- Placental transfusion
- Anaemia
- Hydrops fetalis
- Aplasia
- Polycythaemia
- Bleeding and coagulation disorders
- Thrombocytopenia
- Haemorrhagic disease of the newborn
- Disseminated intravascular coagulation
- Inherited disorders of coagulation
- Congenital deficiency of anticoagulant proteins (hypercoagulable states)
Introduction
Haematological disorders in the fetus and newborn infant arise from the conditions which primarily affect blood constituents such as red blood cells (RBCs), haemoglobin and platelets. Some of the conditions are inherited and amenable to prenatal diagnosis or screening at birth. Symptoms in affected infants may start either in utero or after birth. Diagnosis of some of the haematological conditions may be complex, requiring interpretation by a paediatric haematologist. Treatment includes prevention and specific therapies. It should be borne in mind that the normal haematological indices in newborns are different from those in older children and vary according to postnatal age.
Placental Transfusion
The blood volume and red cell mass at birth and in the neonatal period depend on the volume of the placental transfusion and subsequent readjustments of blood volume.
This occurs within 3 min of delivery and can contribute up to 25% of the total neonatal blood volume. This amount will be increased in the following situations:
- elevated maternal blood pressure
- use of oxytocic drugs
- late clamping or milking of the cord
- infant held in a low, dependent position at birth.
On the other hand, the amount will be reduced by early cord clamping, or holding the infant above the level of the attached placenta.
The average blood volume of a newborn infant is 85–90 mL/kg, but ranges from 75 to 100 mL/kg. The practice of delay in clamping the umbilical cord or milking the cord from the placenta to the baby may have both advantages and disadvantages. Although this can result in improved blood volume and reduced iron deficiency in childhood, there may be associated disadvantages too as inadvertently high red cell mass can result in symptomatic pulmonary plethora and hyperbilirubinaemia. This subject of ‘early’ versus ‘late’ (i.e. physiological) clamping of umbilical cord is still a matter of debate. The UK Resuscitation Council recommends delaying cord clamping for 1 min in uncompromised babies.
Anaemia
Anaemia is usually defined by a haemoglobin (or haematocrit) level and classed as mild if the haemoglobin level (Hb) is 10–12, moderate if it is between 8–10 and severe if it is less than 8 g/dL. The causes of neonatal anaemia are shown in Box 20.1 and broadly fall under the following headings:
- physiological
- haemorrhage
- haemolysis
- hypoplasia or aplasia (diminished production).
- Physiological anaemia
- Anaemia of prematurity
- Haemorrhage
Antepartum haemorrhage
Fetomaternal transfusion
Twin-to-twin transfusion
Neonatal internal haemorrhage
- Haemolysis (see Box 20.2)
- Aplasia: Blackfan–Diamond syndrome
Physiological Anaemia
The full-term infant is born with a haemoglobin concentration in the range 15–23.5 g/dL, whereas in the premature infant the haemoglobin level is slightly lower. Initially there is a slight increase due to haemoconcentration, but then haemoglobin gradually drops and remains low for most of the first year of life (Fig. 20.1). This is known as physiological anaemia.
Figure 20.1 Physiological anaemia. The two graphs show the normal fall in haemoglobin with postnatal age in mature and premature infants.
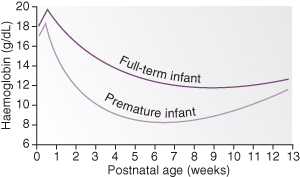
Anaemia of Prematurity
In the preterm infant physiological anaemia occurs earlier, is more severe and prolonged than in the term infant, and is termed anaemia of prematurity. It is caused by a number of factors.
Lack of Erythropoietin
At birth the infant moves from a relatively hypoxic fetal state to become relatively hyperoxic. This suppresses erythropoietin secretion for the first 7–8 weeks of life. In addition the bone marrow is probably more resistant to the stimulatory effect of erythropoietin. When this ends, reticulocytes start appearing in the peripheral blood film.
Repeated Blood Sampling
The preterm infant is often subjected to daily repeated blood sampling for laboratory investigation. This is the commonest cause of anaemia in babies admitted to neonatal units.
Relative Haemodilution
There is an increase in plasma volume over the first months of life and, together with poor red cell production, the haemoglobin falls. This is referred to as ‘early anaemia’.
