- The immune system
- Susceptibility of the neonate to infection
- Congenital infection
- Intrapartum (early-onset) infection
- Postnatal (late-onset) infection
Introduction
Infection may be acquired in utero (congenital), during labour and delivery (intrapartum or early onset) or after birth (postnatal or late-onset). In the neonate, infection can present in many different ways and may involve almost any system in the body. Infection must be considered in almost every differential diagnosis of any condition affecting the newborn. Infection poses a significant risk of mortality and is associated with major morbidity. The incidence of infection is approximately 5 per 1000 live births, and is more common in premature infants.
The Immune System
To allow growth and development, the fetal and neonatal immune system must achieve a number of important tasks, including:
- avoidance of inflammatory responses that can induce alloimmune reactions between mother and fetus
- transition between the normally sterile intrauterine environment to the antigen-rich outside world
- defence against viral and bacterial pathogens
- primary colonization of the skin and intestinal tract by microorganisms.
The immune system develops from early in fetal life, but is not functionally fully integrated until about 1 year of age. Protection is initially provided by physical and chemical barriers in the epithelial and mucous membranes. The next line of defence is provided by the immune system and can be divided into non-specific (or innate) and specific (or adaptive) components. While these components are considered separately, they are integrally related and interact with each other to enhance and fine tune the immune response.
Non-Specific Immunity
Non-specific immunity is the first line of defence against invasive pathogenic organisms in the neonate. It can be further divided into humoral and cellular responses.
- The humoral response includes complement, interferons, lactoferrin and lysozymes. Complement activation causes a cascade of events that leads to lysis of cell membranes. Alpha and beta interferon are proteins produced by cells infected with virus and make other cells resistant to infection. Gamma interferon increases the immune response by increasing killing of intracellular organisms. Lactoferrin (present in human milk) binds iron and reduces growth of E. coli. Lysozymes (found in tears, saliva and neutrophils) have antibacterial activity.
- The cellular response consists primarily of phagocytic white cells (neutrophils, macrophages, monocytes, natural killer cells) which adhere to and ingest bacteria. Enzymes and free oxygen radicals are then released which kill the organism. Phagocytic cells are attracted to sites of infection by chemicals released in the acute inflammatory response.
Specific Immunity
Specific immunity is mediated through antibodies (produced by B lymphocytes) and specific cytotoxic cells (T lymphocytes). When a foreign antigen binds to a specific receptor, either antibody or a specific T lymphocyte is produced. Activation of specific immunity results in immunological memory.
B Lymphocytes
When stimulated, B lymphocytes (or B cells) transform to plasma cells and produce immunoglobulin (Ig). IgM is the first type to be produced at 15 weeks’ gestation, and IgG is first produced at 20 weeks. Initially fetal levels of the three major Ig types are minimal and they remain very low at birth. IgM is normally the first antibody produced in the primary immune response. Its major role is to fix complement. Adult levels of IgM are not normally attained until 5 years of age. IgG is the only immunoglobulin that crosses the placenta. Maternal IgG starts to cross the placenta from as early as 9 weeks but maximum transfer occurs after 32 weeks’ gestation and peaks at term, hence premature infants may miss out on this important antibody transfer. At birth the baby has high levels of maternally derived IgG, giving the neonate effective passive immunity, but the levels fall in the months after birth and are not detectable by 9 months of age. By 2 months of age infants are able to produce a good IgG response, but adult levels are not attained until 7–8 years of age. The physiological nadir in IgG levels occurs between 3 and 6 months of age. IgG leads to bacterial cell lysis by opsonization and complement fixation and also neutralizes viruses and toxins. IgA is produced by secretory cells and is found in mucous membranes and breast milk. Levels rise slowly and may not peak until the teenage years. Its major role is to protect the gastrointestinal tract from infection.
T Lymphocytes
T lymphocytes (or T cells) are produced in the fetal bone marrow and migrate to the thymus – hence the term thymus (or T)-related lymphocytes. They can be divided into T helper (TH) cells and cytotoxic T cells. TH cells are CD4+ and once switched on can develop into TH1 cells or TH2 cells. TH1 cells activate cytotoxic T cells to kill intracellular organisms. TH2 cells secrete cytokines to help B lymphocytes produce antibodies. Cytotoxic T cells are CD8+ and are responsible for killing infected cells and preventing viral replication.
Susceptibility of the Neonate to Infection
A number of differences exist between the neonatal immune system and adult immune systems and these are listed in Box 10.1. In particular, the decreased production of TH1-cell-polarizing cytokines leaves the newborn susceptible to microbial infection and contributes to the impairment of neonatal immune responses to most vaccines. Exogenous factors may also predispose the infant to infection and are also listed in Box 10.1.
- Decreased numbers and function of neutrophils
- Decreased production of tumor necrosis factor (TNF) by monocytes
- Phagocytic action is less effective in the newborn
- Decreased complement levels compared with adults
- Low levels of immunoglobulins, particularly IgM and IgA
- Premature infants fail to receive normal passive IgG transfer during the last trimester of pregnancy
- Decreased levels of the IgG G2 subclass (increases risk of infection with encapsulated organisms)
- Naive T lymphocytes are harder to switch on
- Neonatal lymphocytes do not function as efficiently as mature lymphocytes owing to a reduced production of cytokines.
