Fig. 6.1
Uterine section showing an intramural fibroid in uterine body
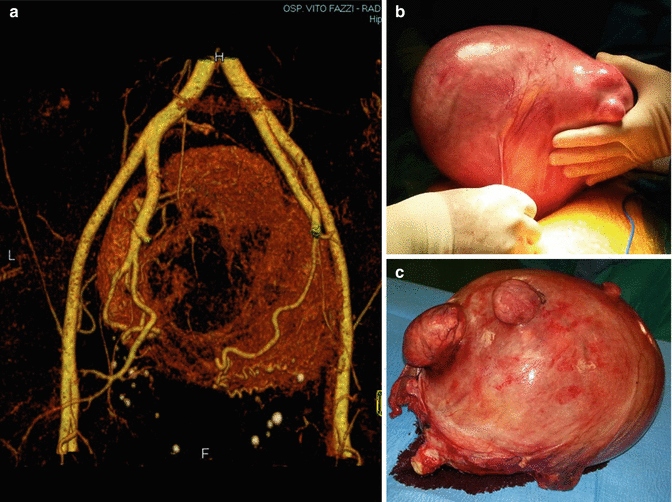
Fig. 6.2
A giant fibroid of 24 cm of diameter: (a) an angio-CT reconstruction of the fibroid peripheral vascularization, as a surrounding vascular network; (b) laparotomic hysterectomy; (c) the uterus on the servant table after surgery
Several treatments are available to remove fibroids and alleviate symptoms, such as conservative surgery [7], medical therapy [8], or various novel radiological interventions [9]. Despite the frequency with which fibroids are diagnosed and treated, there remains considerable uncertainty and controversy among clinicians and women regarding the best way to manage them.
The problem of uncertain management is also due to interference which causes the fibroid on the myometrium. During its growth, fibroid causes compressive phenomena on the surrounding myometrium, that for ischemic phenomena, produce a sort of pseudocapsule, constituted by a surrounding network of collagen fibers, neurofibers and blood vessels, as a separate fibroneurovascular tissue (Fig. 6.3) [10].
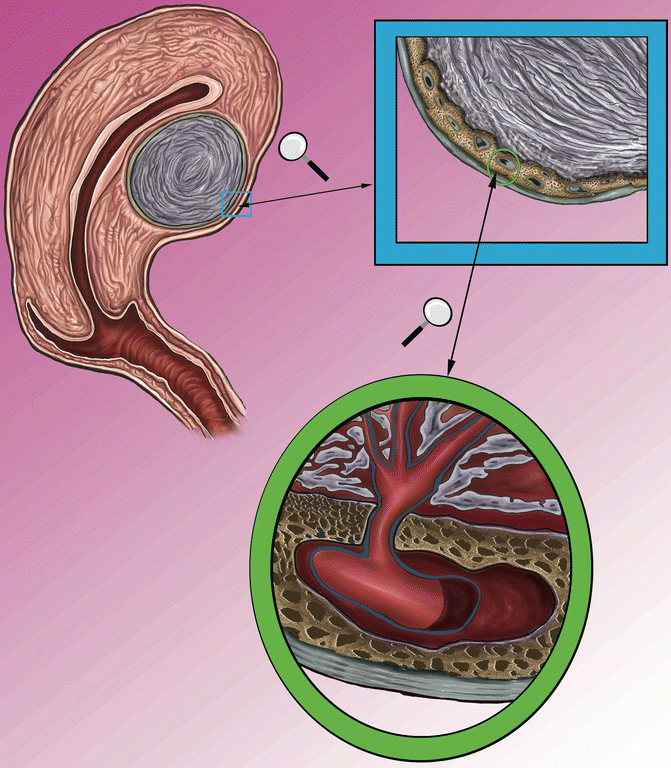

Fig. 6.3
On the top of the left side, is represented the uterine section of with intramural fibroid; the inset highlights the pseudocapsule, which is magnified on the right. Below is represented the pseudocapsule in the ring, constituted by a surrounding network of collagen fibers, neurofibers and blood vessels
Occasionally the pseudocapsule surface is interrupted by collagen fibers and vessels that anchor the fibroid to myometrium, well represented in microscopic sections (Fig. 6.4). It allows a constitution of a macroscopically clear cleavage plane between fibroid and the pseudocapsule, and between the pseudocapsule and the surrounding myometrium (Fig. 6.5) [11]. The pseudocapsule allows to fibroid only a displacement action (but not destructive) on myometrium, retaining the integrity and contractility of uterine structure [12].