Iron Deficiency
The full-term infant is born with sufficient iron stores for the first 4 months of life, but in preterm infants these stores are low and exhausted more quickly because of the infant’s rapid growth rate. An infant of 1.5 kg at birth has half the iron stores of a 3.0 kg neonate. Iron deficiency mostly causes the ‘late anaemia’ that accounts for low haemoglobin levels after 4 months of age, characterized by hypochromic red cells seen on a blood film.
Haemolysis
Haemolysis may occur in preterm infants as a result of vitamin E deficiency. Administration of vitamin E may reduce the extent of late anaemia of prematurity but is not used in practice.
Treatment
A daily dose of elemental iron is associated with a good response in most cases, but routine iron supplementation from an early age to ‘prevent’ anaemia is controversial and not practised universally.
Transfusion (10–20 mL/kg) may be necessary in premature infants if the haemoglobin falls below 7–8 g/dL and the infant is symptomatic. Symptoms include breathlessness with feeds, tachycardia, apnoea and bradycardia or failure to gain weight. However, blood transfusion can also suppress erythropoietin activity, and hence if the infant shows a reticulocyte count of more than 5%, transfusion should be delayed if possible, depending on the infant’s condition. Premature infants receiving intensive care do end up receiving repeated blood transfusions. In such situations, exposure to multiple blood donors can be avoided by using the same batch blood from a single donor and stored in mini packs. Donor blood should be screened for blood-borne viruses, particularly cytomegalovirus (CMV).
The following formula may be used to calculate the volume of blood to be transfused for an anaemic infant (Hct is the haematocrit):
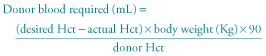
The administration of subcutaneous recombinant human erythropoietin to preterm infants has been shown to stimulate red blood cell production, thereby avoiding the need for frequent blood transfusions. But this treatment has not been shown to be cost-effective and is not widely used.
Haemorrhage
Causes are listed in Box 20.2 and investigations in Box 20.3.
- Haemorrhage before and during delivery from:
- Neonatal haemorrhage:
- Trauma – bleeding may occur into skull, brain, lung, peritoneum or bowel
- Haemorrhagic disease of the newborn
- Trauma – bleeding may occur into skull, brain, lung, peritoneum or bowel
- Haemoglobin and haematocrit
- Kleihauer’s test (to assess the presence of fetal cells in maternal blood, indicating fetomaternal transfusion)
- Coagulation studies (indicated if a bleeding diathesis is suspected)
- Investigations to determine site of bleeding (e.g. ultrasound of head or abdomen)
Treatment
Acute blood loss, if significant, is more likely to present with signs of hypovolaemia and shock as the initial manifestation such as changes in blood pressure, heart rate, tissue perfusion and urine output. In acute haemorrhage, the haemoglobin may be normal in the beginning as enough time has not passed for haemodilution to occur.
Severe haemorrhage may present as a neonatal emergency and require immediate transfusion with blood or blood substitute (such as crystalloid or 4.5% albumin) to prevent irreversible shock. In an emergency, unmatched group O rhesus-negative blood may be used, but formal cross-matching should be done whenever possible. Remember babies rarely die of anaemia; hypovolaemia is much more damaging.
Haemolysis
The causes of neonatal haemolysis are shown in Box 20.4. Haemolysis is usually associated with unconjugated hyperbilirubinaemia and reticulocytosis. Causes of haemolytic anaemia can be broadly divided into two groups; immune and non-immune.
- Rhesus incompatibility
- ABO incompatibility
- Minor blood group incompatibility (e.g. Kell, Duffy, Kidd)
- Maternal autoimmune diseases (e.g. SLE)
- Congenital infection
- Disseminated intravascular coagulation (DIC)
- G6PD deficiency
- Pyruvate kinase deficiency
- Hereditary spherocytosis (HS)
- α-Thalassaemia
- Infantile pyknocytosis
- Vitamin E deficiency
Rhesus Haemolytic Disease
This occurs because the mother’s immune system has been sensitized by rhesus-positive cells from her fetus. Sensitization may be due to
- fetomaternal transfusion (during previous delivery or from miscarriage)
- rhesus-incompatible transfusions.
