Background
Little is known about fertility choices and pregnancy outcome rates among HIV-infected women in the current combination antiretroviral treatment era.
Objective
We sought to describe trends and factors associated with live-birth and abortion rates among HIV-positive and high-risk HIV-negative women enrolled in the Women’s Interagency HIV Study in the United States.
Study Design
We analyzed longitudinal data collected from Oct. 1, 1994, through Sept. 30, 2012, through the Women’s Interagency HIV Study. Age-adjusted rates per 100 person-years live births and induced abortions were calculated by HIV serostatus over 4 time periods. Poisson mixed effects models containing variables associated with live births and abortions in bivariable analyses ( P < .05) generated adjusted incidence rate ratios and 95% confidence intervals.
Results
There were 1356 pregnancies among 2414 women. Among HIV-positive women, age-adjusted rates of live birth increased from 1994 through 1997 to 2006 through 2012 (2.85-7.27/100 person-years, P trend < .0001). Age-adjusted rates of abortion in HIV-positive women remained stable over these time periods (4.03-4.29/100 person-years, P trend = .09). Significantly lower live-birth rates occurred among HIV-positive compared to HIV-negative women in 1994 through 1997 and 1997 through 2001, however rates were similar during 2002 through 2005 and 2006 through 2012. Higher CD4 + T cells/mm 3 (≥350 adjusted incidence rate ratio, 1.39 [95% CI 1.03-1.89] vs <350) were significantly associated with increased live-birth rates, while combination antiretroviral treatment use (adjusted incidence rate ratio, 1.35 [95% CI 0.99-1.83]) was marginally associated with increased live-birth rates. Younger age, having a prior abortion, condom use, and increased parity were associated with increased abortion rates among both HIV-positive and HIV-negative women. CD4 + T-cell count, combination antiretroviral treatment use, and viral load were not associated with abortion rates.
Conclusion
Unlike earlier periods (pre-2001) when live-birth rates were lower among HIV-positive women, rates are now similar to HIV-negative women, potentially due to improved health status and combination antiretroviral treatment. Abortion rates remain unchanged, illuminating a need to improve contraceptive services.
Introduction
Pregnancy and family planning among women living with HIV involves complex decision-making and clinical management. Considerable improvements in the treatment and care of HIV-positive women has led to increased life expectancy and improved health. Additionally, improved clinical management of pregnant women has led to near elimination of perinatal HIV transmission among those on effective combination antiretroviral treatment (cART) throughout pregnancy. The potential of cART to reduce maternal-to-child transmission of HIV has affected the reproductive decisions of women living with HIV, with more women now choosing to have children. However, family planning is often incompletely addressed within the current HIV care paradigm. Many pregnancies occurring among HIV-positive women, similar to the general population, are unplanned. Although data are scant, it has been estimated that up to 80% of pregnancies occurring among HIV-positive women in the United States are unintended, compared to approximately 50% in the general population. This highlights the importance of understanding reproductive health needs of this cohort and providing targeted family planning options as part of routine HIV care.
It is still unclear how pregnancy rates and outcomes have changed over the past 20 years among HIV-positive women, spanning the introduction of cART, compared to HIV-negative women. A 1994 through 2002 Women’s Interagency HIV Study (WIHS) (an ongoing multicenter prospective cohort study of HIV infection and outcomes among women with and without HIV) analysis found pregnancy rates of 7.4 and 15.2 per 100 person-years among HIV-positive and high-risk HIV-negative women, respectively. In a more recent 2002 through 2009 WIHS, Linas et al reported that HIV infection was associated with a 40% reduction in pregnancy incidence among HIV-positive vs HIV-negative women adjusting for age, number of sexual partners, contraception, parity, and alcohol use.
This study aims to describe trends in pregnancy and pregnancy outcomes, including live birth and abortion, over the past 20 years in HIV-positive and HIV-negative women enrolled in the WIHS cohort. We further determine factors associated with live births and abortion, specifically focusing on the role of HIV viral load and cART, to better understand the impact of improved immune function and other factors in pregnancy outcomes.
