Abdominal Trauma
Steven Stylianos
Barry A. Hicks
Columbia University College of Physicians and Surgeons and Babies, and Children’s Hospital of New York, New York 10032.
University of Texas-Southwestern School of Medicine and Children’s Medical Center of Dallas, Dallas, Texas 75235.
The development of pediatric expertise within regional trauma systems has led to advances in triage and transport of seriously injured children to facilities prepared for the unique challenges of these patients (1). Recent review of the National Pediatric Trauma Registry (NPTR) indicate that 8% to 12% of children suffering blunt trauma have an abdominal injury (2). Fortunately, more than 90% of them survive. Only 22% of the deaths in the NPTR were related to the abdominal injury.
The treatment of children with major abdominal injuries has changed significantly since the mid-1980s. Surgical restraint has been the theme in recent years and increased awareness of the anatomic patterns and physiologic responses characteristic of pediatric trauma has resulted in the successful nonoperative treatment of many abdominal solid organ injuries. Our colleagues in adult trauma care have acknowledged this success and applied many of the principles learned in pediatric trauma to their patients (3).
Historically, trauma surgeons unfamiliar with the nonoperative management of solid organ injuries often raised doubts about the wisdom of this approach. Their concerns included the potential for increased transfusion requirements, increased length of hospital stay, and missed associated injuries. Some even questioned the need for involvement of pediatric surgeons in pediatric trauma care. The clinical experience accumulated over the past 20 to 30 years, which has settled this controversy, is outlined as follows.
Few surgeons have extensive experience with massive abdominal solid organ injury requiring immediate surgery. It is imperative that surgeons familiarize themselves with current treatment algorithms for life-threatening abdominal trauma. Important contributions have been made in the diagnosis and treatment of children with abdominal injury by radiologists and endoscopists. The resolution and speed of computed tomography (CT), screening capabilities of focused abdominal sonography for trauma (FAST), and the percutaneous, angiographic, and endoscopic interventions of nonsurgeon members of the pediatric trauma team have all enhanced patient care and improved outcomes. This chapter focuses on the more common blunt injuries and unique aspects of care in children. The readers are referred to standard trauma texts for detailed discussion of penetrating injuries and operative maneuvers (4).
DIAGNOSTIC MODALITIES
The initial evaluation of the acutely injured child is similar to that of the adult. Plain radiography of the cervical spine, chest, and pelvis are obtained following the initial survey and evaluation of A (airway), B (breathing), and C (circulation). Plain abdominal films offer little in the acute evaluation of the pediatric trauma patient. In a child with a suspected intraabdominal injury, treatment algorithms have changed significantly as imaging modalities have improved. Prompt identification of potentially life-threatening injuries is now possible in the vast majority of children.
Computed Tomography
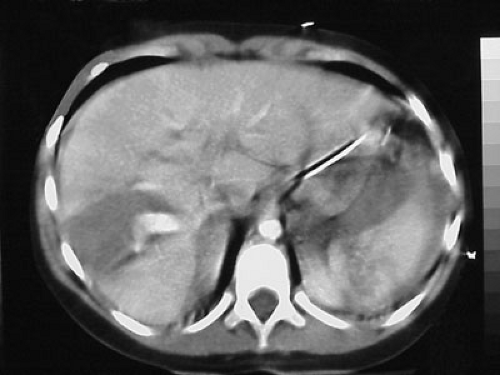
CT has become the imaging study of choice for the evaluation of injured children. CT is readily accessible in most health care facilities, is noninvasive, is a very accurate method of identifying and qualifying the extent of abdominal injury, and has reduced the incidence of non-therapeutic exploratory laparotomy (Fig. 26-1).
Use of intravenous contrast is essential and utilization of “dynamic” methods of scanning have optimized vascular and parenchymal enhancement. The finding of a contrast “blush” on CT in children with blunt liver injury has been associated with larger transfusion requirements and a higher morality rate in a small series (5). A head CT,
if indicated, should be performed first without contrast, to avoid contrast concealing a hemorrhagic brain injury. Enteral contrast for enhancement of the gastrointestinal tract is generally not required in the acute trauma setting and can lead to aspiration (6,7).
if indicated, should be performed first without contrast, to avoid contrast concealing a hemorrhagic brain injury. Enteral contrast for enhancement of the gastrointestinal tract is generally not required in the acute trauma setting and can lead to aspiration (6,7).