- T lymphocyte response is predominantly TH2 rather than TH1 which may predispose to listeria and salmonella infection (as they rely on a TH1 response).
- Breaches of the skin barrier such as long lines, cannulas and venepuncture for blood tests may allow entry of bacteria to the baby
- Fat emulsions. Agents such as Intralipid may impair the phagocytic function of the white cells
- The baby is born bacteriologically sterile, with little competition from existing bacterial flora when exposed to potential pathogens. Babies exposed to very early antibiotic use, either as newborns or as fetuses, may be predisposed to colonization with potentially pathogenic organisms
- Drugs may further impair immune function, with corticosteroids being the main offenders
- Hyperbilirubinaemia reduces immune function in several different ways
Congenital Infection
Intrauterine infections may have devastating effects on the fetus. Maternal infection may be completely asymptomatic or have only non-specific symptoms such as fever, malaise, myalgia, headache and nausea. Maternal infection may result in vertical transmission to the fetus. Several congenital infections have a similar clinical picture, and it is convenient to think about, and investigate, the TORCH group as a whole. TORCH is an acronym derived from the first letter of the following conditions: toxoplasmosis, other (e.g. Coxsackie B virus, varicella, HIV), rubella, cytomegalovirus (CMV), herpes simplex.
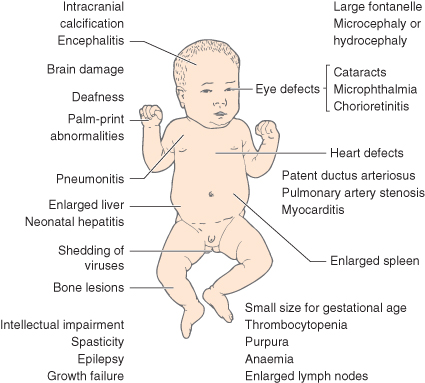
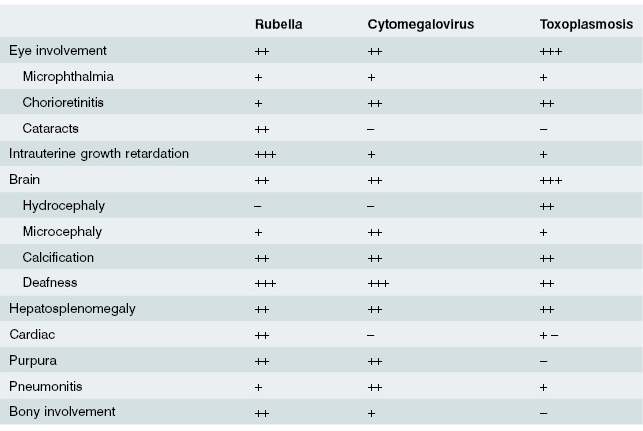
Infection at the embryonic stage (first 12 weeks) may lead to multiple abnormalities (Fig. 10.1). With infection occurring later, the baby may be born with a viraemia and may have neonatal illness associated with jaundice, enlarged liver and spleen, anaemia and thrombocytopenia. The predominant features of the three most common prenatal infections are shown in Table 10.1.
Table 10.1 Principles of management of congenital infection
| Organism | Maternal treatment | Infant treatment |
| CMV | Ganciclovir for 3 months | |
| Toxoplasmosis | Spiramyicin and sulfadiazine or pyrimethamine + sulfadiazine | Spiramycin alternating with pyrimethamine + sulfadiazine for 1 year |
| Syphilis | Penicillin | Penicillin for 10 days |
| Hepatitis B | Vaccination at birth, 1 and 6 months | |
| HIV | Antiretroviral treatment from 28 weeks | Oral zidovudine for 4–6 weeks Deliver by elective caesarean section Avoid breastfeeding |
| Varicella | ZIG and, if vesicles, start aciclovir |
The investigation of infants with suspected congenital infections should include:
- review of maternal history for immunization and exposure to infectious agents
- serological tests, including quantitative IgM and specific antibody serology for TORCH infections, and polymerase chain reaction (PCR) to amplify DNA particles
- urine for CMV PCR
- throat and nose swabs, cerebrospinal fluid (CSF) and faeces for viral culture
- cranial ultrasound and/or MRI to detect intracranial calcification in toxoplasmosis, CMV and rubella, and radiographs of long bones to show periostitis in syphilis and viral osteopathy in rubella and CMV
- ophthalmological examination to detect chorioretinitis (toxoplasmosis, CMV) and cataracts, retinitis and microphthalmia in rubella.
Cytomegalovirus
CMV is the commonest congenital infection and may affect as many as 0.3 to 2% of live births in Australia. In primary maternal CMV infection there is a 50% risk of vertical transmission and in secondary maternal infection the risk of transmission is 1–13% depending on the length of time elapsed since the primary infection (higher risk with duration <4 years). Only 10% of congenitally infected infants will develop symptoms in primary maternal infection and in secondary maternal infection less than 1% will develop symptoms. The risk of serious adverse neurological outcome increases if primary infection occurs in the first half of pregnancy.