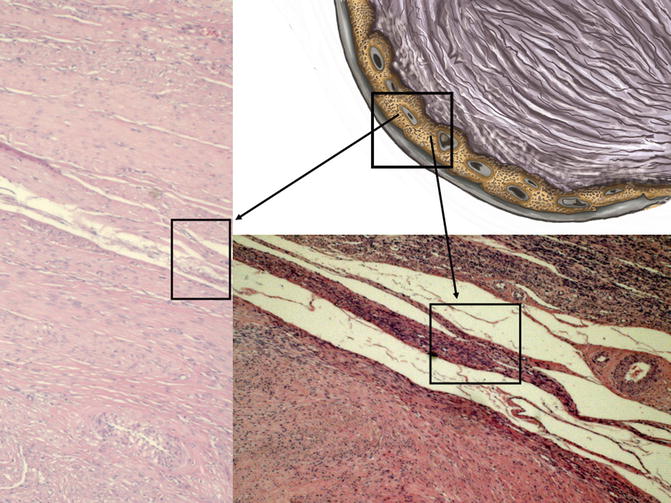
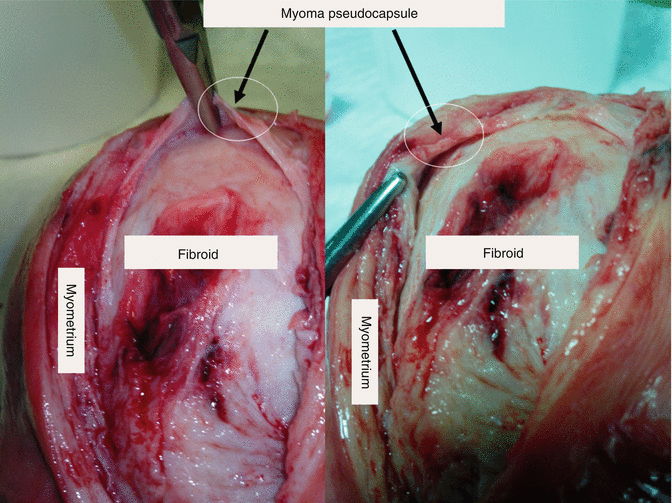

Fig. 6.4
In the drawing of fibroid to the upper right side, the pseudocapsule is highlighted into the box; to the left and below, black arrows show, in the two box, histological microphotographs, stained by hematoxylin & eosin, of myoma pseudocapsule: detection of compressed myometrial muscular smooth tissue, collagen fibers and blood vessels

Fig. 6.5
A sectioned uterus with an intramural fibroid; it is macroscopically clear the cleavage plane between fibroid and the pseudocapsule, in the white ring, gripped by the forceps
In this chapter we will discuss the importance of this small structure in the context of the myometrium, for the purpose anatomical, pathophysiological and reproductive systems.
Anatomy of Fibroid Pseudocapsule
Microscopically, the pseudocapsule seems to be as a continuous layer between the fibroid and myometrium and is made of a thickening of collagen fibers and blood vessels that form a vascular ring, sonographically called “ring of fire” by echo-color Doppler: the pseudocapsule is separated from the surrounding myometrium, sonographically forming a hyperechogenic ring that surrounds and defines myoma (Fig. 6.6) [10]. To help with understanding of the micro-neuroanatomy of the fibroid peripheral area, we should get close with the story of pseudocapsule entity. An anatomical textbook, published at the end of last century, asserted: “myoma shows a nodular aspect, a round image, well circumscribed, to enucleate even if they miss a pseudocapsule” [13]. These authors confirmed the existence of the fibroid pseudocapsule and cleavage plane to identify and to respect during a myomectomy [14]. The first paper that described a fibrovascular system over a fibroid as “a mass of proliferating arteries” was in 1944 [15] and then, Farrer-Brown in 1970 [16] and Awataguchi in 1982 [17] found a fibrovascular plexus around the periphery of the fibroid. Lately, Casey et al. reported significantly higher microvasculature density in the surrounding myometrium in large fibroids (Fig. 6.7); the author discovered that the vascular capsule was a substantial feature of all fibroids, excluding those smaller, and that it reaches the highest density of blood vessels in large tumors [18]. Pathologists examined the relationship between ultrasound and histological findings of the fibroids vascular capsule in the last century, in a series of women using Gn-RH analogues in preoperative treatment before myomectomy [19–21].