Not all children with potential abdominal injuries are candidates for CT evaluation. Obvious penetrating injury often necessitates immediate operative intervention. The hemodynamically unstable child should not be taken out of an appropriate resuscitation room for a CT. These children may benefit from an alternative diagnostic study, such as a diagnostic peritoneal lavage, focused abdominal sonogram, or urgent operative intervention. The greatest limitation of abdominal CT scanning in trauma is the lack of ability to reliably identify intestinal rupture (8,9). Findings suggestive but not diagnostic of intestinal perforation are pneumoperitoneum, bowel wall thickening, free intraperitoneal fluid, bowel wall enhancement, and dilated bowel (10). A high index of suspicion should exist for the presence of a bowel injury in the child with intraperitoneal fluid and no identifiable solid organ injury on CT scanning (11).
Focused Abdominal Sonography for Trauma
Clinician-performed sonography for the early evaluation of the injured child is currently being evaluated to determine its optimal use. Examination of Morrison’s pouch, the pouch of Douglas, the left flank to include the perisplenic anatomy, and a subxiphoid view to visualize the pericardium is the standard four-view FAST exam. This bedside examination may be useful as a rapid screening study, particularly in those patients too unstable to undergo an abdominal CT scan. Early reports have found FAST to be a useful screening tool in children, with a high specificity (95%), but a low sensitivity (33%) in identifying intestinal injury (12). A lack of identifiable free fluid does not exclude a significant injury. FAST may be very useful in decreasing the number of CT scans performed for “low-likelihood” injuries. The study may need to be repeated, dependent on clinical correlation and the finding of free fluid in itself is not an indication for surgical intervention. A FAST scan with no abnormality noted may preclude the need for other abdominal imaging studies; a positive FAST scan may necessitate other imaging studies or laparotomy.
Angiography
Angiography is rarely required today in the evaluation of the acutely injured child. New generation CT scanners are capable of identifying sites of bleeding and injuries that in the past were seen only with angiography. The role of the angiographer in pediatric abdominal trauma is generally reserved for therapeutic embolization of solid organ or pelvic bleeding, and is discussed in later sections of this chapter.
Diagnostic Peritoneal Lavage
Since its description in 1965, the use of diagnostic peritoneal lavage (DPL) has diminished significantly, especially in the pediatric trauma population, because the availability of high-resolution CT scanners and use of nonoperative treatment have increased. Although very accurate for intraabdominal hemorrhage, retroperitoneal injuries may be missed. Also, and perhaps more important, a positive DPL, may lead to nontherapeutic laparotomy. Because the majority of solid organ injuries do not require surgical intervention, intraperitoneal blood identified by DPL has little clinical significance. The need for operative management is determined by clinical instability and the requirement for ongoing blood replacement. Incisional pain may also interfere with following serial examinations in a child being managed nonoperatively following a negative DPL.
There are clinical situations in which a DPL may prove to be very useful. A hemodynamically unstable child may rapidly undergo a DPL to rule out the abdomen as a source of blood loss. Children with lap-belt injury pose diagnostic challenges, particularly if a concomitant neurologic injury is present. The initial abdominal CT is frequently normal in those with lap-belt injury, but a DPL may document the presence of an occult hollow viscus injury with the return of bile, bacteria, feculent matter, or an increased white blood cell (WBC) count. Infusion of 10 mL per kg normal saline into the peritoneal cavity is followed by allowing the infusate to drain. Criteria for a positive lavage include 10 mL gross blood return with lavage catheter insertion, greater than 100,000 red blood cells (RBCs) per mm3, greater than 500 WBCs per mm3, an amylase greater than 175 IU per dL, or the presence of bile, bacteria, or vegetable matter on microscopic examination.
Diagnostic and Therapeutic Laparoscopy
Laparoscopy of the injured child may have its place in the evaluation of the hemodynamically stable patient. The sensitivity is comparable to DPL, but the specificity is higher, as one would expect by actually visualizing the injury (13). A decrease in the number of nontherapeutic laparotomies has been demonstrated in adult series (14). Studies have also shown that not only may the traumatic injury be identified with laparoscopy, but also the definitive repair may frequently be performed (15).
SOLID ORGAN INJURY
Spleen and Liver
The spleen and liver are the organs most commonly injured in blunt abdominal trauma, with each accounting for one-third of the injuries. Nonoperative treatment of isolated splenic and hepatic injuries in stable children is now standard practice. Although nonoperative treatment of children with isolated, blunt spleen or liver injury has been universally successful, there has been great variation in the management algorithms used by individual pediatric surgeons. Review of the NPTR and surveys of the American Pediatric Surgical Association (APSA) membership confirm the wide disparity in practice (16,17). Controversy also exists regarding the utility of CT grading as a prediction of outcome in liver and spleen injury (18,19,20). More recently, the APSA Trauma Committee has defined consensus guidelines for resource utilization in hemodynamically stable children with isolated liver or spleen injury based on CT grading by analyzing a contemporary, multiinstitution database of 832 children treated nonoperatively at 32 centers in North America from 1995 to 1997 (Table 26-1) (21). Consensus guidelines on intensive care unit (ICU) stay, length of hospital stay, use of follow-up imaging, and physical activity restriction for clinically stable children with isolated spleen or liver injuries (grades I to IV) were defined by analysis of this database (Table 26-2).