The rhesus factor is complex, comprising CDE/cde antigens. The commonest antigen is D, and this accounts for 95% of cases. Approximately 83% of the population are D positive, that is, rhesus positive (Rh +ve). If sensitization occurs, maternal immunoglobulin G (IgG) crosses the placenta to cause haemolysis of ‘foreign’ fetal erythrocytes. IgG remains present in the neonatal circulation for up to 3 months and neonatal haemolysis may continue to occur for some weeks after birth.
Prevention
Rh IgG prophylaxis (anti-D gammaglobulin) is indicated in the management of all non-immunized pregnant women who are Rh negative. Current recommendations include the routine administration of IgG at 28 weeks’ gestation to all pregnant women who are RhD negative, and within 72 h of delivery to all rhesus-negative women who give birth to rhesus-positive infants. If antibodies are already present anti-D is not required. The standard dose of 300 µg is sufficient for protection for up to 30 mL of fetal blood. This provides satisfactory prophylaxis for 99% of all term deliveries.
Anti-D gammaglobulin should also be given to at-risk rhesus-negative women after every sensitizing event, such as miscarriage, termination of pregnancy or amniocentesis.
Anti-D gammaglobulin is ineffective against non-D rhesus antigen (usually C, E). If a large transfusion of fetal blood occurs, the standard dose of 300 µg/kg may be insufficient. In women at high risk, a Kleihauer–Berke smear test can be performed to quantitate for the fetal blood present. For every 30 mL of fetal blood detected, an additional 300 µg of IgG can be administered.
Management during Pregnancy
This includes routine testing for antibodies, monitoring of antibodies, assessment of bilirubin in amniotic fluid, monitoring of fetal well-being and monitoring for signs of fetal complications.
- Routine testing. Rhesus-negative women should be screened for rhesus antibodies at their first antenatal visit, and at subsequent visits according to local guidelines. If antibodies are detected at any of these times, more frequent testing will be necessary.
- Antibodies present. If antibodies are present, then depending on the level and/or whether the titre is rising, an amniocentesis should be performed. Amniocentesis is commonly done at 30–32 weeks but may be performed earlier, depending on the level of antibodies, and particularly on the history of previous pregnancies. A Kleihauer test is done before and after amniocentesis. The timing of delivery will depend on the antibody levels, amniocentesis result and previous history of affected infants.
- Assessment of bilirubin in liquor. Fetal rhesus disease is now a relatively rare condition, and affected pregnancies should be managed in recognized regional centres experienced in treating these women and their babies.
- Assessment of fetal well-being. High-resolution ultrasound is an essential adjunct to the assessment of isoimmunized pregnancies. It allows early detection of hydrops, assessment of fetal behaviour (biophysical profile, which provides important evidence of fetal well-being), and interventions such as fetal blood samplings and transfusion. Ultrasound is used to assess the amniotic fluid index, hepatomegaly, subcutaneous oedema, ascites and increases in middle cerebral artery blood flow velocity.
Management of the Rhesus-Immunized Infant
The baby should be assessed for maturity, pallor, jaundice, hepatosplenomegaly, oedema, ascites, ecchymoses, heart failure and respiratory distress. The placenta is examined for the presence of oedema, weighed and sent to the pathology department for confirmation of the diagnosis.
- Investigations at birth. Cord blood is taken for grouping, direct Coombs’ test, haemoglobin and platelet count and total bilirubin estimation. The Coombs’ test is always positive in rhesus incompatibility, unless an intrauterine transfusion with rhesus-negative blood has recently been performed. The more positive the Coombs’ test, the more severely affected the infant is likely to be.
- Indications for exchange transfusion are listed in Box 20.5.
- Cord haemoglobin <8 g/dL* (recent in utero transfusion may lead to falsely reassuring Hb)
- Hydrops fetalis.
- Cord bilirubin >85 µmol/L (5 mg/100 mL)
- Cord haemoglobin 8–12 g/dL
- Rapidly rising serum bilirubin that crosses the level for exchange transfusion on the charts (see Chapter 19)
- A very strongly positive Coombs’ test
Interval exchange transfusion is done to prevent the serum unconjugated bilirubin reaching a potentially dangerous level (e.g. 450 in >38 weeks and 350 at 35 weeks). This is usually carried out as an adjunct to phototherapy, and guideline graphs are useful (see Chapter 19).
Exchange transfusion is performed with warmed (37 °C) whole
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