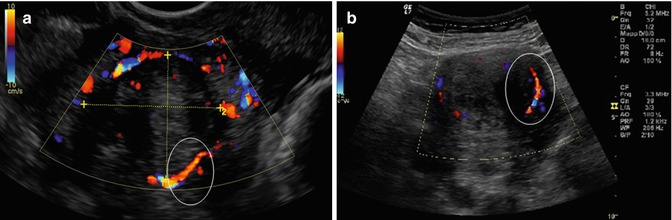

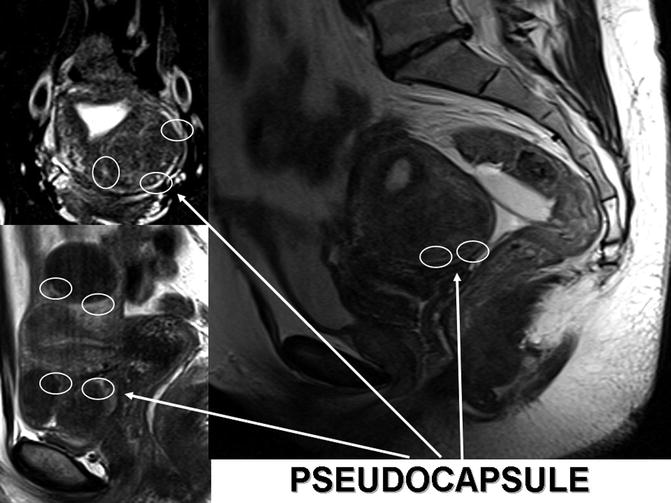


Fig. 6.6
A uterine fibroid Doppler ultrasonography showing, in the white ring, the fibroid pseudocapsule as a “ring of fire” around fibroid (a, b, d); in the image (c), it is enhanced, in the white box, the myoma pseudocapsule as an hyperecogenic white outline

Fig. 6.7
MRI reconstructions of myomatosic uteries. The white arrows indicates white rings enhancing myoma pseudocapsules
Walocha et al. [22] performed a microstructural evaluation of the fibroid pseudocapsule and found that the density of blood vessels increases around myoma. As the fibroid grows, new blood vessels penetrate the tumor from its periphery where “the vascular capsule” network is formed. Some of the vessels in the pseudocapsule connect at the base of the myoma and form a little foot which often bleeds during an extra-capsular myomectomy. These authors analyzed the fibroid vascular system using corrosion casting combined with scanning electron microscopy and affirmed that the pre-existing blood vessels undergo in regression and new vessels invade the tumor from the periphery during the development of myomas. Myoma pseudocapsule originates from small vessels entering the tumor from the periphery, forming a “vascular network” around the myoma, with flattened veins compressed by the tumor (Fig. 6.2, image a) [22].
Forssman et al. reported the vascular capsule surrounding myoma of 2 cm of diameter and found that the inner aspect of the capsule contained large vessels that invade the capsule from the periphery [23].
Neurotransmitters in Myoma Pseudocapsule
The fibroid pseudocapsule is a structure which surrounds the uterine fibroid and separates it from the uterine tissue. At the ultrastructural level, visualized by transmission electron microscopy, the pseudocapsule cells have the features of smooth muscle cells similar to the myometrium. So, the pseudocapsules are part of the myometrium which compresses the leiomyoma. This ultrastructural feature suggests that when removing fibroids their pseudocapsules should be preserved to preserve myometrium and post-surgical uterine anatomy [24].
Wei et al. demonstrated that in large uterine fibroids, the most biologically active zone is the region next to the periphery with a higher level of gene expression, a higher density of blood vessels, a higher proliferative rate, and a lower level of hyaline degeneration [25].
These studies confirms preliminary evidence that pseudocapsules contain neuropeptides together with their related fibers, as a neurovascular bundle, containing a vascular network rich in neurotransmitters like a neurovascular bundle (Fig. 6.8) [26].