TABLE 26-1 Resource Utilization and Activity Restriction in 832 Children with Isolated Spleen or Liver Injury. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 26-2 Proposed Guidelines for Resource Utilization in Children with Isolated Spleen or Liver Injury. | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
The guidelines were then applied prospectively in 312 children with liver or spleen injuries treated nonoperatively at 16 centers from 1998 to 2000 (22). Patients with other minor injuries such as nondisplaced, noncomminuted fractures or soft tissue injuries were included as long as the associated injuries did not influence the variables in this study. The patients were grouped by severity of injury defined by CT grade. Compliance with the proposed guidelines was analyzed for age, organ injured, and injury grade. All patients were followed for 4 months after injury. It is imperative to emphasize that these proposed guidelines assume hemodynamic stability. The extremely low rates of transfusion and operation document the stability of the study patients.
There were no differences in compliance with the proposed guidelines by age, gender, or organ injured. Deviation from guidelines was the surgeon’s choice in 90% and patient related in 10%. Six (1.9%) patients were readmitted, although none required operation. Compared with the previously studied 832 patients, the 312 patients managed prospectively by the proposed guidelines had a significant reduction in ICU stay, hospital stay, follow-up imaging, and interval of physical activity restriction within each grade of injury.
From these data, we concluded that prospective application of specific treatment guidelines based on injury severity resulted in conformity in patient management, improved utilization of resources, and validation of guideline safety.
The attending surgeon’s decision to operate for spleen or liver injury is best based on evidence of continued blood loss, such as low blood pressure, tachycardia, decreased urine output, and falling hematocrit unresponsive to crystalloid and blood transfusion. The rates of successful nonoperative treatment of isolated blunt splenic and hepatic injury now exceed 90% in most pediatric trauma centers and adult trauma centers with strong pediatric commitment (21,22,23,24). A more recent study of more than 100 patients from the NPTR indicated that nonoperative treatment of spleen or liver injury is indicated, even in the presence of associated head injury, if the patient is hemodynamically stable (25). Rates of operative intervention for blunt spleen or liver injury were similar with or without an associated closed head injury.
Surgeons unfamiliar with current treatment algorithms for blunt splenic injuries in children occasionally question the nonoperative approach. This is important because the majority of seriously injured children are treated outside dedicated pediatric trauma centers. Although several adult trauma services have reported excellent survival rates for pediatric trauma patients, analysis of treatment for spleen and liver injuries reveals an alarmingly high rate of operative treatment (23,26,27,28). It is possible that trauma surgeons, influenced by their past experience with adult patients, are more likely to favor operative treatment than their pediatric surgical colleagues. Adult trauma surgeons caring for injured children must consider the anatomic, immunologic, and physiologic differences between pediatric and adult trauma patients, and incorporate these differences into their treatment protocols. The major concerns are related to the potential risks of increased transfusion requirements, missed associated injuries, and increased length of hospital stay. Each concern has been shown to be without merit (17). In addition, adult trauma surgeons may cite that in their experience they have never had a patient who underwent splenectomy who subsequently developed postsplenectomy sepsis or died due to sepsis. However, many trauma surgeons do not have long-term follow-up of their patients. Thus, an adverse result in postsplenectomy sepsis 2 years after splenectomy may never be reported back to the surgeon.
Missed Associated Abdominal Injuries
Advocates of surgical intervention for splenic trauma cite their concern about missing associated abdominal injuries if no operation is performed. Morse and Garcia reported successful nonoperative treatment in 110 of 120 children (91%) with blunt splenic trauma, of whom 22 (18%) had associated abdominal injuries. Only 3 of these 120 patients (2.5%) had gastrointestinal injuries, and each was found at early celiotomy done for a specific indication. There was no morbidity from missed injuries or delayed surgery. Similarly, a review of the NPTR from 1988 to 1998 revealed 2,977 patients with solid abdominal visceral injury; only 96 (3.2%) had an associated hollow viscus injury. Higher rates of hollow viscus injury were observed in assaulted patients and those with multiple solid visceral injury or pancreatic injury. Differences in mechanism of injury may account for the much lower incidence of associated abdominal injuries in children with splenic trauma. There is no justification for an exploratory celiotomy solely to avoid missing potential associated injuries in children.