Materials and Methods
We performed a longitudinal analysis of WIHS data collected from Oct. 1, 1994, through Sept. 30, 2012. HIV-positive and high-risk HIV-negative women were recruited from similar sites with more than half of the participants living below the federally defined level of poverty and frequency matched based on demographics and key risk factors for acquiring HIV, such as drug use and number of partners, with recruitment targets adjusted when imbalances arose. Enrollment for WIHS began in 1994 with 6 sites (Bronx and Brooklyn, NY; Chicago, IL; Washington, DC; Los Angeles and San Francisco, CA), and has continued with 6-month visits using trained interviewers, where visits include detailed questionnaires including antiretroviral treatment (ART) use, medical history, and medication use; a clinical examination; and comprehensive laboratory testing (including CD4 T-cell count and HIV viral load). WIHS had replenishment enrollment in 2001 through 2002, 2011 through 2012, and 2013 through 2015, now with a total of 9 US sites. Data from the 6 original sites are included in this study. Institutional review board approval was obtained from each WIHS site prior to study enrollment. This evaluation utilized deidentified data.
As obstetrical care was received outside of the study, pregnancy outcomes and associated dates were reported by the participant. Outcomes were classified as: induced abortion for those who terminated the pregnancy, live birth for a baby born alive, and nonviable for spontaneous abortion, ectopic pregnancy, or stillbirth.
Women were included in this analysis if they attended at least 2 WIHS visits. Women not at risk for pregnancy due to history of hysterectomy, tubal ligation, or bilateral oophorectomy and women age >45 years were excluded/censored from analysis.
Rates for induced abortion, live birth, nonviable pregnancy, and all pregnancy outcomes combined were the number of pregnancies divided by the person-years at-risk over the entire study, stratified by time: 1994 through 1997, 1998 through 2001, 2002 through 2005, and 2006 through 2012. Pregnancy rates per 100 person-years were directly adjusted using the population age distribution of women with pregnancy outcomes in 1996, similar to the methods used in the prior publication by Massad et al in 2004. Due to the 0 birth rate among older age groups, 95% confidence intervals (CI) for directly age-adjusted rates are based on a modified gamma distribution. The Mantel-Haenszel χ 2 quantified whether age-adjusted pregnancy rates differed by HIV serostatus within each time period. Trend tests evaluated trends in adjusted rates by time interval by serostatus.
Bivariable associations between covariates (both baseline variables and time-varying) and live birth and abortion were determined using a Poisson regression model with an offset for years in each risk group. Baseline covariates of interest were recorded at the participant’s first WIHS visit and included race/ethnicity, income, marital status, education, employment status, insurance status, WIHS site, number of male sexual partners, parity, history of abortion, prior sexually transmitted infection, and year of first cART, where cART was defined as ≥3 antiretroviral drugs used concurrently by self-report. Time-varying covariates reflect activity for the time since the last WIHS visit and included contraception use, characterized as hormonal or long-acting reversible contraceptive (LARC) including intrauterine contraception and implants compared to none or other method use (condoms, withdrawal, diaphragm). Condom use was dichotomized into any condom use since the last visit vs no use. Alcohol use was dichotomized into self-report of 0-7 drinks/wk compared to >7 drinks/wk. Current cigarette smoking and illicit drug use (marijuana/hash, crack/cocaine, methamphetamine, or intravenous drug use) were positive if the individual reported any use since the last visit. A time-varying composite for chronic medical conditions (stroke, myocardial infarction, diabetes, hypertension, deep vein thrombosis, pulmonary embolus, cancer, or tuberculosis) was created. HIV-specific factors were incorporated for the HIV-positive cohort and included use of cART at the visit, viral load (categorized as <4 and ≥4 log 10 copies or 10,000), and CD4 + T-cell count (<350 and ≥350 cells/mm 3 ). Categorization for viral load and CD4 + T-cell count was determined a priori based on clinical significance and the approximate median value for the WIHS cohort in 2004 based on prior publications.
Poisson mixed effects models with a random intercept and offset for years since last visit were created to evaluate multivariate associations with live birth or abortion including all variables with significance by bivariable association ( P < .05), unadjusted crude incidence rate ratios (cIRR), and adjusted incidence rate ratios (aIRR) with 95% CI.
Materials and Methods
We performed a longitudinal analysis of WIHS data collected from Oct. 1, 1994, through Sept. 30, 2012. HIV-positive and high-risk HIV-negative women were recruited from similar sites with more than half of the participants living below the federally defined level of poverty and frequency matched based on demographics and key risk factors for acquiring HIV, such as drug use and number of partners, with recruitment targets adjusted when imbalances arose. Enrollment for WIHS began in 1994 with 6 sites (Bronx and Brooklyn, NY; Chicago, IL; Washington, DC; Los Angeles and San Francisco, CA), and has continued with 6-month visits using trained interviewers, where visits include detailed questionnaires including antiretroviral treatment (ART) use, medical history, and medication use; a clinical examination; and comprehensive laboratory testing (including CD4 T-cell count and HIV viral load). WIHS had replenishment enrollment in 2001 through 2002, 2011 through 2012, and 2013 through 2015, now with a total of 9 US sites. Data from the 6 original sites are included in this study. Institutional review board approval was obtained from each WIHS site prior to study enrollment. This evaluation utilized deidentified data.
As obstetrical care was received outside of the study, pregnancy outcomes and associated dates were reported by the participant. Outcomes were classified as: induced abortion for those who terminated the pregnancy, live birth for a baby born alive, and nonviable for spontaneous abortion, ectopic pregnancy, or stillbirth.
Women were included in this analysis if they attended at least 2 WIHS visits. Women not at risk for pregnancy due to history of hysterectomy, tubal ligation, or bilateral oophorectomy and women age >45 years were excluded/censored from analysis.
Rates for induced abortion, live birth, nonviable pregnancy, and all pregnancy outcomes combined were the number of pregnancies divided by the person-years at-risk over the entire study, stratified by time: 1994 through 1997, 1998 through 2001, 2002 through 2005, and 2006 through 2012. Pregnancy rates per 100 person-years were directly adjusted using the population age distribution of women with pregnancy outcomes in 1996, similar to the methods used in the prior publication by Massad et al in 2004. Due to the 0 birth rate among older age groups, 95% confidence intervals (CI) for directly age-adjusted rates are based on a modified gamma distribution. The Mantel-Haenszel χ 2 quantified whether age-adjusted pregnancy rates differed by HIV serostatus within each time period. Trend tests evaluated trends in adjusted rates by time interval by serostatus.
Bivariable associations between covariates (both baseline variables and time-varying) and live birth and abortion were determined using a Poisson regression model with an offset for years in each risk group. Baseline covariates of interest were recorded at the participant’s first WIHS visit and included race/ethnicity, income, marital status, education, employment status, insurance status, WIHS site, number of male sexual partners, parity, history of abortion, prior sexually transmitted infection, and year of first cART, where cART was defined as ≥3 antiretroviral drugs used concurrently by self-report. Time-varying covariates reflect activity for the time since the last WIHS visit and included contraception use, characterized as hormonal or long-acting reversible contraceptive (LARC) including intrauterine contraception and implants compared to none or other method use (condoms, withdrawal, diaphragm). Condom use was dichotomized into any condom use since the last visit vs no use. Alcohol use was dichotomized into self-report of 0-7 drinks/wk compared to >7 drinks/wk. Current cigarette smoking and illicit drug use (marijuana/hash, crack/cocaine, methamphetamine, or intravenous drug use) were positive if the individual reported any use since the last visit. A time-varying composite for chronic medical conditions (stroke, myocardial infarction, diabetes, hypertension, deep vein thrombosis, pulmonary embolus, cancer, or tuberculosis) was created. HIV-specific factors were incorporated for the HIV-positive cohort and included use of cART at the visit, viral load (categorized as <4 and ≥4 log 10 copies or 10,000), and CD4 + T-cell count (<350 and ≥350 cells/mm 3 ). Categorization for viral load and CD4 + T-cell count was determined a priori based on clinical significance and the approximate median value for the WIHS cohort in 2004 based on prior publications.
Poisson mixed effects models with a random intercept and offset for years since last visit were created to evaluate multivariate associations with live birth or abortion including all variables with significance by bivariable association ( P < .05), unadjusted crude incidence rate ratios (cIRR), and adjusted incidence rate ratios (aIRR) with 95% CI.
Results
Among 4346 women (1089 HIV-negative, 3232 HIV-positive, 25 seroconverters) enrolled in WIHS during the study period, about 56% (n = 2414) were included in this analysis. Of the 1932 women excluded, 36% (n = 707) were age >45 years, 61% not at risk for pregnancy (n = 568 hysterectomy, n = 608 tubal ligation, n = 7 bilateral oophorectomy), and 3% were excluded for other reasons (n = 13 HIV seroconverters, n = 25 did not have 2 eligible visits to analyze, n = 4 pregnancy outcome data were incomplete). This analysis included 1750 HIV-positive and 664 HIV-negative women with a total of 1356 pregnancies reported >16,670 person-years (8.13/100 person-years, unadjusted). Of these pregnancies, 586 (43.2%) ended in live birth, 469 (34.6%) in abortion, and 301 (22.2%) in a nonviable pregnancy. Supplemental Tables 1 and 2 show that about 50% were African American, single, had at least a high-school education, and were employed and insured. Over half of the women had a prior abortion and over half had at least 1 live birth. HIV-positive women were older than HIV-negative women. Contraception use, other than condoms, among all women was low at approximately 15% of included visits. HIV-positive women were on cART during about half of study visits, and for >60% of all visits viral loads were satisfactorily suppressed (<4 log 10 copies/mL) and CD4 + T-cell counts adequate (≥350 cell/mm 3 ).
Pregnancy outcome rates over time by HIV serostatus
For all-pregnancy outcomes combined, age-adjusted rates ( Figure , A, and Supplemental Table 3 ) among HIV-positive women demonstrated a nonsignificant increasing trend over time ( P trend = .088). While the overall age-adjusted rates were lower among HIV-positive women compared to HIV-negative women, significantly lower rates occurred during 1994 through 1997 and 1998 through 2001 for HIV-positive women compared to HIV-negative, whereas rates during 2002 through 2005 and 2006 through 2012 were similar to HIV-negative women.
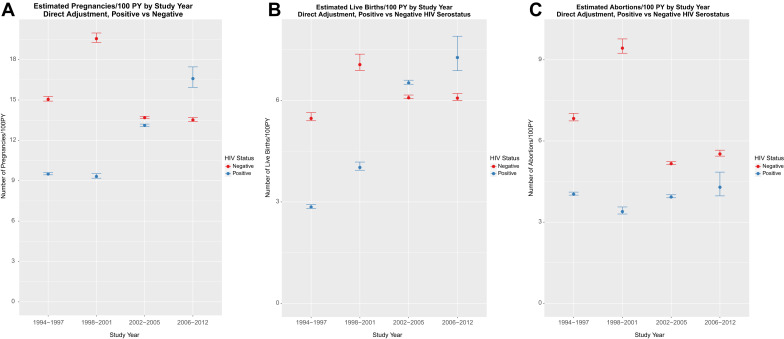
Similar results were seen in age-adjusted live-birth rates. There was a significant increase observed over time (HIV-positive P trend < .0001) ( Figure , B) for HIV-positive women, with no significant change observed among HIV-negative women ( P trend = .76). The overall age-adjusted live-birth rate was lower for HIV-positive compared to HIV-negative women, with significantly lower rates at earlier time periods (1994 through 1997, 1998 through 2001) and no significant difference at the later time periods (2002 through 2005, 2006 through 2012).
Age-adjusted abortion rates remained relatively stable over time (HIV-positive P trend = .093) ( Figure , C) with age-adjusted rates remaining between 3.4-4.4/100 person-years. While age-adjusted abortion rates among HIV-negative women decreased over time ( P trend = .0015), the rates for HIV-positive women were significantly lower during the earlier time periods (1994 through 1997, 1998 through 2001) with no difference at periods 2002 through 2005 and 2006 through 2012. Of the 301 pregnancies (181 HIV-positive and 120 HIV-negative) that ended in nonviable pregnancies, there was no difference in the overall age-adjusted nonviable pregnancy rate between HIV-negative and HIV-positive ( P = .611) and no significant trend over the years of observation ( P trend = .534 and .149 for HIV-positive and HIV-negative, respectively).
Associations with live-birth rates
Bivariable associations are presented in Supplemental Tables 4 and 5 . Notably, among HIV-negative and HIV-positive women, income, insurance status, and contraception use were not associated with live-birth rates. For HIV-positive women, cART use (cIRR, 1.69; 95% CI, 1.32–2.16), lower viral loads (<4 vs ≥4 log 10 copies/mL, cIRR, 2.90; 95% CI, 2.17–3.87), and higher CD4 + T-cell counts (≥350 vs <350 cells/mm 3 , cIRR, 2.70; 95% CI, 2.00–3.57) were associated with increased live-birth rates in crude analyses.
In multivariable models among HIV-negative women ( Table 1 ), those living with a partner (vs married, aIRR, 1.65; 95% CI, 1.07–2.54), and with less than a high-school education (aIRR, 1.38; 95% CI, 1.02–1.85) had higher live-birth rates, while older age (≥35 vs <25 years, aIRR, 0.22; 95% CI, 0.14–0.35), history of abortion (aIRR, 0.73; 95% CI, 0.55–0.98), using condoms (aIRR, 0.55; 95% CI, 0.41–0.73), marijuana use (aIRR, 0.57; 95% CI, 0.39–0.84), or drug use (aIRR, 0.32; 95% CI, 0.15–0.66) were associated with decreased live-birth rates. For HIV-positive women ( Table 1 ), older age (≥35 vs <25 years, aIRR, 0.14; 95% CI, 0.08–0.23), alcohol consumption (>7 vs <7 drinks/wk, aIRR, 0.47; 95% CI, 0.22–0.97), using condoms (aIRR, 0.73; 95% CI, 0.56–0.97), and CD4 + T-cell count (<350 vs ≥350 cells/mm 3 , aIRR, 0.72; 95% CI, 0.53–0.97) were associated with lower live-birth rates. There was a nonsignificant trend toward increased live-birth rates among those using cART compared to nonusers (aIRR, 1.35; 95% CI, 0.99–1.83) in the multivariable model that included variables such as CD4 + T-cell count and viral load. In multivariate models for both HIV-positive and HIV-negative women, time period was not significantly associated with live birth.
| Variable | HIV-negative | HIV-positive | ||||
|---|---|---|---|---|---|---|
| aIRR | 95% CI | aIRR | 95% CI | |||
| Age 25–29 vs <25 y | 0.59 | 0.39 | 0.89 | 0.87 | 0.55 | 1.40 |
| Age 30–34 vs <25 y | 0.46 | 0.30 | 0.70 | 0.52 | 0.32 | 0.84 |
| Age ≥35 vs <25 y | 0.22 | 0.14 | 0.35 | 0.14 | 0.08 | 0.23 |
| Living with partner vs married | 1.65 | 1.07 | 2.54 | – | – | – |
| <High school vs ≥high school education | 1.38 | 1.02 | 1.85 | – | – | – |
| Prior abortion vs no | 0.73 | 0.55 | 0.98 | – | – | – |
| ≥7 vs <7 Drinks/wk | – | – | – | 0.47 | 0.22 | 0.97 |
| Use condom vs no | 0.55 | 0.41 | 0.73 | 0.73 | 0.56 | 0.97 |
| Marijuana use vs no | 0.57 | 0.39 | 0.84 | – | – | – |
| Drug use vs no | 0.32 | 0.15 | 0.66 | – | – | – |
| cART use vs no | – | – | – | 1.35 a | 0.99 | 1.83 |
| CD4 + T-cell count <350 vs ≥350 mm 3 | – | – | – | 0.72 | 0.53 | 0.97 |
Associations with abortion rate
Bivariable associations with abortion rate are presented in Supplemental Tables 4 and 5 . Notably, among HIV-negative and HIV-positive women, income, marital status, insurance status, contraception use, and number of male sexual partners were not associated with abortion rates. For HIV-positive women, cART use was significantly associated with reduced abortion rates (cIRR, 0.61; 95% CI, 0.44–0.85) while lower viral loads (<4 vs ≥4 log 10 copies/mL, cIRR, 1.43; 95% CI, 1.03–1.98) and higher CD4 + T-cell counts (≥350 vs <350 cells/mm 3 , cIRR, 1.82; 95% CI, 1.32–2.50) were each significantly associated with increased abortion rates.
Multivariable models for abortion rates for HIV-negative and HIV-positive women are presented in Table 2 . Among HIV-negative women, older age was associated with decreased abortion rates (≥35 vs <25 years, aIRR, 0.07; 95% CI, 0.04–0.13), while increased parity (≥3 vs 0, aIRR, 3.14; 95% CI, 1.66–5.93), having a history of abortion (aIRR, 1.56; 95% CI, 1.06–2.30), and use of condoms (aIRR, 2.18; 95% CI, 1.58–3.01) were associated with increased rates of abortion. Further, study site was also associated with abortion rate, with significantly fewer abortions among participants at the Washington, DC, and Chicago, IL, sites compared to the Bronx, NY, site (aIRR, 0.47; 95% CI, 0.22–0.99 and aIRR, 0.18; 95% CI, 0.06–0.52, respectively).
| Variable | HIV-negative | HIV-positive | ||||
|---|---|---|---|---|---|---|
| aIRR | 95% CI | aIRR | 95% CI | |||
| Age 25–29 vs <25 y | 0.49 | 0.33 | 0.74 | 0.44 | 0.26 | 0.76 |
| Age 30–34 vs <25 y | 0.27 | 0.17 | 0.43 | 0.29 | 0.16 | 0.51 |
| Age ≥35 vs <25 y | 0.07 | 0.04 | 0.13 | 0.14 | 0.07 | 0.25 |
| Brooklyn vs Bronx, NY | – | – | – | 1.96 | 1.07 | 3.59 |
| Washington, DC, vs Bronx, NY | 0.47 | 0.22 | 0.99 | – | – | – |
| Chicago, IL, vs Bronx, NY | 0.18 | 0.06 | 0.52 | – | – | – |
| Baseline parity 1–2 vs 0 | 2.55 | 1.54 | 4.23 | 1.73 | 0.99 | 3.03 |
| Baseline parity ≥3 vs 0 | 3.14 | 1.66 | 5.93 | 2.64 | 1.41 | 4.93 |
| Prior abortion vs no | 1.56 | 1.06 | 2.30 | 1.72 | 1.16 | 2.55 |
| Prior STI vs no | – | – | – | 0.63 | 0.43 | 0.92 |
| Use condom vs no | 2.18 | 1.58 | 3.01 | 2.40 | 1.52 | 3.79 |
For HIV-positive women, abortion rates were negatively associated with age (≥35 vs <25 years, aIRR, 0.14; 95% CI, 0.07–0.25) and positively associated with prior abortion (aIRR, 1.72; 95% CI, 1.16–2.55), study site (Brooklyn vs Bronx, NY, aIRR, 1.96; 95% CI, 1.07–3.59), and condom use (aIRR, 2.40; 95% CI, 1.52–3.79). Prior self-reported sexually transmitted infection was associated with decreased abortion rates (aIRR, 0.63; 95% CI, 0.43–0.92). Time period, cART use, CD4 + T-cell count, and viral load were not associated with abortion rates in the multivariate model.
Comment
Although reduced pregnancy and live-birth rates had been the reported among HIV-positive vs HIV-negative women during the earlier HIV epidemic, we demonstrate that in the current cART era these rates have increased among HIV-positive women and are now comparable to rates among HIV-negative women. Studies in sub-Saharan Africa have suggested a similar trend toward increasing pregnancy rates with ART use, however this trend among women in developed countries has not been previously demonstrated. Increased contraception, sterilization, and sexual abstinence may have led to initial drops in pregnancy rates among those living with HIV, perhaps driven by fear of transmission, poor overall health status, and stigma. Increases in pregnancy and live-birth rates may be reflective of improved fecundability, improved health, and changing fertility intentions. Fertility intention may be influenced by overall improvement in perceived well-being, life expectancy, reduced fear of perinatal HIV transmission, and more positive attitudes about pregnancy among HIV care providers and communities. Women with lower CD4 + T-cell counts, with higher viral loads, and not using cART continued to have lower live-birth rates. Further, while age-adjusted live-birth rates were significantly increased, when factors such as cART use, viral load, and CD4 + T-cell count were included in our models, time period was no longer associated with live-birth rate. This suggests that HIV-related clinical factors play a central role in the increased trend. Interestingly, although cART use, CD4 count, and viral load were significant in the bivariate analysis, after including them in the multivariable model and adjusting for other important covariates, only CD4 + T-cell count was significantly associated with increased live-births rates.
Approximately half of all pregnancies in the United States are unintended; among those, 40% will end in induced abortion. Estimates for unintended pregnancy among women living with HIV may be much higher. A 2014 study among HIV-positive pregnant women found 23% had an unplanned pregnancy, 58% were ambivalent, and only 19% reported a planned pregnancy. Previous analysis from the WIHS cohort had documented reduced abortion rates during the early HIV epidemic in conjunction with fewer overall pregnancies. While our data and others showed a decrease in abortion rates over the last decade among HIV-negative women, we demonstrate that the rate among those living with HIV has not altered. Notably, cART use, CD4 + T-cell count, and viral load were not associated with abortion rates among HIV-positive women, suggesting that for many women the complex decision-making regarding pregnancy continuation and abortion may not be strongly influenced by their HIV disease state. Similar to previous literature, predictors of abortion among both HIV-positive and HIV-negative high-risk women include younger age, increased parity, and history of having had an abortion. These high-risk groups should be targeted for contraceptive counseling.
Condoms were the dominant form of contraception in our cohort. Given high, typical-use pregnancy rates of 18% per year with condom use, condoms alone have limited utility for unintended pregnancy prevention. Use of other contraceptives among our cohort was low, with women reporting either a hormonal or LARC method at only 15% of visits. Ineffective contraception use with a high reliance on condoms alone among WIHS participants has been reported, where HIV-positive women in this cohort have not experienced an increase in LARC use seen in HIV-negative women. As contraceptive use was not associated with live-birth rates, we can conclude that many women may be offered or choose less effective methods, do not use them consistently or correctly, and continue with pregnancy when contraceptives fail. Previous studies demonstrated poor utilization of the most effective long-term contraceptives among women living with HIV, despite increasing uptake nationally. Long-acting contraceptive options, such as intrauterine devices and contraceptive implants, are safe for HIV-positive and at-risk women, have high continuation rates, and reduce unintended pregnancy rates. With potentially increased health engagement among women in HIV care, opportunities for integrated family planning services must be sought to reduce barriers to safe contraception. Educational messaging must shift from condom use alone to encourage dual protection, with a hormonal or LARC method for pregnancy prevention and concurrent use of condoms for effective sexually transmitted infection and HIV prevention. Novel approaches may be needed to successfully integrate reproductive health services into HIV care.
This study has several limitations. Information on pregnancy outcomes and covariates were based on self-report and are thus prone to potential misclassification; however self-reported data used in other WIHS were strongly correlated with clinical outcomes and several personal self-reported questions gathered throughout the WIHS cohort may not be routinely available in clinical chart reviews or provider-generated database evaluations. Study intervals of 6 months for assessment of time-varying covariates limited our ability to evaluate changes during those intervals; however this is likely to be more generalizable to data that could be collected in a clinical setting. Moreover, we do not have reliable data on pregnancy intention or abortion access. Eligibility criteria in this longitudinal cohort potentially limits external validity. The average age of our cohort is relatively advanced, thus results may not reflect the younger HIV-positive or high-risk HIV-negative women or those with perinatally acquired HIV. Lastly, we dichotomized CD4 T-cell count and viral load based on predetermined values to reflect poor HIV control, however other lower cut-offs are often used for defining virologic suppression.
In conclusion, we demonstrate that overall pregnancy rates are now similar between HIV-positive and high-risk HIV-negative women, possibly due to improved health status among HIV-positive women coupled with shifting attitudes due to reduced risk of maternal-to-child transmission. With that said, abortion rates have remained consistent and do not appear to be associated with HIV-related health variables, indicating that unintended pregnancy among all individuals need to be addressed. Given high failure rates with condom use alone, dual protection with hormonal contraceptives or nonhormonal intrauterine devices should be strongly encouraged for women seeking contraception. Integrating reproductive health services into HIV clinical care can reduce barriers and improve care for high-risk women and provide an important opportunity to address family planning and safe conception practices.
Acknowledgment
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health. WIHS (principal investigators): University of Alabama, Birmingham WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta, GA WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx, NY WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn, NY WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago, IL WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington, DC WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami, FL WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; University of North Carolina, Chapel Hill WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; and Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV).
Appendix
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