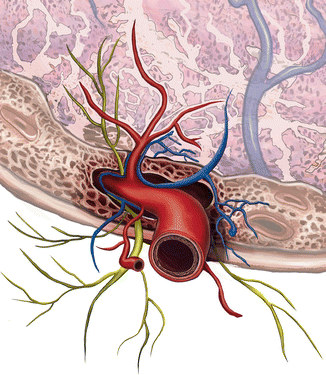

Fig. 6.8
A 3D reconstruction of myoma pseudocapsule as a neurovascular bundle: the fibroneurovascular network surrounding fibroid is rich in neuropeptides and neurotransmitters
Literature data confirm that pseudocapsules contain many neuropeptides and neurotransmitters [27–29], physiologically active. Moreover, these substances may play a significant role in wound healing [30–32] and innervation repair [33], and may be essential for reproductive [34–36] and sexual function [37, 38]. Indeed, the lower urinary tract neuropeptide–receptor systems may represent a potential target for therapeutic interventions [39, 40].
Comparison Between Myoma Pseudocapsule and Prostate Capsule
Mettler et al. studied the myoma pseudocapsule by endocrinological side, affirming it has a delicate vascular network rich with neurotransmitters, as a neurovascular bundle containing neuropeptides and related fibers surrounding prostate [26].
The idea of a neurovascular bundle surrounding a myoma, inside pseudocapsule, derives from a multidisciplinary discussion among gynecologists and urologists, on analogies of myoma pseudocapsule with the prostate capsule. Based on the purpose of reducing the probability of impotence associated prostate cancer treatment, urologist generally must preserve neurovascular bundles surrounds prostate. Anatomically, the neurovascular bundles are situated on the peripheral to the prostate. The neurovascular bundles is placed in the lateral pelvic fascia, deep, lateral, and cephalad to Denonvilliers’ fascia and the prostatic fascia and the levator fascia [41]. As evidenced by Takenaka et al. [42] and Costello et al. [43], the cavernous branches connect the capsular arteries and veins in a spray-like distribution to form the neurovascular bundles 20–30 mm distal to the junction of the bladder and prostate. At the apex of the prostate, the branches of the nerves to the cavernous bodies and striated sphincter also have a spray-like distribution both anteriorly and posteriorly with large variation. This anatomical framework has been defined the neurovascular bundles by Walsh [44]. As analyzed by surgeons, the neurovascular bundles passes through two distinct fascial planes that surround the prostate called “the prostatic fascia and levator fascia”: the nerves cross in the neurovascular bundle and innervate the corpora cavernosa, rectum, prostate, and levator ani musculature. The last three receive a blood supply from vessels running in the neurovascular bundle. Nowadays, in most men who are candidates for radical prostatectomy, it is usually safe to preserve both neurovascular bundles and rarely necessary to excise both of them. If nerve sparing is performed correctly, the prostatic fascia must remain intact on the prostate. Moreover, when tumor extends through the capsule, it seldom penetrates more than 1–2 mm, this tissue can often be removed likewise, with preservation of the neurovascular bundles. Even in extra-prostatic extension in the region of the neurovascular bundles, it is feasible to partially excise the bundle, preserve potency, and achieve negative margins of excision [45]. The prostatic neurovascular bundle provide both somatic and autonomic innervations to the continence mechanism. The excision of neurovascular bundle causes more incontinence and impotency than when the neurovascular bundles are preserved [46]. In order to spare the prostatic neurovascular bundles, laparoscopic or robotic assisted prostatectomy are both useful [47], since the magnification facilitates a more gentle dissection with less traction and careful dissection [48]. Surgically, a midline vertical incision is made into Denonvilliers’ fascia—along the entire gland extending up the urethra—and the fascia is sharply retracted to the right and left to expose the posterior prostatic capsule. This incision must completely release the neurovascular bundles to allow correct restore of the retractor blades and thus to avoid a traction injury to the neurovascular bundles. Blunt or sharp dissection is often needed to release the bundles far enough laterally to achieve adequate exposure to the posterior prostate base. Although these nerves are microscopic [49], their anatomic location can be evaluated intraoperatively through the use of the capsular vessels as a landmark. An opening in the levator fascia is performed by sharp incision along the anterolateral surface of the prostate starting at the base of the prostate and proceeding toward the apex. This maneuver releases the bundle laterally, thus making it easier to make the next step, where the bundle is released posteriorly at the apex. Once the superficial fascia has been released, the site of the neurovascular bundles can be identified by the presence of a thin “groove” on the posterolateral edge of the prostate. The interfascial plane (i.e., between the levator and prostatic fascia) is developed gently using blunt dissection with a fine curved dissector and a gentle diathermocoagulation. Dissection continues in close approximation to the surface of the prostatic fascia in efforts to optimize quantitative cavernous nerve preservation. If bleeding occurs from periprostatic vessels, insufflation pressure can be in the meanwhile increased and pressure applied to the source of bleeding with hemostatic gauze. Hemostasis with high wattage diathermocoagulation or ultrasonic heat energy should be always avoided during dissection near the neurovascular bundles, as these energy sources have been shown to be injurious to cavernous nerve function in the canine model [50]. The neurovascular bundles may be observed through the use of ultrasound Doppler, which has become an important technique for non-invasive flow measurement in medical applications [51]. On the bases of these findings about the importance of the prostatic capsule and the important and physiologic role of nerve-sparing techniques for prostatectomy, and comparing this structure to the fibroid pseudocapsule, the knowledge of peripheral neurovascular bundle of the uterine leiomyoma was challenged and revisited.
