormal bleeding, especially when heavy or prolonged.
After excluding pregnancy, the most important question to answer is whether the patient is ovulating, because the causes and clinical management of ovulatory and anovulatory uterine bleeding are quite different. When the menstrual history alone does not allow a confident conclusion, a well timed serum progesterone determination during the putative luteal phase of the cycle can help to document ovulation or anovulation. A logical strategy is to obtain the test between cycle day 22 and 24, after ovulation in the longest normal cycle and before the end of the shortest normal cycle; any value greater than 3 ng/mL provides reliable evidence that ovulation has occurred recently.85 However, when bleeding episodes are frequent or poorly documented, proper timing for a progesterone measurement can be difficult to determine. It also is important to remember that many women with abnormal bleeding, especially perimenarcheal and perimenopausal women, ovulate at least occasionally. Although most commonly applied to assess ovulatory function in women with infertility and now seldom used even for that purpose, basal body temperature recordings can be very informative in women with a confusing pattern of bleeding. Endometrial biopsy also can be used to assess ovulatory function (proliferative vs. secretory endometrium) but cannot be justified for that purpose alone when a less costly and less invasive serum progesterone measurement provides the same qualitative information; endometrial sampling should be reserved for those at risk for endometrial hyperplasia or neoplasia, as discussed below.
In sexually active women, a nucleic acid based test for chlamydia and gonorrhea and a wet prep to exclude trichomonas infection merit consideration, particularly in those with evidence of vaginitis and/or cervicitis. In presumed or proven anovulatory women, a serum thyroid-stimulating hormone (TSH) level excludes any associated thyroid disorder. Liver or renal function tests are indicated only for those with known or strongly suspected disease.
Adolescents, women with a suspicious personal or family history of bleeding symptoms (easy bruising, frequent gum bleeding when flossing or brushing teeth, epistaxis), and women with unexplained menorrhagia warrant evaluation with coagulation studies to exclude coagulopathies, such as von Willebrand disease, factor deficiencies, and platelet function abnormalities.66, 68, 86, 87 In addition to a platelet count, screening should include
both a prothrombin (PT), which evaluates the extrinsic and final common clotting pathways, and an activated partial thromboplastin time (aPTT), which tests the intrinsic and common pathways of coagulation. Although the PT and aPTT have relatively low positive and negative predictive value for detecting underlying bleeding disorders,88 they are adequate screens for severe factor deficiencies.89 The high prevalence of von Willebrand disease among women with menorrhagia (approximately 13%) warrants specific exclusion of the diagnosis and justifies measurement of von Willebrand factor, ristocetin cofactor activity (von Willebrand factor activity), the factor VIII level, and blood typing.87, 90, 91 It is important to note that test results can fluctuate over time,92 and also may vary across the menstrual cycle; repeated testing, ideally during the first few days of the cycle, may be required to establish the diagnosis of von Willebrand disease.90,93 The blood type is helpful because von Willebrand factor and factor VIII levels are 25% lower in patients with type O blood than in those with other blood types.94 Although the bleeding time is the traditional method for evaluating platelet function, an automated laboratory test (Platelet Function Analyzer, PFA-100) is taking its place because it has greater sensitivity and reproducibility and is less invasive.95, 96 The instrument exposes platelets in citrated whole blood to high shear inside a capillary tube and monitors the drop in flow rate as the platelets form a plug in the center of a membrane coated with collagen and either adenosine diphosphate or epinephrine. For patients with abnormal coagulation studies, consultation with a hematologist is recommended.97, 98
both a prothrombin (PT), which evaluates the extrinsic and final common clotting pathways, and an activated partial thromboplastin time (aPTT), which tests the intrinsic and common pathways of coagulation. Although the PT and aPTT have relatively low positive and negative predictive value for detecting underlying bleeding disorders,88 they are adequate screens for severe factor deficiencies.89 The high prevalence of von Willebrand disease among women with menorrhagia (approximately 13%) warrants specific exclusion of the diagnosis and justifies measurement of von Willebrand factor, ristocetin cofactor activity (von Willebrand factor activity), the factor VIII level, and blood typing.87, 90, 91 It is important to note that test results can fluctuate over time,92 and also may vary across the menstrual cycle; repeated testing, ideally during the first few days of the cycle, may be required to establish the diagnosis of von Willebrand disease.90,93 The blood type is helpful because von Willebrand factor and factor VIII levels are 25% lower in patients with type O blood than in those with other blood types.94 Although the bleeding time is the traditional method for evaluating platelet function, an automated laboratory test (Platelet Function Analyzer, PFA-100) is taking its place because it has greater sensitivity and reproducibility and is less invasive.95, 96 The instrument exposes platelets in citrated whole blood to high shear inside a capillary tube and monitors the drop in flow rate as the platelets form a plug in the center of a membrane coated with collagen and either adenosine diphosphate or epinephrine. For patients with abnormal coagulation studies, consultation with a hematologist is recommended.97, 98
Endometrial Sampling
An endometrial biopsy can exclude endometrial hyperplasia or cancer. Age over 35 or 40 years is widely considered a risk factor for endometrial disease and cited as an indication for biopsy in women with abnormal bleeding. Endometrial hyperplasia and cancer are more commonly detected in older than in younger women, but the duration of exposure to unopposed estrogen stimulation is the more critical risk factor. Longterm exposure is more likely in older than in younger women, but women under age 30, and even teenagers, can develop endometrial cancer.99,100,101 and 102 In premenopausal women, the likelihood of abnormal endometrial histology is relatively high (14%) when menses are irregular, but very low (< 1%) when cycles are regular.103 The small flexible suction cannulas now widely available cause less discomfort than older traditional biopsy instruments and yield comparable results.104,105 and 106 Unfortunately, hospital-based curettage without hysteroscopy is still commonly performed, even though it is no longer the gold standard.
In addition to revealing any intrinsic endometrial disease, such as chronic endometritis, hyperplasia, or adenocarcinoma, biopsy can help to direct further evaluation or to guide the choice of treatment in women with a confusing history of abnormal bleeding. An inactive or atrophic endometrium identifies women unlikely to respond to progestational therapy. In women with no recent exposure to exogenous progestins, a secretory endometrium provides reliable evidence of recent ovulation and signals the need to search for an anatomical cause.
Imaging
Imaging can help to differentiate anovulatory bleeding from anatomical causes, myomas and endometrial polyps being the most common examples. Standard transvaginal ultrasonography can provide accurate information about the size and location of any uterine fibroids that may explain abnormal bleeding or exaggerate the bleeding due to other causes.107
Ultrasonography may reveal an obvious cavitary lesion or an abnormally thin or thick endometrium. A very thin endometrial “stripe” (<5 mm), like a biopsy that yields minimal tissue, suggests an attenuated or denuded endometrium best treated first with estrogen rather than with a progestin or an estrogen-progestin combination (discussed below).
In perimenopausal and postmenopausal women with abnormal bleeding, endometrial biopsy generally is considered unnecessary when the endometrial thickness is less than 4 or 5 mm because the risk of endometrial hyperplasia or cancer is remote.108,109 and 110 It seems logical to apply the same criterion for the same reason in premenopausal women with abnormal bleeding, although there is no substantial direct evidence to support the extrapolation. Otherwise, the decision to biopsy or not should be based primarily on clinical suspicion and risk factors rather than on ultrasonographic measurements of endometrial thickness. That does not mean that endometrial thickness has no bearing on the decision whether to perform a biopsy; a grossly increased endometrial thickness (>12 mm) increases the risk of disease and is an indication for sampling, even when clinical suspicion of pathology is otherwise low.111 In summary, we believe that biopsy is unnecessary when the endometrial thickness is less than 5 mm, that biopsy is indicated when the clinical history suggests long-term unopposed estrogen exposure even when the endometrial thickness is “normal” (5-12 mm), and that biopsy should be performed when endometrial thickness is greater than 12 mm even when clinical suspicion of disease is low.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