Complications of Nonoperative Treatment
Nonoperative treatment protocols have been the standard for most children with blunt liver and spleen injury since the mid-1980s. The cumulative experience gained allows us to evaluate both the benefits and risks of the nonoperative approach. Fundamental to the success of the nonoperative strategy is the early, spontaneous cessation of hemorrhage. Transfusion rates for children with isolated spleen or liver injury have fallen below 10%, confirming the lack of continued blood loss in the majority of patients (20,21,22,23,24,29). Despite many favorable observations, isolated reports of significant delayed hemorrhage with adverse outcome continue to appear (30,31,32,33). Shilyansky et al. reported two children with delayed hemorrhage 10 days after blunt liver injury (32). Both children had persistent right upper quadrant (RUQ) and right shoulder pain, despite normal vital signs and stable hematocrits. The authors recommended continued inhouse observation until symptoms resolve. More recent reports described patients with significant bleeding 38 days after grade II spleen injury and 24 days after grade IV liver injury (31,33). These rare occurrences create anxiety in identifying the minimum safe interval prior to resuming unrestricted activities.
Sequelae of Damage Control Strategies
The vast majority of solid organ injuries can be treated without surgery if there is prompt response to resuscitation (34). In patients who are hemodynamically unstable, despite fluid and packed RBC transfusion, emergency laparotomy is indicated. Most spleen and liver injuries requiring operation are amenable to simple methods of hemostasis, using a combination of manual compression, direct suture, and topical hemostatic agents. In young children with significant hepatic injuries, the sternum can be divided rapidly to expose the suprahepatic or intrapericardial IVC. Children will tolerate clamping of the inferior vena cava (IVC) above the liver as long as their blood volume is replenished. With this exposure, the liver and
major perihepatic veins can be isolated and the bleeding controlled to permit direct suture repair or ligation of the offending vessel.
major perihepatic veins can be isolated and the bleeding controlled to permit direct suture repair or ligation of the offending vessel.
The early morbidity and mortality of severe hepatic injuries are related to the effects of massive blood loss and replacement with large volumes of cold blood products. The consequences of prolonged operations with massive blood product replacement include hypothermia, coagulopathy, and acidosis. Although the surgical team may keep pace with blood loss, life-threatening physiologic and metabolic consequences are inevitable, and many of these critically ill patients are unlikely to survive once their physiologic reserves have been exceeded. A multiinstitutional review identified exsanguination as the cause of death in 82% of 537 intraoperative deaths at 8 academic trauma centers (35). The mean pH was 7.18 and mean core temperature was 32°C prior to death. Moulton reported survival in only 5 of 12 (40%) consecutive operative cases of retrohepatic vascular or severe parenchymal liver injury in children (36).
Maintenance of physiologic stability during the struggle for surgical control of severe bleeding is a formidable challenge, even for the most experienced operative team, particularly when hypothermia, coagulopathy, and acidosis occur. This triad creates a vicious cycle in which each derangement exacerbates the others, and the physiologic and metabolic consequences of the triad often preclude completion of the procedure. Lethal coagulopathy from dilution, hypothermia, and acidosis can rapidly occur (37).
Increased emphasis on physiologic and metabolic stability in emergency abdominal operations has led to the development of staged, multidisciplinary treatment plans, including abbreviated laparotomy, perihepatic packing, temporary abdominal closure, angiographic embolization, and endoscopic biliary stenting (38,39,40). Asensio et al. reported 22 patients with grade IV or V hepatic injuries treated between 1992 and 1997 (41). Mean blood loss was estimated at 4.6 L and mean packed red cell transfusion was 15 units. Ten patients underwent packing of the hepatic injuries at the first operation. Fifteen patients had postoperative angiographic embolization in an attempt to control hemorrhage. Survival was 92% in 13 grade IV patients and 78% in 9 grade V patients.
Abbreviated laparotomy with packing for hemostasis, allowing resuscitation prior to planned reoperation, is an alternative in unstable patients where further blood loss would be untenable. This “damage control” philosophy is a systematic, phased approach to the management of the exsanguinating trauma patient (42,43,44). The three phases of damage control are detailed in Table 26-3. Although controversial, several resuscitative end points have been proposed beyond the conventional vital signs and urine output, including serum lactate, base deficit, mixed venous oxygen saturation, and gastric mucosal pH. Once patients are rewarmed, coagulation factors replaced, and oxygen delivery optimized, the patient can be returned to the operating room for pack removal and definitive repair of injuries. Review of nearly 700 adult patients treated by abdominal packing from several institutions demonstrated hemostasis in 80%, survival of 32% to 73%, and abdominal abscess rates of 10% to 40% (45,46). Although abdominal packing (PACKS) with planned reoperation has been used with increasing frequency in adults since the mid-1980s, there is little published experience reported in children (47,48,49,50,51,52,53,54). Nevertheless, we believe this technique has a place in the management of children with massive intraabdominal bleeding, especially after blunt trauma.
TABLE 26-3 “Damage Control” Strategy in the Exsanguinating Trauma Patient.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|