Intracapsular Myomectomy
Fibroids enucleating or myomectomy, the removal of fibroids surgically without hysterectomy, is the second most common surgical procedure for this condition, to restore uterine anatomy [7]. Myomectomy is the most common conservative treatment in gynecology, performed by classical open surgery or, currently, by laparoscopy with a less traumatic fibroid removal and better recovery [7].
Literature showed the possibility to perform myomectomy by removing the fibroid from its surrounding structure, the fibroid pseudocapsule [14, 52, 53], called by authors “intracapsular myomectomy”. It performs by stretching and extracting fibroid directly from the surrounding fibromuscular skeleton, breaking up the fibrous bridges.
Researches on pseudocapsule started wit Ito et al., who performed an histopathologic evaluation of uterine fibroid and its pseudocapsule, to determine the scientific reason for less blood loss during a intracapsular myomectomy. They demonstrated how a pseudocapsule is formed by extra-cellular matrix around the myoma, separating fibroid from normal myometrium. The authors sustained that the fibroid is anchored to the pseudocapsule by connective bridges, but lacks its own true vascular pedicle [54]. Also Dapunt et al. showed a vascular network surrounding myoma, as a pseudocapsule, so that if the detachment of the myoma occurring into the pseudocapsule, surgeon has less bleeding during myomectomy [55]. Fox et al. studied fibroids by ultrastructural microscopy and showed an anatomical structure different than the normal myometrium: fibroids had a well-defined regular outline and a surrounding pseudocapsule of compressed muscle fibers [56]. The hypothesis of presence of fibroid pseudocapsule was also asserted by Vizza and Motta, whose demonstrated that the pseudocapsule contains the fibers that tend to bulge out from the surrounding myometrium and have a firm, whorled or trabeculate surface [57]. Furthermore, ultrasonographic evaluations have been performed on myomas and their connecting structures: pseudocapsule appeared as an echogenic line around the myoma, with a wall 1 cm or less, and with reinforcement of distal echoes [58]. Additional histological investigations have been performed on the fibroid vascular pseudocapsule to better understand the role in the modern minimally invasive myomectomy [17]. The macroscopic evaluation of the pseudocapsule and of the adjacent myometrium showed that parallel arrays of extremely dense capillaries and larger vessels form the capsule and this is separated from the myometrial vasculature by a narrow avascular cleft. The pseudocapsule vessels from the surrounding myometrium formed clusters in the center of the vascular network to form a sort of pedicle and the veins surrounding the myoma circulated under the pseudocapsule arranged in a plexus [17, 25]. The main principle of myomectomy is to perform all manipulations as precisely and bloodlessly as possible, and the new surgical technique of intracapsular myomectomy meets this requirement. Nevertheless, the impact of surgical myomectomy differs depending on the technique used [59]. The laparotomic myomectomy is different from the laparoscopic technique, also with same intracapsular method: the laparoscopic access route proved to be the most beneficial. The advantages of the laparoscopic approach are the significantly reduced parameters of both intra- and post-surgical blood loss, decreased bladder pain after Foley removal, the lower number of patients requiring pain relief medication and the shorter hospital stay. In addition, laparoscopic intracapsular myomectomy resulted in slightly improved short-term outcomes in relation to postoperative fever, myometrium scar hematomas, ileus and antibiotic treatment compared to open surgery. Finally, the laparoscopic myomectomy has a favorable impact on blood loss by intracapsular method, aiming to preserve musculature under hysterotomy. The CO2 insufflation can influence blood loss during intracapsular myomectomy, as the increased intra-peritoneal pressure can lead to the occlusion of the small blood vessels and capillaries of the pseudocapsule. This effect, combined with less traumatic endoscopic micro-manipulations, could result in beneficial outcomes of surgery [59].
To better clarify these results, we remember that our intracapsular myomectomy was standardized and published [53]. Laparoscopic myomectomies are performed under general anesthesia by endotracheal intubation with a standardized four port approach: one port for the laparoscope and three lower quadrant ancillary ports (one suprapubic central 10 mm port and two lateral 5 mm ports). The 10 mm central suprapubic port is often changed to 15–20 mm for the introduction of the morcellator at the end of the procedure. All patients receive an intrauterine manipulator prior to laparoscopy, to better mobilize uterus. Intracapsular laparoscopic myomectomy of sub mucous and intramural fibroids is generally performed without injection of ischemic solution into the myometrium. The visceral peritoneum is incised in the midline longitudinal plane, by monopolar scissors or crochet needle electrode, proceeding in depth into myometrium to reach the right plane under myometrium, to detect myoma pseudocapsule with the fibroid below.
Once identified the myoma pseudocapsule, it is well exposed by atraumatic clamp or by irrigator cannula, to provide a panoramic laparoscopic view of pseudocapsule of all subserous-intramural leiomyomas. Then surgeon affects the pseudocapsule by a longitudinal cut, performed by monopolar scissors or Hook electrode at low wattage (30 W), to expose the myoma surface. Then fibroid is hooked by a myoma screw or Collins laparoscopic forceps to perform the traction necessary for its gentle enucleating, helping by irrigator cannula to be inserted in the space under myoma pseudocapsule and fibroid. Hemostasis of the small vessels bleeding is selectively achieved by a low wattage bipolar clamp or by Hook electrode or monopolar scissors, always at 30 W, to free the base of the myoma and the connective bridges from the pseudocapsule (Fig. 6.9). In such way, complete minimal traumatic fibroid removal from its pseudocapsule was accomplished with a minimal blood loss and pseudocapsule sparing. In case of pseudo-pedunculated myomas, the pedicle is coagulated by bipolar forceps and cut by laparoscopic scissors or cut after placement of loops or staples, without suturing. In cases of deep intramural myomas, chromopertubation is always applied via a cervical cannula not only to check tubal patency but also to facilitate the direct recognition of an inadvertently opened uterine cavity.
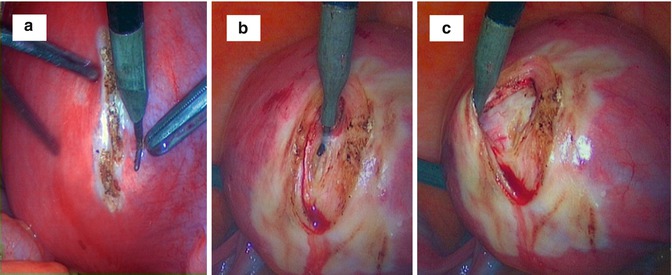
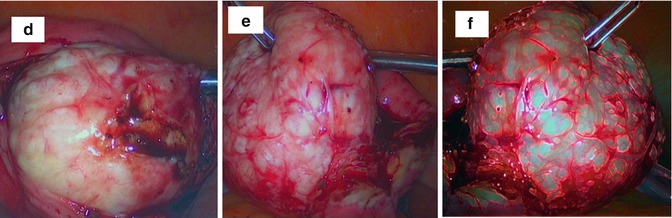


Fig. 6.9
An intraoperative sequence of laparotomic myomectomy images evidencing fibroid pseudocapsule during myoma stretching by surgeon from myometrium: (a) the uterine serosa is plotted by a Hook electrode at low wattage (30 W); (b) the vertical cut proceeds in depth up to the fibroid, cutting gently the pseudocapsule (always by low wattage energy) to expose the myoma surface; (c) surgeon expose the fibroid with a lateralization and sparing of the pseudocapsule during myoma removal; (d) after inserting a mini-drill in fibroid, the surgeon gently pulls the fibroid, with its enucleation from the uterine fovea; (e) when exposed to the fibroid, the surgeon show the residual branches of pseudocapsule connecting fibroid to uterus, to be cut with scissors or by Hook electrode; (f) the fibroid is enucleated; residues of fibrous strands, cut during myomectomy, appear on fibroid surface
The myometrium closuring is performed by a single (for subserous fibroids) or double layer (for intramurals), including overlying serosa, with a round CT-1 curved needle, using intra or extracorporeal knots. In sub-serosal myomectomies, the edges of the uterine defect were approximated with introflecting U-inverted stitches (myometrium/serosa-serosa/myometrium direction) with intra myometrial knot, at 1 cm increments from the edge of the incision (as a “baseball-type” suture). The closure was by surgeon choice: interrupted closure or traditional unidirectional running suture, stared at the end of one of hysterotomic sides.
Deep intramural fibroids required a two-layer myometrial closure with introflecting sutures, ever by a “baseball-type” suture. If the uterine cavity was accidentally opened during fibroid enucleating, 2–3 deep myometrial single or continuous sutures were applied on the uterine cavity edges. After hysterorraphy, fibroids are usually morcellated.
The Functional Autonomy of Pseudocapsule and Its Impact on Muscular Healing
The biochemical growth factors evaluated in the pseudocapsule vessels cause intense angiogenesis in pseudocapsule, probably promoted by the fibroids [60]. The angiogenesis of the myoma pseudocapsule likely leads to the formation of a “protective” vascular capsule responsible for the supply of blood to the growing tumor [61]. However, studies demonstrated a dysregulation of various growth factors and their receptors in uterine myomas [17, 61].
In fact, a research on gene expression analysis in uterine leiomyoma pseudocapsule revealed an angiogenic profile in pseudocapsule [60]. In this investigation authors performed, by quantitative real-time RT-PCR method (qRT-PCR), a gene expression analysis of PC, matching it with the same analysis in UL and UM, evaluating the expression levels of IGF-2, used as tumoral marker, and COL4A2, CYR61/CCN1, CTGF/CCN2, VEGF-A and vWF, known to be involved in angiogenic processes.
The results clearly indicated that the pseudocapsule was a structure anatomically distinguishable from the myometrium and the surrounding fibroid, displaying a significant and specific gene expression profile. The pseudocapsule, as the fibroid, exhibited a significantly reduced expression of the IGF-2 gene, known to be a tumor growth marker, if compared to the fibroid, suggesting that it has a non-fibroid origin and that it has a structural continuity with myometrium. The pseudocapsule also showed a statistical relevant over-expression of the endoglin/CD105 gene, when compared to the myometrium and to the fibroid. Based on these evidences, the over-expression of the endoglin gene, rather than of other angiogenic genes, seemed to indicate the presence of an active angiogenesis correlated with reparative process in the pseudocapsule. All together these data clearly depicted the pseudocapsule as a site of intense angiogenesis linked to the endoglin activation rather than other angiogenic factors such as VEGF-A or vWF. The presence of an active angiogenesis is concordant with the histological studies that describe a parallel array of extremely dense capillaries in the pseudocapsule and in the adjacent myometrium, that are absent in fibroid. This can define the structural and functional features of pseudocapsule, that could explain its possible roles in the uterine regenerative process [62].
Moreover, in such regenerative process, there is also the involvement of neuropeptides and neurotransmitters, extremely important in wound healing. In fact, there is evidence that the nervous system and its neurotransmitters, such as Substance P (SP), Vasoactive Intestinal Peptide (VIP), neuropeptide Y (NPY), Oxytocin, Vasopressin (VP), PGP9.5, calcitonin gene-related peptide (CGRP), growth hormone-releasing hormone (GHRH), play a role in mediating inflammation and healing [62–64]. Referring to uterine musculature scar physiology, these peptides sparing enhances a correct healing of an hysterotomy, as evidenced by Mettler et al. [26].
Most of these neuropeptides have been highlighted in the pseudocapsule.
Malvasi et al. [27] evaluated the distribution of two neuropeptides, the SP and the VIP in pseudocapsule of uterine fibroids: they proved that these neurofibers are present in the pseudocapsule of the fibroid as well as in the normal myometrium of the non-pregnant uterus [27].
Then, Malvasi et al. [28] showed also the presence of NT, NPY and PGP 9.5 presence in myometrium as well as in fibroid pseudocapsule, underlying their possible impact on muscular physiology. Finally, the same authors [29] evaluated the opioid neuropeptides, as enkephalin (ENK) and oxytocin (OXT), in the nerve fibers within pseudocapsule and their possible influence in human reproduction. An absence of ENK-positive nerve fibers was seen in the uterine fundus and corpus fibroid pseudocapsules, whereas they were observed in the nerve fibers in fibroid pseudocapsules obtained from the isthmian–cervical region. OXT-positive fibers were present in fibroid pseudocapsules of all uterine regions, whereas the distribution of OXT-positive fibers was significantly higher in the isthmian–cervical region. Authors speculated that ENK- and OXT-positive nerve fibers are present mainly in the isthmian–cervical pseudocapsules (Fig. 6.10) than in the corporal pseudocapsules [29]. The present findings are important to discern the pathophysiology of the female reproductive system and sexual disorders manifesting after surgical procedures in the cervix, including complications during pregnancy and delivery (i.e. miscarriage and cervical dystocia during labor).
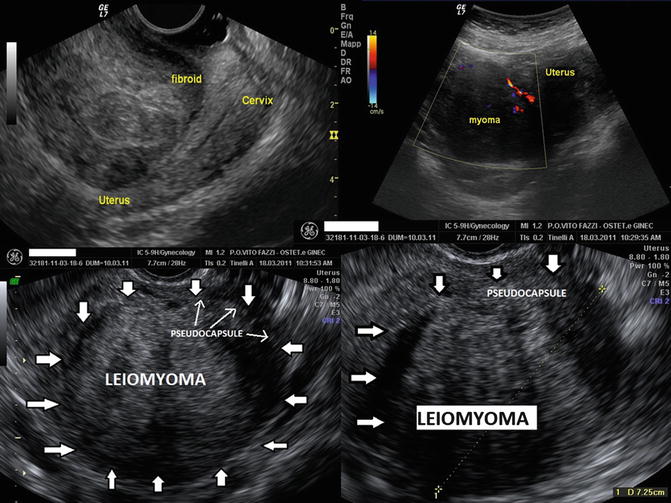

Fig. 6.10
The ultrasonographic figure shows isthmian–cervical pseudocapsules, rich of ENK-positive and OXT-positive nerve fibers
As the neuropeptides are transported to the tissues by the neurovascular network, the myoma pseudocapsule, as neurovascular bundle [26], is therefore a structure rich of neuropeptides. Pseudocapsule vessels were also studied by a preliminary three-dimensional mathematical model [65], who showed an increase vascular tortuosity, disarray, an abnormal branching and the presence of “cul-de-sac” pseudocapsule vessels. All of these features are similar to malignant neoplastic tissue vessels features, present in malignant tumors. It was not possible to clarify if the pseudocapsule vasculature network could be sustained by mechanical and inflammatory effects of myoma on myometrium, or produced by a sort of neoangiogenesis “neoplastic-type”, due to the fibroid growth or even to a muscle and tissue healing process, such as a neurovascular preparative reaction of female body to a fibroid expulsion (pedunculated fibroids), necrosis or degeneration, allowing a normal uterine restoration [65].
In human body, difficulties in obtaining serial samples of the hysterotomic scar during myomectomy or cesarean section, are a major barrier to our understanding of the events involved in the post-myomectomy and post-cesarean section remodeling processes of the uterine wound. They can be only monitored by ultrasound or MRI [30].
However, irrespective of the ultimate result, wound healing is a dynamic, interactive process involving neuromediators, angiogenetic factors, neuropeptides, blood cells, extracellular matrix, and parenchymal cells that follows three complex and overlapping phases: inflammation, tissue formation, and tissue remodeling [66]. And this healing process is be involved also for post-operative adhesion development, as consequence of myomectomy and associated with a high risk of de-novo adhesion formation, that may ever decrease fertility [67].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