Signs and symptoms of congenital CMV infection include small for gestational age, thrombocytopenia, seizures, hepatomegaly, splenomegaly, pneumonia, pleural effusions, pericardial effusions, intra-abdominal calcifications, ascites, hydrops fetalis, intracranial calcifications, microcephaly, hydrocephalus, sensory neural hearing loss, and chorioretinitis. Mortality rates for symptomatic congenital CMV are 10–30%.
CMV acquired postnatally is an important source of this condition and may be contracted from caregivers, infected blood (all blood products should be screened for CMV) and breast milk.
In infants with symptomatic congenital CMV there is preliminary evidence that intravenous ganciclovir started within 28 days of birth may improve neurological outcome.
Rubella
Because of high immunization rates, congenital rubella syndrome currently affects less than 50 children each year in the UK. When maternal infection occurs before 8 weeks of pregnancy, 80–90% of infants will have symptoms; this rate falls to 50% if infection occurs at 8–12 weeks and to 20% if infection occurs between 12 and 20 weeks. The risk of congenital infection is less than 1% after 20 weeks’ gestation. The risk of a fetus being damaged as a result of inadvertent rubella vaccination given to a pregnant woman is very small. Clinical features of congenital rubella are highlighted in Table 10.2.
Table 10.2 Relative clinical features of prenatal rubella, cytomegalovirus and toxoplasmosis
Toxoplasmosis
Toxoplasmas are protozoal organisms that rarely cause congenital infection in the UK and Australia; such infection is considerably more common in France. Infection may be prevented by avoiding raw/undercooked meat, washing hands after gardening and washing raw vegetables. Fetal infection after maternal seroconversion may be diagnosed by PCR or serological testing on fetal blood obtained by cordocentesis. Maternal treatment is with sulfadoxine and pyrimethamine alone or alternating with spiramycin (not before 16 weeks’ gestational age). Maternal infection in the first trimester has low risk of transmission (5–15%) but a high risk of damage to the fetus if it occurs (60–80%). Maternal infection in the second trimester has a 25–40% risk of vertical transmission and a 15–25% risk of damage to the baby. Maternal infection in the last trimester of pregnancy has a high risk of vertical transmission but a low risk (2–10%) of damage to the baby. The classical features are the triad of chorioretinitis, hydrocephalus and periventricular calcification. Even if asymptomatic at birth, signs and symptoms may not appear for several years.
The congenitally infected baby (even if asymptomatic) should be treated with with pyrimethamine and sulfadiazine (plus folinic acid) continuously or alternating with spiramycin.
Syphilis
The incidence of this condition has increased recently in developed countries. Congenital infection is seen if maternal infection occurs after the fourth month of gestation. Penicillin is an effective treatment for mother and fetus. Classically the infant at birth is found to have persistent snuffles, skin eruptions and widespread metaphyseal bony lesions. However, many infants show no symptoms at birth.
Interstitial keratitis is the commonest feature of congenital infection, and hepatomegaly is present in almost all cases. While treatment of maternal syphilis in pregnancy usually eradicates infection in the infant, the mother will still have positive tests for VDRL (Venereal Disease Research Laboratory), TPHA (Treponema Pallidum Haemagglutination Assay), and RPR (Rapid Plasma Reagin). The infant will also be positive as a result of passive transfer of IgG to the fetus. More specific investigations are necessary.
The fluorescent treponema antibody absorption (FTA-ABS) test should be performed for both IgG and IgM. The IgG response will remain positive for several weeks but should be negative by 6 months. The IgM response should be negative at birth. If the IgM is positive or if the VDRL/RPR titre is four times higher in the baby than in the mother, the infant should be treated for congenital syphilis with parenteral benzylpenicillin for at least 10 days. The CSF should be examined before treatment.
Hepatitis B
This is largely a disease of developing countries, but with worldwide travel it is now relatively common in the UK and Australia. Mothers who develop acute hepatitis B infection in the first or second trimester of pregnancy have a 10% risk of perinatal transmission. Mothers who develop acute hepatitis B infection in the third trimester of pregnancy have a 75% risk of the infant developing the disease, but the neonatal disease is rarely severe or fatal. More commonly, mothers who are chronically hepatitis B ‘surface antigen’ positive pass the infection vertically to their baby via the placenta. Forty per cent of children with persistent hepatitis B infection will die in adult life of hepatocellular carcinoma or chronic liver disease.
All women should be screened for hepatitis B at booking, with serological tests for surface antigen (HBsAg). They are at greatest risk of infecting their babies if they are also HBe antigen (HBeAg) positive. If a woman has antibody to HBe (antiHBeAb), then the risk of serious disease to her infants is very small. Therefore, women who are HBsAg positive and negative for HBeAg and have antiHBeAb, are at low risk of their infants developing severe disease. Their infants may become chronic carriers of HBsAg and are at risk of developing carcinoma of the liver. Symptoms of liver disease in the neonatal period are rare in affected babies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree