● TETRALOGY OF FALLOT
Definition, Spectrum of Disease, and Incidence
Tetralogy of Fallot (TOF) is characterized by a subaortic (malaligned) ventricular septal defect (VSD), an aortic root that overrides the VSD, and infundibular pulmonary stenosis (Fig. 25.1). Right ventricular hypertrophy, which represents the fourth anatomic feature of the “tetralogy,” is typically not present prenatally. The typical form is the TOF with pulmonary stenosis, but the spectrum of TOF includes severe forms, such as TOF with pulmonary atresia and TOF with absent pulmonary valve, both of which will be discussed in more detail later in this chapter. TOF is one of the most common forms of cyanotic congenital heart disease (CHD) and is found in about 1 in 3,600 live births and accounts for 3% to 7% of infants with CHD (1–3). The classic form of TOF with pulmonary stenosis accounts for about 80% of all newborns with TOF.
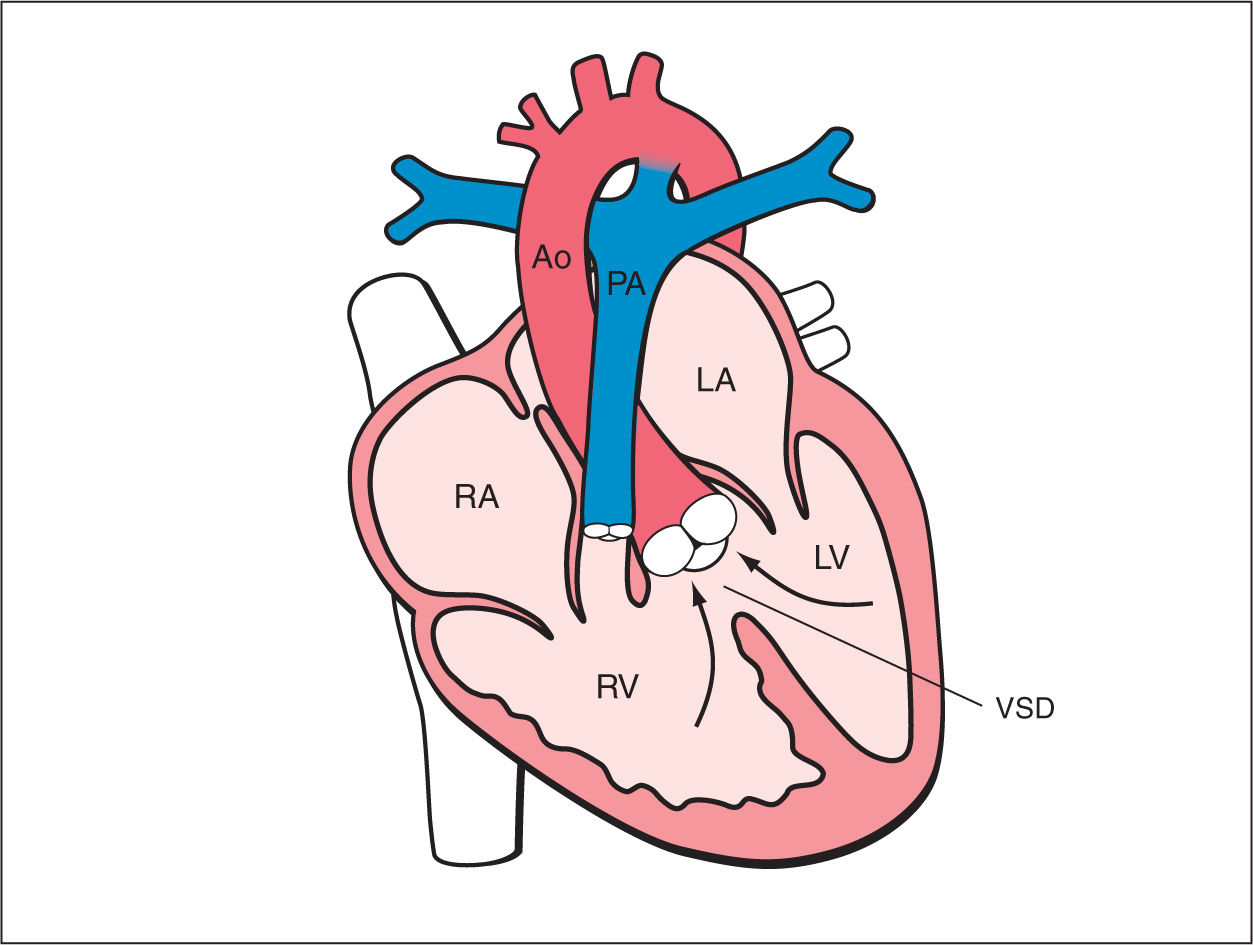
Figure 25.1: Schematic drawing of tetralogy of Fallot. See text for details. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; Ao, aorta; VSD, ventricular septal defect; PA, pulmonary artery.
Ultrasound Findings
Gray Scale
In TOF, the four-chamber view appears normal (Fig. 25.2A) unless the VSD is large and visible in this plane. TOF is typically detected in the five-chamber view, which demonstrates a perimembranous subaortic VSD with an aortic root override (4) (Figs. 25.2B and 25.3 to 25.5). This aortic override is due to a discontinuity between the interventricular septum and the medial aortic wall (so-called malalignement VSD), with a partial connection of the aorta to the right ventricle (Fig. 25.3). The aorta is thus slightly shifted to the right, a condition termed aortic dextroposition. Generally the aortic root, which receives blood from both the right and left ventricles, appears dilated, especially in the third trimester, which may provide the first hint to the presence of TOF (5). Furthermore, in TOF, the overriding aorta assumes a parallel course to the ventricular septum in contrast to the ascending aorta in a normal heart (Fig. 25.3). The diagnosis of TOF also requires the demonstration of a narrow but patent main pulmonary artery “pulmonary stenosis,” which is best demonstrated in the short-axis or the three-vessel view (Figs. 25.4 and 25.5). In some milder forms of TOF, especially in midgestation, the size discrepancy between the pulmonary trunk and the aorta may be subtle. The discrepancy in size, however, becomes more obvious with advancing gestation. Occasionally, TOF is missed on second trimester ultrasound. In general, these are mild cases, where the discrepancy of vessel diameter at this stage is not evident and the VSD not easily seen. The authors have also observed a few cases of perimembranous VSDs, diagnosed in early gestation with normal-sized great vessels. With advancing gestation, these VSDs became more malaligned and the preferential flow toward the ascending aorta resulted in discrepant great vessel diameters, developing into mild forms of TOF.
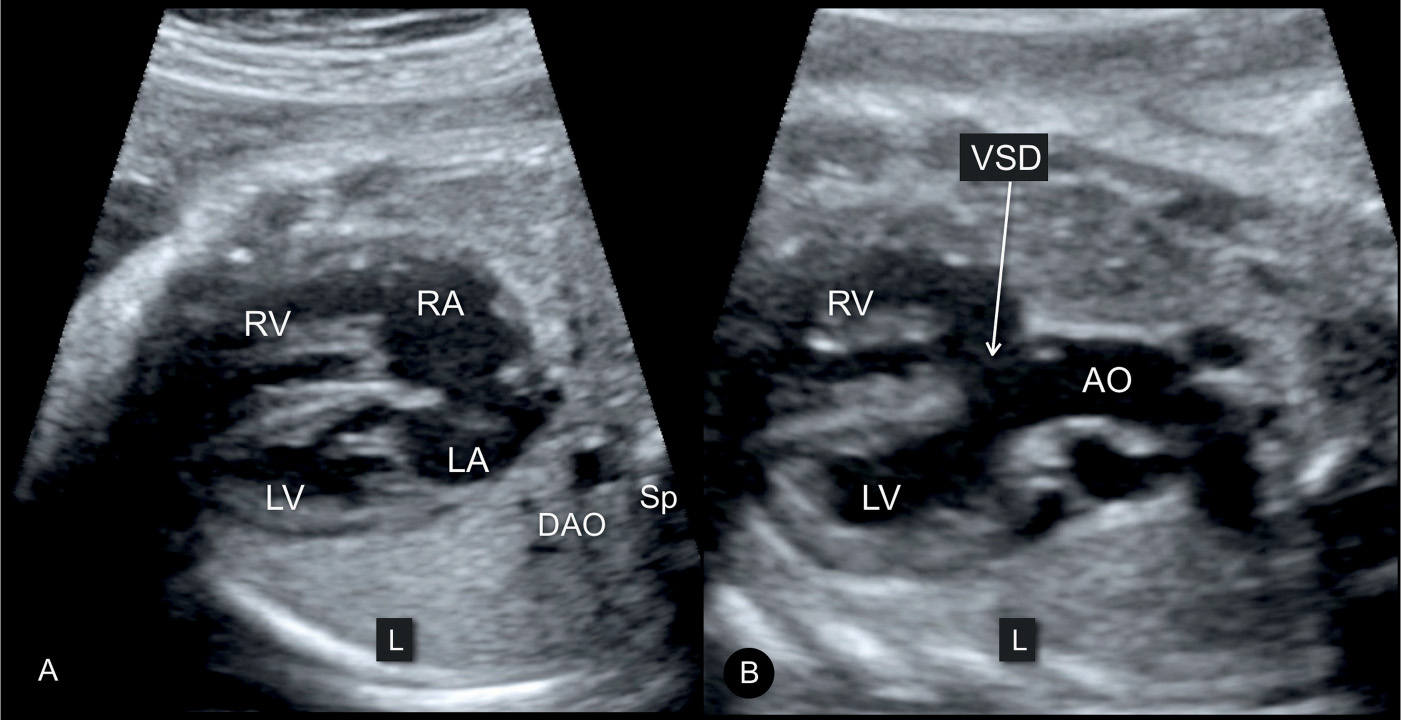
Figure 25.2: Transverse planes of the fetal chest in gray scale at the level of the four-chamber view (A) and the five-chamber view (B) in a fetus with tetralogy of Fallot. Note the normal appearance of the four-chamber view in A and the large ventricular septal defect (VSD) (arrow) and dilated overriding aorta (AO) in B. The descending aorta (DAO) is seen to the left to the spine (Sp) in A. LV, left ventricle; RV, right ventricle; RA, right atrium; LA, left atrium.
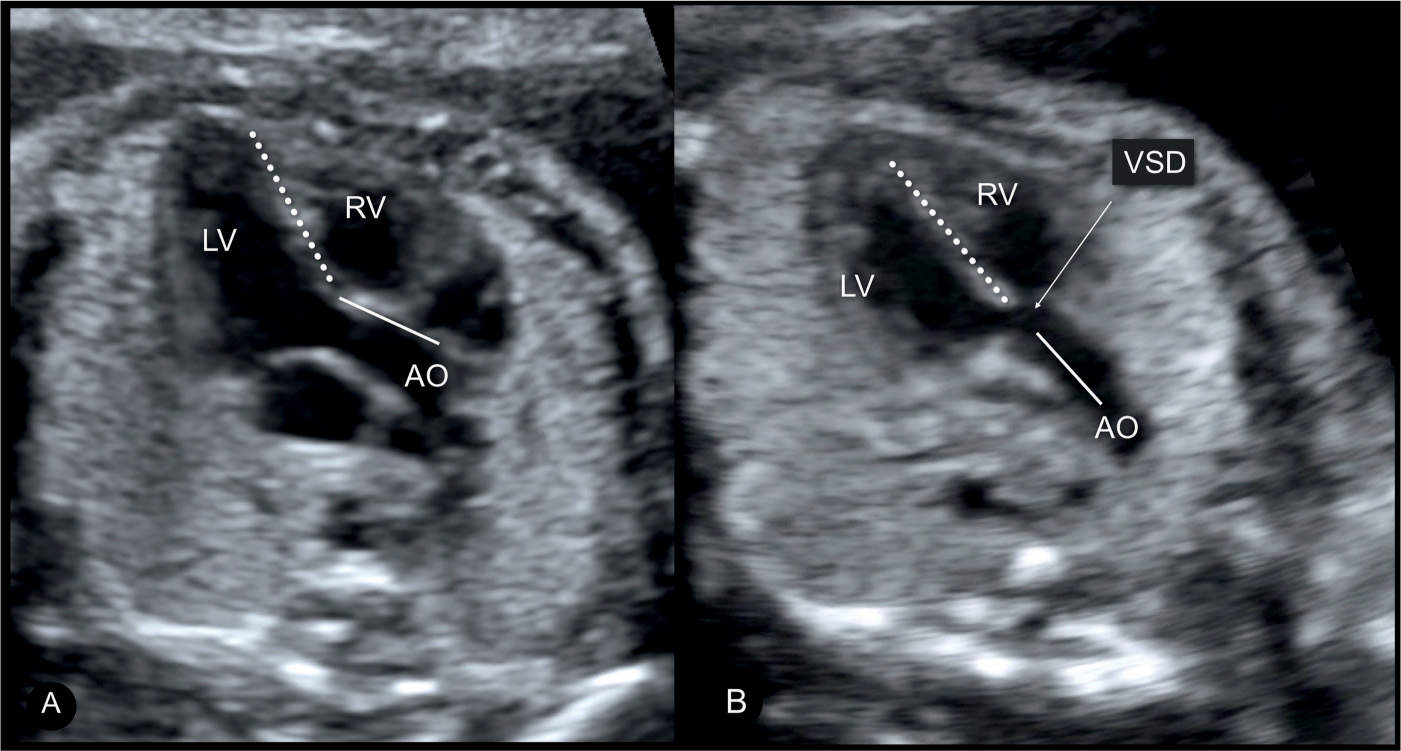
Figure 25.3: Comparison of apical five-chamber views in a normal fetus (A) and a fetus with tetralogy of Fallot with aortic override and a large ventricular septal defect (VSD) (B). In the normal fetus (A), the ascending aorta (AO) points to the right fetal shoulder with a wide angle between the direction of the ventricular septum (dashed line) and the anterior wall of the ascending aorta (solid line). In the fetus with aortic override (B), the course of the ascending aorta (solid line) is parallel to the ventricular septum (dashed line). This finding is also noted in other anomalies involving aortic override. LV, left ventricle; RV, right ventricle.
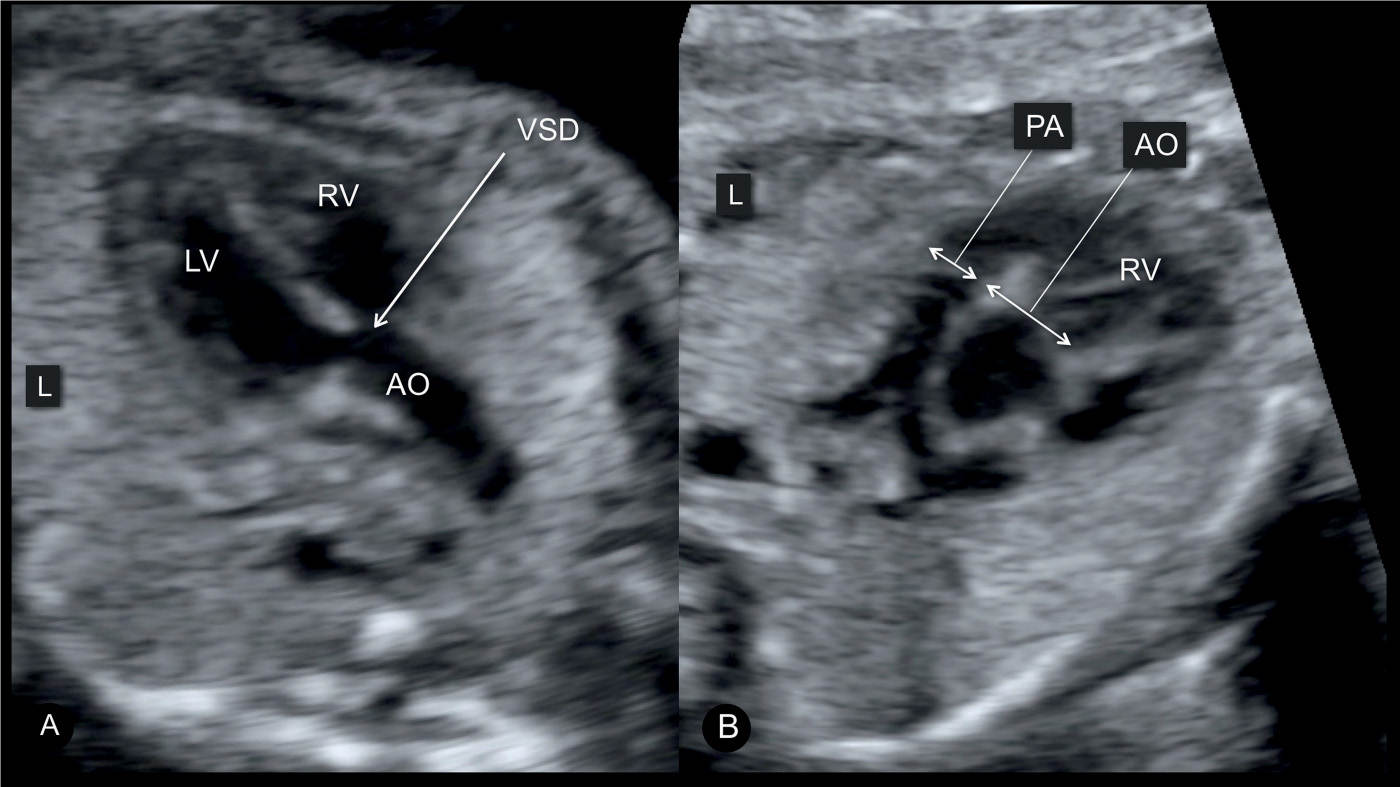
Figure 25.4: Two typical diagnostic planes for tetralogy of Fallot: the five-chamber view plane (A) and the three-vessel view plane (B). In the five-chamber view (A), the ventricular septal defect (VSD) with an overriding aorta (AO) is demonstrated. In the three-vessel view plane (B), the main pulmonary artery (PA) is small due to the presence of pulmonary stenosis and the aorta is dilated due to increased blood flow. L, left; LV, left ventricle; RV, right ventricle.
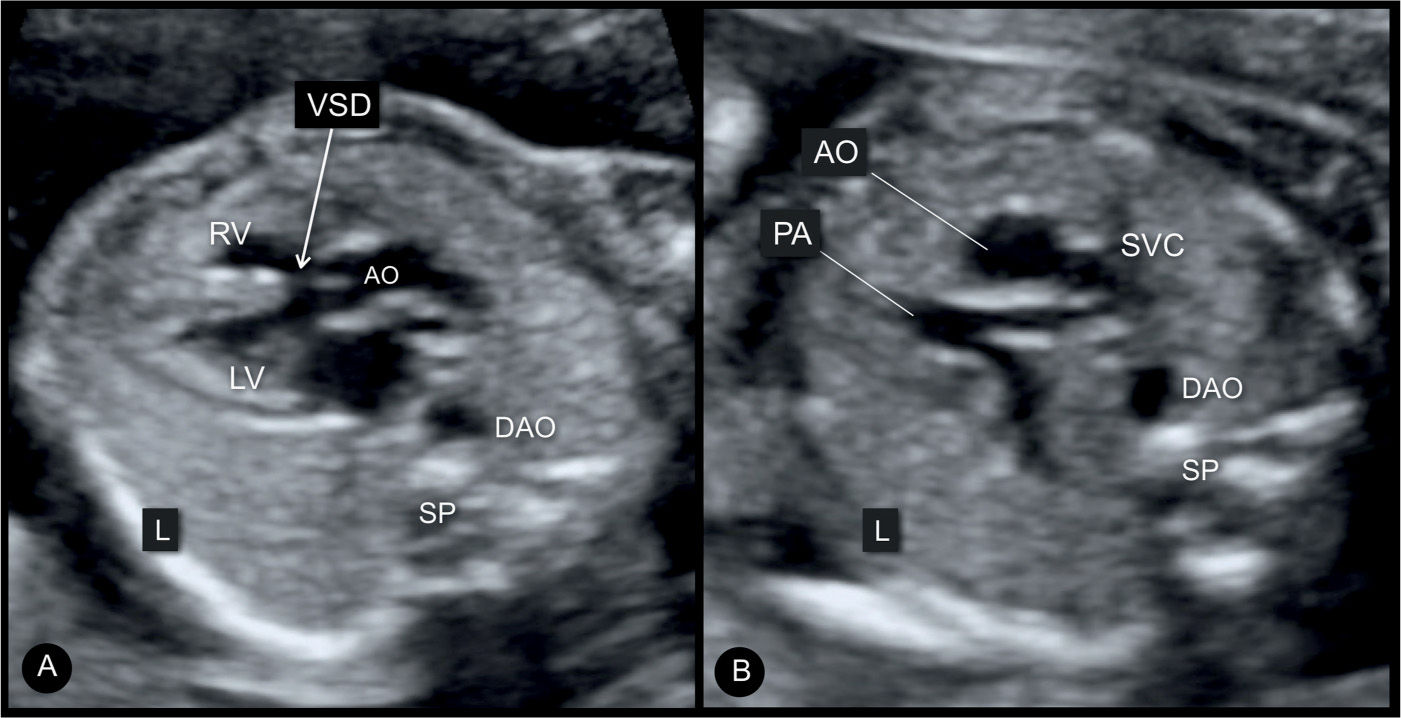
Figure 25.5: Axial five-chamber view plane (A) and three-vessel view plane (B) in a fetus with tetralogy of Fallot. Note the large ventricular septal defect (VSD) (arrow) with the typical aortic override in A and in a small pulmonary artery (PA), when compared to the dilated aorta (AO) in B. Note that in this fetus the descending aorta (DAO) is on the right side of the spine (SP) as an associated right aortic arch. L, left; LV, left ventricle; SVC, superior vena cava; RV, right ventricle.
Color Doppler
Color Doppler facilitates the demonstration of VSD (shunting of blood across the VSD) and confirms the presence of an overriding aorta with blood draining from both ventricles into the aortic root (Fig. 25.6). Color Doppler at the three-vessel-trachea view can also demonstrate a small pulmonary artery (Figs. 25.7 and 25.8). Often, the inflow into the aorta appears aliased on color Doppler due to the high perfusion (Fig. 25.6). Color and pulsed Doppler velocities in the pulmonary artery are generally normal or only mildly increased in the fetus, in contrast to postnatal findings (6) (Figs. 25.8 and 25.9). Flow across the ductus arteriosus is antegrade in mild TOF, but can also be reversed in more severe cases (Fig. 25.9). In such cases, postnatal ductus dependency of the pulmonary circulation can be associated with cyanosis of the newborn. Fetal echocardiography along with color Doppler can differentiate various subgroups of TOF as shown in Table 25.1.
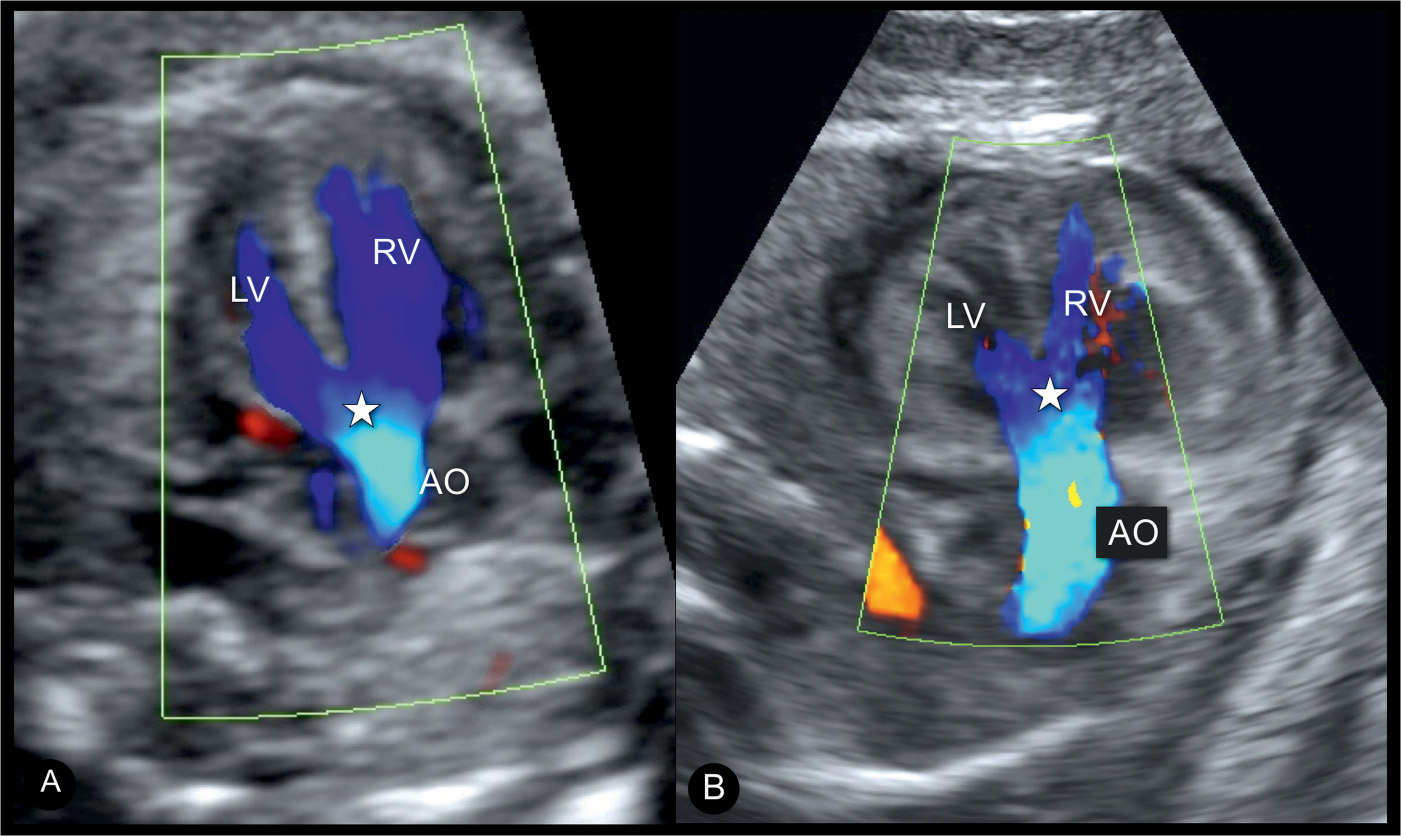
Figure 25.6: Apical five-chamber views with color Doppler in a fetus (A) with an isolated tetralogy of Fallot (TOF) and in a fetus (B) with TOF in association with an atrioventricular septal defect and thickened myocardium. Color Doppler demonstrates blood filling the overriding, dilated aorta (AO) from both right (RV) and left (LV) ventricles in both fetuses. Star denotes the site of the ventricular septal defect and the aortic override. The fetus in B demonstrates that TOF can be part of a complex cardiac malformation.
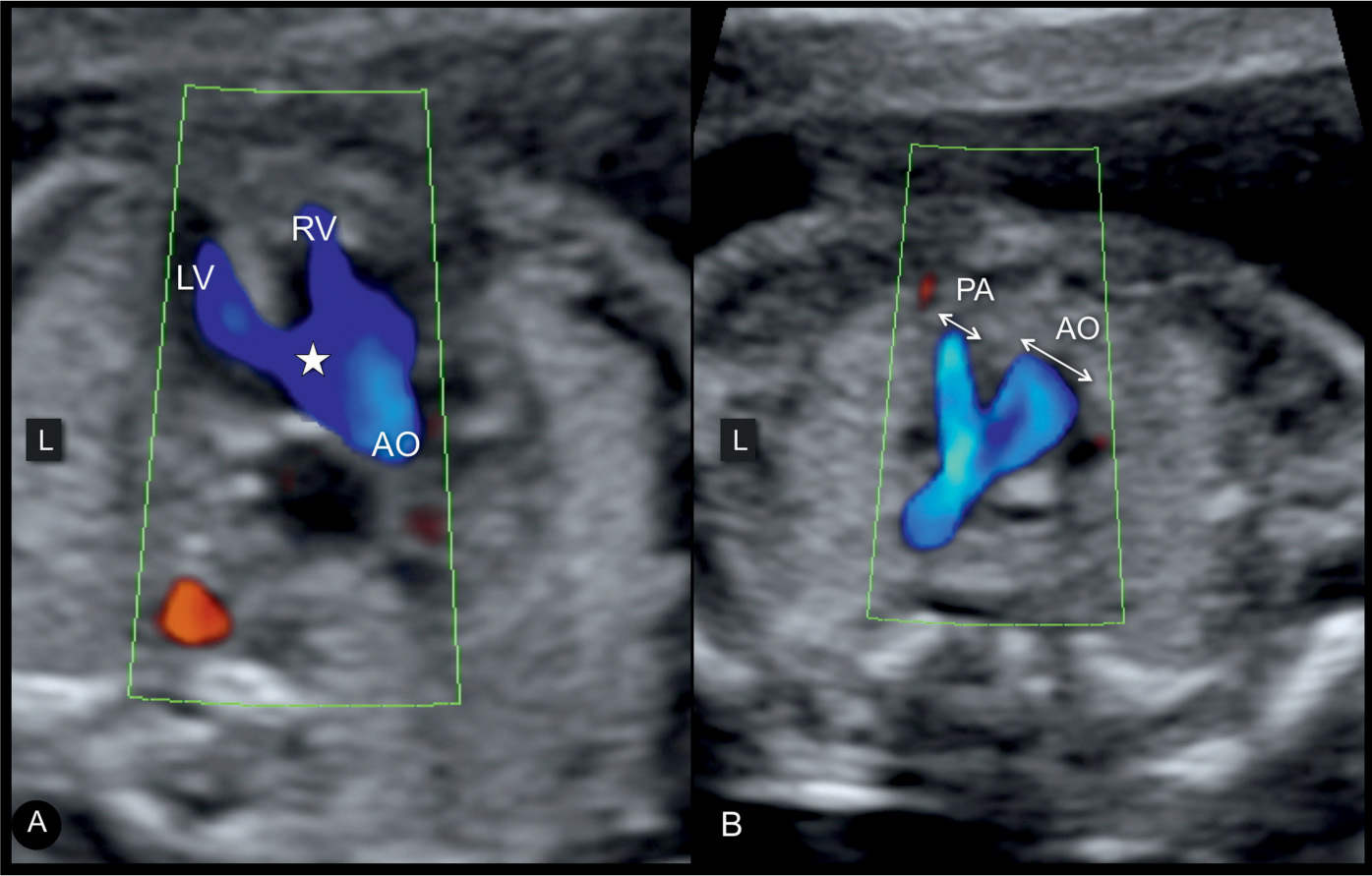
Figure 25.7: Axial five-chamber view plane (A) and three-vessel-trachea view plane (B) in color Doppler in a fetus with tetralogy of Fallot (TOF). Note the three main sonographic findings in TOF demonstrated on color Doppler: a ventricular septal defect (star) in A, an overriding aorta (AO) shown in A, and pulmonary artery (PA) stenosis with antegrade flow shown in B. Compare the color Doppler findings in this figure with the grayscale findings in Figures 25.4 and 25.5. L, left; LV, left ventricle; RV, right ventricle.
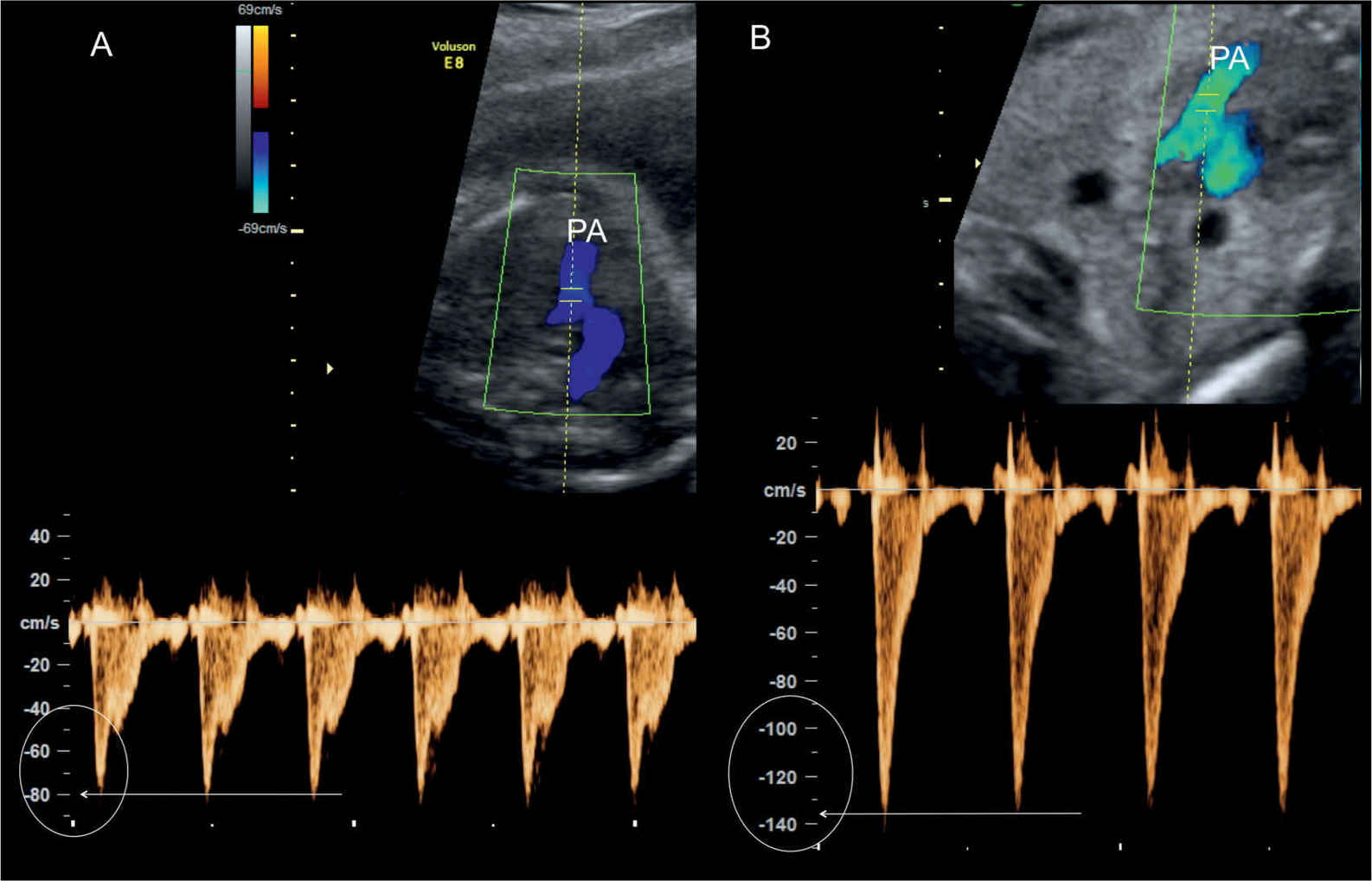
Figure 25.8: Color and pulsed Doppler across the pulmonary valves in two fetuses with tetralogy of Fallot (TOF). In fetus A, Doppler velocities are within the normal range at 80 cm/s, and in fetus B, Doppler velocities are slightly elevated at 130 cm/s. The diagnosis of pulmonary stenosis in TOF primarily relies on the diminutive pulmonary artery (PA) rather than the measured peak velocity.
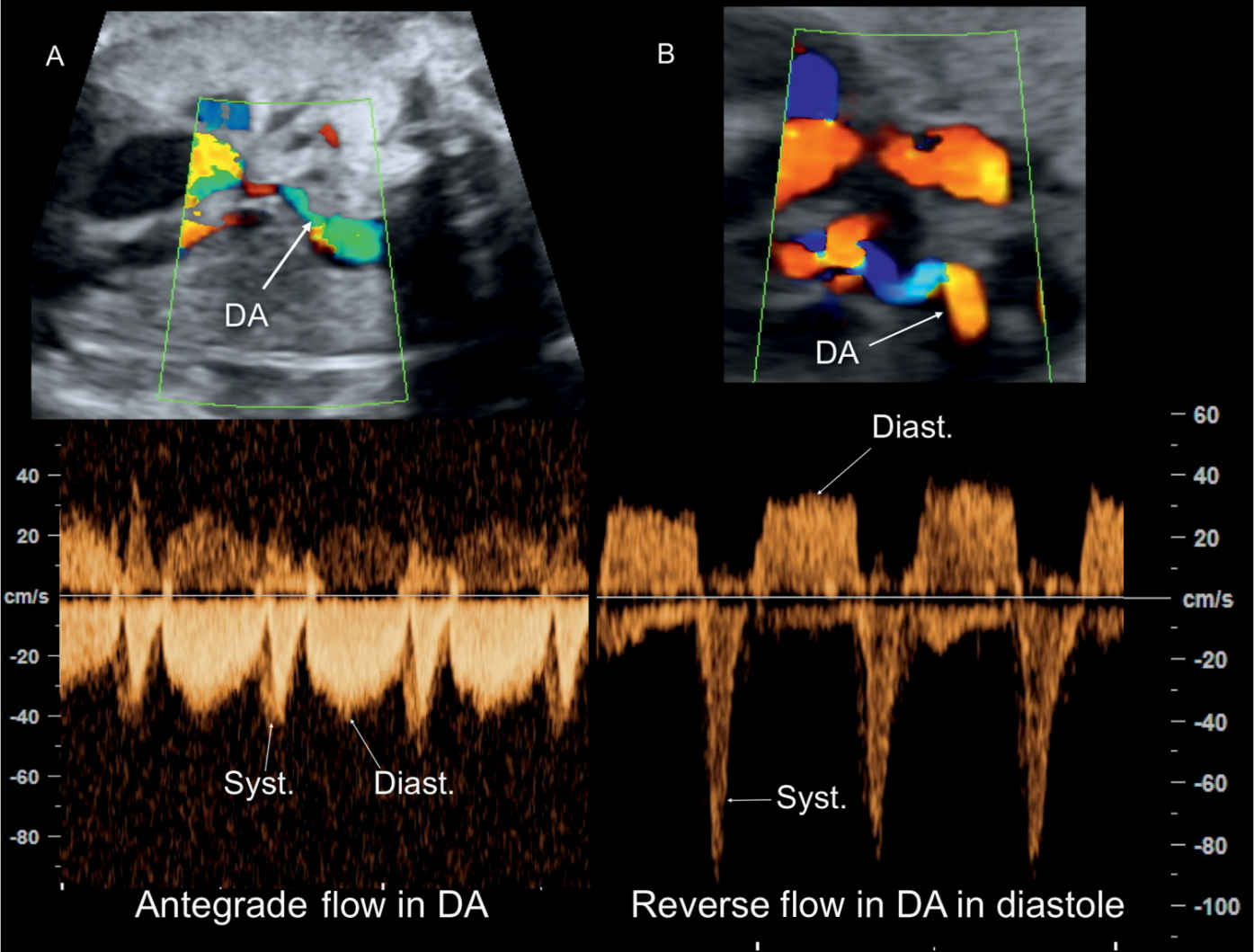
Figure 25.9: Color and pulsed Doppler across the ductus arteriosus (DA) in two fetuses with tetralogy of Fallot (TOF) and diminutive DA. In A, antegrade flow in the DA is shown throughout the cardiac cycle. In B, reverse flow in the DA is shown during diastole. Fetus B is at risk of ductal-dependent circulation postnatally. Syst., systole; Diast., diastole.
Differentiating Features of the Three Subtypes of Tetralogy of Fallot (TOF) |
TOF 1 Pulmonary stenosis | TOF 1 Pulmonary atresia | TOF 1 Absent pulmonary valve | |
Four-chamber view | Normal | Normal | RV dilated, especially in late gestation |
Five-chamber view | VSD + Overriding aorta | VSD + Overriding aorta | VSD + Overriding aorta |
Aortic root | Dilated + | Dilated ++ | Normal size |
Pulmonary artery | Narrow, antegrade flow | Very narrow, or even not visible; retrograde flow | Markedly dilated with to-and-fro flow |
Ductus arteriosus | Antegrade or retrograde flow In right aortic arch, ductus arteriosus is difficult to see as it is under the aortic arch | Tortuous with retrograde perfusion | Typically absent |
MAPCAs | Not typical | Present in more than 20% of cases | Absent |
Prognosis | Good | Guarded | Guarded |
Deletion 22q11 | 10%–15% | 20%–25% | 30%–40% |
MAPCAs, major aortopulmonary collateral arteries; RV, right ventricle; VSD, ventricular septal defect.
Early Gestation
In the late first and early second trimesters, the diagnosis of TOF is possible but in many cases difficult. Clues to the diagnosis include a large aortic root in the five-chamber view on gray scale and color (Fig. 25.10) and/or a small pulmonary artery (Fig. 25.11). The aortic override may not be easily detected. A deviation of the cardiac axis (Fig. 25.11A) may be a first hint for the presence of a cardiac malformation (7). The discrepant size between the aorta and pulmonary artery with antegrade flow in both vessels on color Doppler is an important sign at this early gestation (Figs. 25.10 and 25.11). A strong association between an increased nuchal translucency measurement and the diagnosis of TOF was reported, even in the absence of chromosomal abnormalities, with almost half of the cases in one study showing this association (8).
Three-Dimensional Ultrasound
The tomographic mode applied to a three-dimensional (3D) volume acquired at the level of the four-chamber view allows the demonstration of the VSD, aortic overriding, and the stenotic pulmonary artery in a single view of multiple planes (Fig. 25.12). Surface mode increases the spatial demonstration of aortic override (Fig. 25.13). Spatiotemporal image correlation (STIC) with color Doppler displayed in glass-body mode provides a clear demonstration of the aortic override (Fig. 25.14A) and the lesion in the three-vessel-trachea view with a narrow pulmonary artery when compared to the aorta (compare Fig. 25.14B with Fig. 15.20C).
Associated Cardiac and Extracardiac Findings
Associated cardiac abnormalities can be found in TOF. On prenatal ultrasound, the common findings include a right aortic arch, found in up to 25% of the cases. Occasionally, an atrioventricular septal defect coexists with TOF, and this increases the risk of chromosomal abnormalities (9). A patent foramen ovale or an atrial septal defect has been reported in 83% and a persistent left superior vena cava in 11% of newborns with TOF (10). Variations in coronary artery anatomy are occasionally seen, which may have an impact on the surgical approach to repair (11).
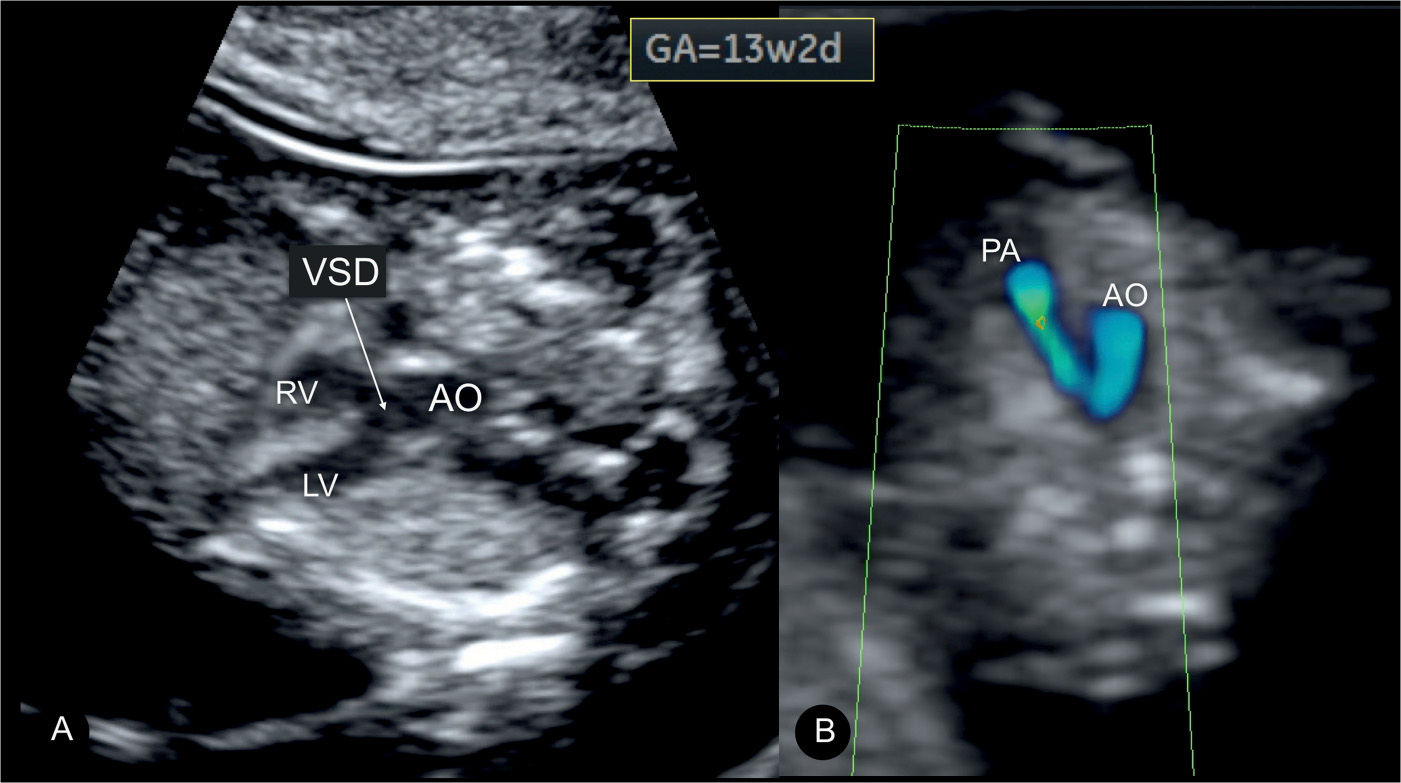
Figure 25.10: Tetralogy of Fallot at 13 weeks’ gestation in a fetus with trisomy 18. In A, a large ventricular septal defect (VSD) is visualized with an overriding aorta (AO) at the five-chamber view. In B, color Doppler at the three-vessel-trachea view shows a small pulmonary artery (PA) with antegrade flow. Findings in the first trimester are similar to those noted in the second trimester (Figs. 25.5A and 25.7B) in TOF. LV, left ventricle; RV, right ventricle.
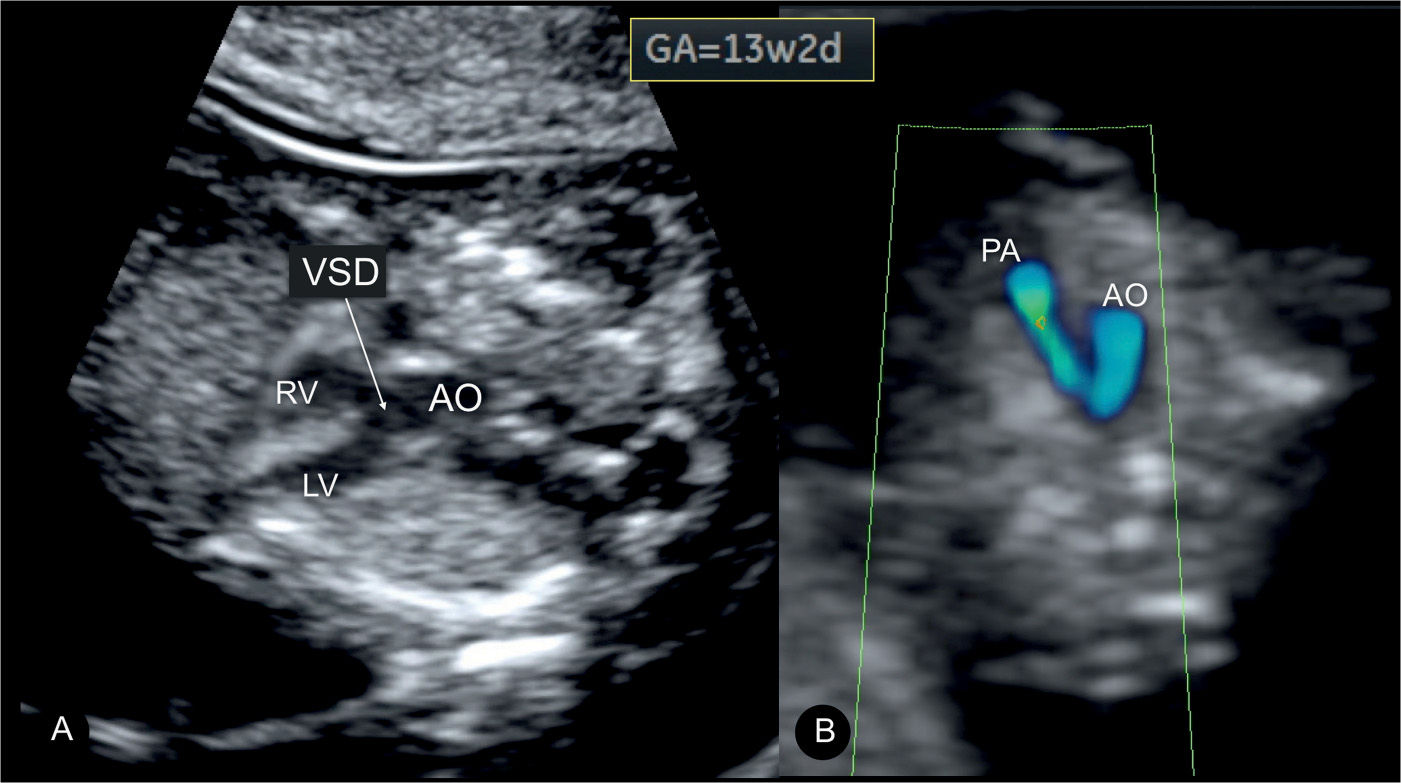
Figure 25.11: Transvaginal ultrasound in a fetus with tetralogy of Fallot at 14 weeks’ gestation. A and B show the four-chamber view in gray scale and color Doppler, respectively. Note the normal appearing four-chamber view in A and B with the exception of mild cardiac axis deviation to the left in A. B demonstrates normal filling during diastole. In C, the five-chamber view in color Doppler shows a dilated aorta (AO) that is overriding the ventricular septal defect (star). In D, the three-vessel-trachea view in color Doppler shows a diminutive pulmonary artery (PA) in comparison to the AO. LV, left ventricle; RV, right ventricle.
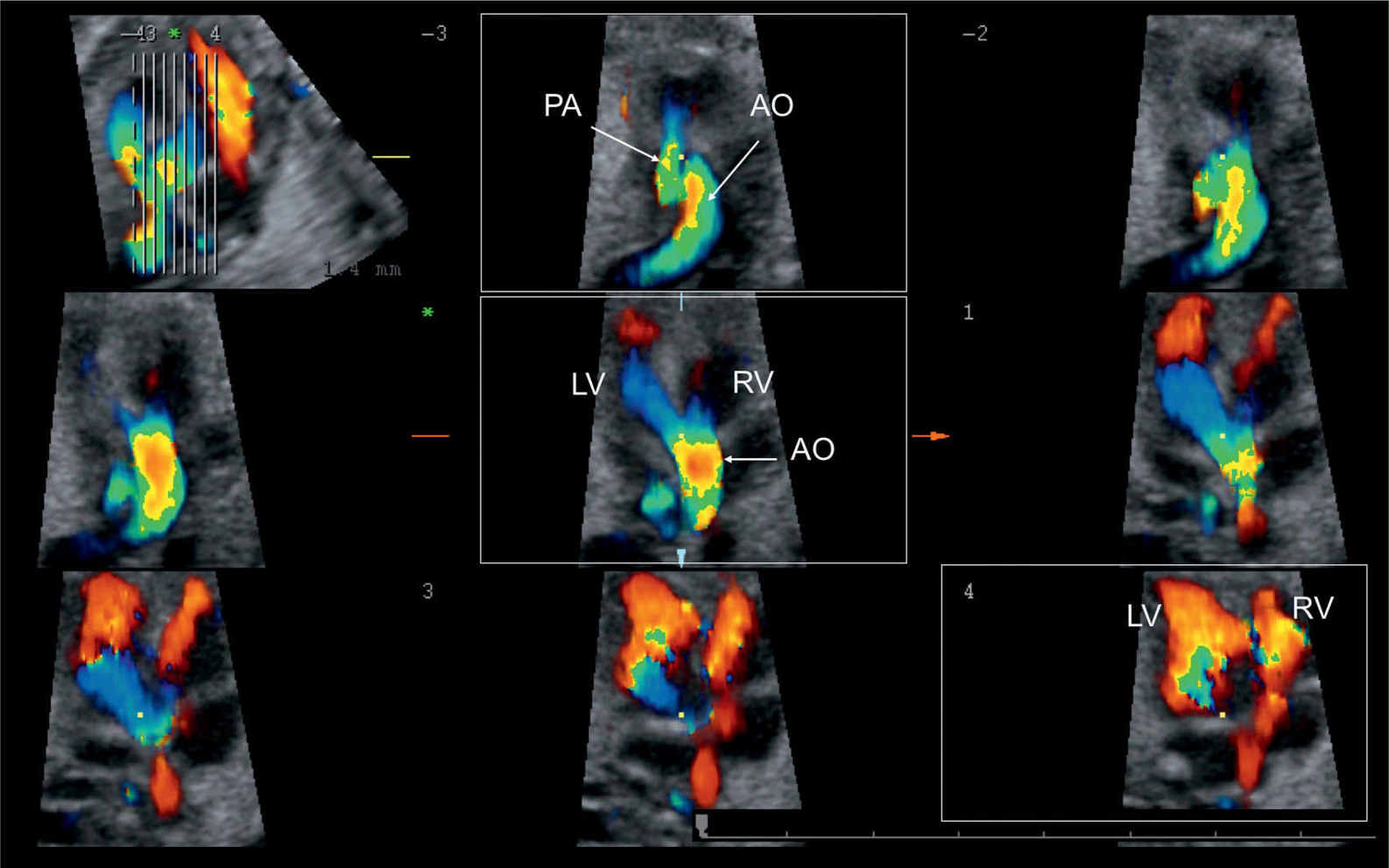
Figure 25.12: 3D ultrasound volume in color spatiotemporal image correlation (STIC) shown in tomographic display in a fetus with tetralogy of Fallot, demonstrating in one view ventricular filling in diastole (box in lower row), aortic override with filling from both ventricles (box in middle row), and a small pulmonary artery (PA) as compared to the aorta (AO) (box in upper row). LV, left ventricle; RV, right ventricle.
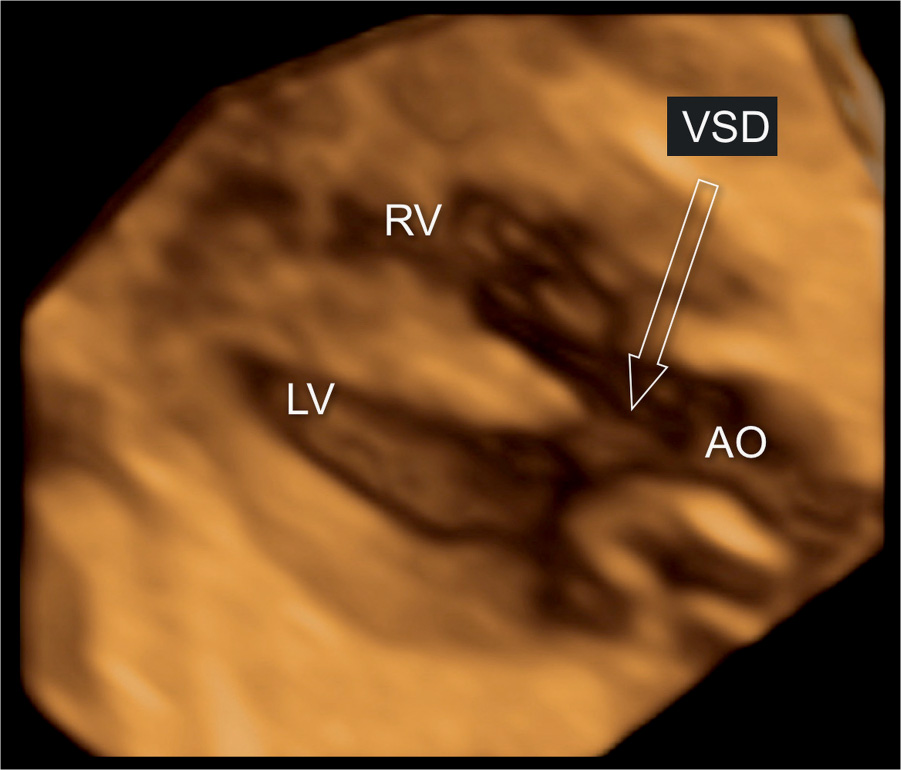
Figure 25.13: 3D ultrasound volume in surface rendering mode display at the five-chamber view in a fetus with tetralogy of Fallot. Note the presence of a large ventricular septal defect (VSD) (arrow) and a dilated, overriding aorta (AO). LV, left ventricle; RV, right ventricle.
Conversely to neonates and infants with diagnosed TOF, the fetus with this cardiac anomaly tends to have a higher incidence of extracardiac malformations, chromosomal anomalies, and genetic syndromes (8). Associated extracardiac congenital anomalies are fairly common with no specific organ involvement. The rate of chromosomal abnormalities is around 30%, with trisomies 21, 13, and 18 accounting for the majority of cases (8). The rate of deletion 22q11 is found in 10% to 15% of fetuses and neonates with TOF (12, 13). The risk of deletion 22q11 in cases of TOF increases when the thymus is hypoplastic (14, 15), the aortic arch is right sided, extracardiac anomalies are noted, or polyhydramnios is found (16). Table 25.2 lists common cardiac and extracardiac abnormalities associated with TOF. TOF can also be found in syndromic diseases, such as Alagille syndrome, CHARGE syndrome (17), and many others. In recent years, there has been an uptake in the use of subchromosomal analysis (microarray) in such complex cardiac malformations, in order to assess for the presence of deletions and duplications (see Chapter 4).
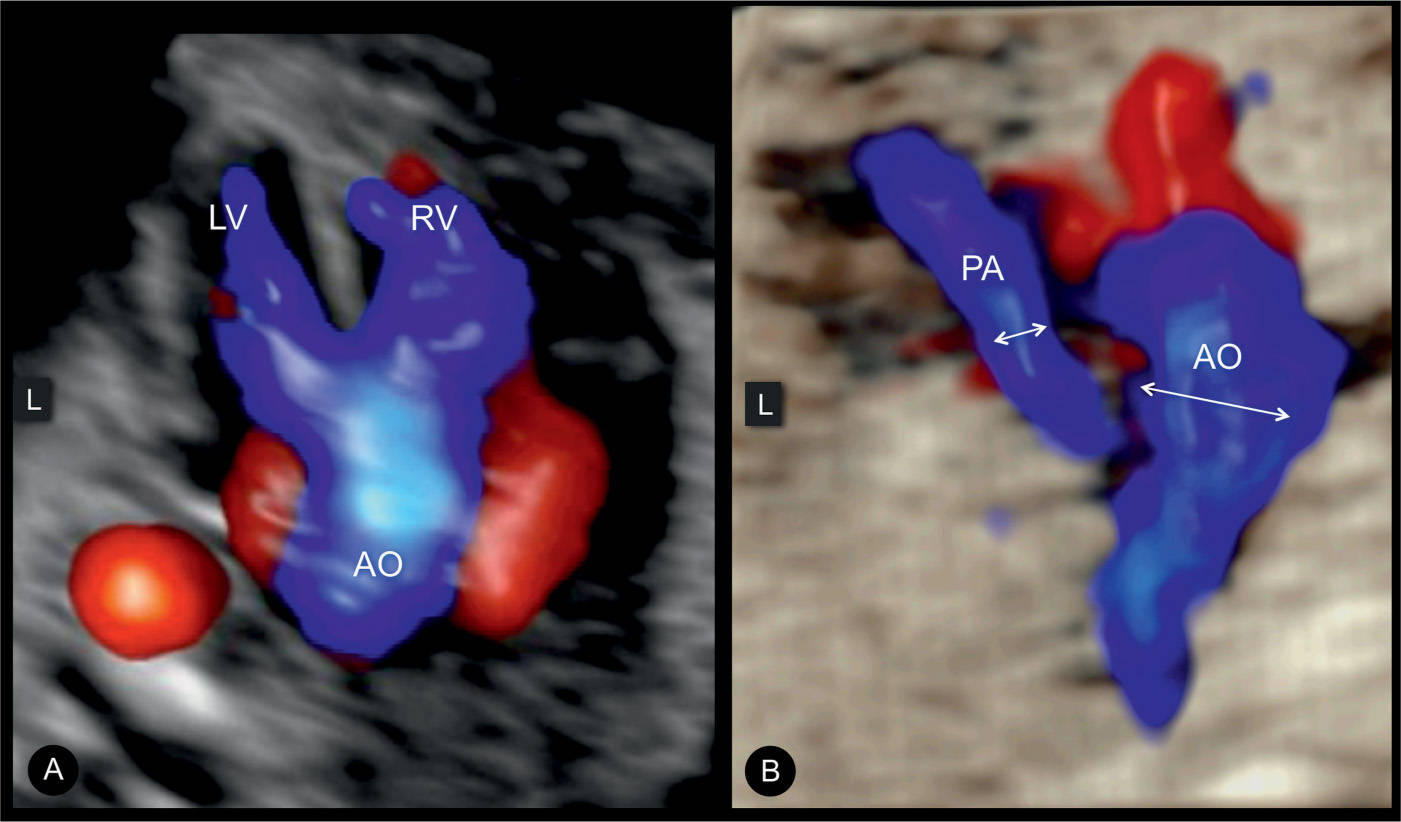
Figure 25.14: 3D ultrasound volume in color spatiotemporal image correlation (STIC), displayed in glass-body mode at the five-chamber view (A) and three-vessel-trachea view (B) in a fetus with tetralogy of Fallot (TOF). Note the overriding aorta (AO) in systole in A with blood flow from the right (RV) and left (LV) ventricles. The diminutive pulmonary artery (PA) with antegrade flow is shown in B. Note the small size of the PA as compared to the AO. L, left.
Common Cardiac and Extracardiac Abnormalities Associated with Tetralogy of Fallot |
Associated cardiac abnormalities | |
Patent foramen ovale/atrial septal defect | 83% |
Right-sided aortic arch | 25% |
Persistent left superior vena cava | 11% |
Atrioventricular septal defect | <5% |
Abnormal coronary circulation | <5% |
Anomaly of pulmonary venous connection | <1% |
Associated extracardiac abnormalities | |
Chromosomal abnormalities | 30% |
Deletion 22q11 | 10%–15% |
Congenital anomaly of anatomic organs | Common |
Differential Diagnosis
Differential diagnosis of TOF includes conditions presenting with an overriding of the aorta, such as pulmonary atresia with VSD, absent pulmonary valve, common arterial trunk (CAT), double outlet right ventricle, and malaligned VSD without great vessels abnormalities. The diagnosis of these abnormalities can be typically achieved by the correct evaluation of the size and origin of the pulmonary trunk, and Table 25.3 lists various diagnostic tools in the differential diagnosis of these cardiac lesions.
Prognosis and Outcome
Serial prenatal ultrasound examinations to document fetal pulmonary artery growth and flow across the ductus arteriosus are critical for counseling and appropriate care of the newborn, as pulmonary artery growth has been shown to be variable and unpredictable (6, 18). In general, prenatally diagnosed cases of TOF have a worse prognosis than postnatal cases due to an increased association with chromosomal aberrations, associated syndromes, or complex extracardiac anomalies (8). Case series and analysis of cardiac surgery databases suggest a short- and long-term survival of infants with TOF upward of 90% (19, 20). Poor prognostic signs include decelerated growth of the pulmonary artery, accelerated growth of the ascending aorta, cessation of forward flow through the pulmonary valve, and reversed flow through the ductus arteriosus (6). Prenatal echocardiographic markers that could predict the need for neonatal intervention in fetuses with right ventricular outflow tract obstruction were recently reviewed in several studies. In a study of 52 fetuses with right ventricular tract outlet obstruction, a pulmonary valve/aortic valve ratio of <0.6 or a pulmonary valve Z-score of −3 at fetal final echocardiogram was highly sensitive (92%) but poorly specific (50%), whereas classifying direction of flow in the ductus arteriosus as either normal (all pulmonary-to-aorta) or abnormal (aorta-to-pulmonary or bidirectional) was both highly sensitive (100%) and specific (95%) for predicting the need for a neonatal intervention (21). In a study involving 23 live-born fetuses with isolated TOF, pulmonary valve peak systolic velocity (PVPSV) ≥87.5 cm/s at 19 to 22 weeks predicted early neonatal intervention with 100% sensitivity and 93.3% specificity (22). Furthermore, a PVPSV at 34 to 38 weeks of ≥144.5 cm/s predicted all cases undergoing early neonatal intervention (22). TOF with an atresia of the pulmonary valve (pulmonary atresia with VSD) or cases of absent pulmonary valve are acknowledged to have a worse prognosis. Table 25.4 lists poor prognostic signs associated with TOF.
Differential Diagnosis of a Great Vessel Override over a Ventricular Septal Defect (VSD) |
Diagnostic clue | Additional signs | |
Tetralogy of Fallot | • Patent, narrow PA • Antegrade flow in PA | • Antegrade or retrograde flow in DA |
Pulmonary atresia with VSD | • Very narrow PA • No antegrade flow in PA | • DA tortuous with retrograde flow |
Absent pulmonary valve | • Very large PA • To-and-fro blood flow in PA | • No DA generally • Aortic root is more narrow than the PA |
Common arterial trunk | • PA arises from the overriding vessel | • Valve of the overriding vessel may show regurgitation |
Double outlet right ventricle | • PA is overriding and aorta courses in parallel | • Mimics a TGA with VSD • Aorta or PA may be of normal size or narrow |
PA, pulmonary artery; DA, ductus arteriosus; TGA, transposition of great arteries.
• Decelerated growth of the pulmonary artery • Accelerated growth of the ascending aorta • Cessation of forward flow through the pulmonary valve • Reversed flow through the ductus arteriosus • TOF with atresia of the pulmonary valve (pulmonary atresia with ventricular septal defect) • Absent pulmonary valve • Associated chromosomal aberrations • Associated extracardiac congenital malformations • Small left ventricle • Associated abnormal venous connection |
KEY POINTS  Tetralogy of Fallot
Tetralogy of Fallot
 TOF is characterized by subaortic VSD, aortic root override, and infundibular pulmonary stenosis.
TOF is characterized by subaortic VSD, aortic root override, and infundibular pulmonary stenosis.
 TOF is one of the most common forms of cyanotic CHD.
TOF is one of the most common forms of cyanotic CHD.
 The classic form of TOF with pulmonary stenosis accounts for about 80% of all cases.
The classic form of TOF with pulmonary stenosis accounts for about 80% of all cases.
 The four-chamber view appears normal in TOF unless the VSD is large and visible in this plane.
The four-chamber view appears normal in TOF unless the VSD is large and visible in this plane.
 TOF is typically detected in the five-chamber view, demonstrating a perimembranous subaortic VSD with an aortic root override.
TOF is typically detected in the five-chamber view, demonstrating a perimembranous subaortic VSD with an aortic root override.
 The aortic root appears dilated in TOF, especially in the third trimester.
The aortic root appears dilated in TOF, especially in the third trimester.
 There is a strong association between an increased nuchal translucency measurement and the diagnosis of TOF.
There is a strong association between an increased nuchal translucency measurement and the diagnosis of TOF.
 Associated cardiac and extracardiac abnormalities are common in TOF.
Associated cardiac and extracardiac abnormalities are common in TOF.
 A patent foramen ovale or an atrial septal defect is found in 83% of TOF cases.
A patent foramen ovale or an atrial septal defect is found in 83% of TOF cases.
 A right-sided aortic arch and a persistent left superior vena cava have been found in 25% and 11% of TOF cases, respectively.
A right-sided aortic arch and a persistent left superior vena cava have been found in 25% and 11% of TOF cases, respectively.
 The rate of chromosomal abnormalities is around 30% in TOF.
The rate of chromosomal abnormalities is around 30% in TOF.
 Microdeletion of 22q11 is found in 10% to 15% of TOF fetuses.
Microdeletion of 22q11 is found in 10% to 15% of TOF fetuses.
 Poor prognostic signs of TOF include decelerated growth of the pulmonary artery, accelerated growth of the ascending aorta, cessation of forward flow through the pulmonary valve, and reversed flow through the ductus arteriosus.
Poor prognostic signs of TOF include decelerated growth of the pulmonary artery, accelerated growth of the ascending aorta, cessation of forward flow through the pulmonary valve, and reversed flow through the ductus arteriosus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


