● PULMONARY STENOSIS
Definition, Spectrum of Disease, and Incidence
Pulmonary stenosis is a cardiac anomaly characterized by an obstruction to pulmonary arterial blood flow. Pulmonary stenosis can be due to pulmonary valve abnormalities (Fig. 24.1), infravalvular (infundibulum) narrowing, or rarely supravalvular narrowing involving the main pulmonary artery or its branches. Pulmonary stenosis can be an isolated cardiac anomaly or as part of other cardiac defects as shown in Table 24.1. In this chapter, we will discuss pulmonary stenosis in its isolated type.
Valvular pulmonary stenosis, primarily due to fusion of the valve commissures, is the most common cause of pulmonary stenosis (Fig. 24.1). On occasion, pulmonary stenosis is due to unfused, thickened dysplastic valve leaflets with associated pulmonary regurgitation. Unfused, dysplastic valve leaflets are seen in cases of pulmonary stenosis with Noonan syndrome (1). Infundibular pulmonary stenosis is a typical feature of tetralogy of Fallot (see Chapter 25). Infundibular thickening due to right ventricular wall hypertrophy is also a common cause of pulmonary stenosis in the recipient twin of twin–twin transfusion syndrome. The severity of stenosis varies from mild lesions, typically missed prenatally, to severe lesions with associated hypertrophy of the right ventricle (Fig. 24.1) and tricuspid regurgitation. Pulmonary stenosis can progressively worsen in utero, resulting in critical pulmonary stenosis and pulmonary atresia. Isolated pulmonary stenosis, which occurs in about 0.73 per 1,000 live births, is the second most common cardiac anomaly to ventricular septal defect and accounts for 9% of live births with congenital heart disease (2, 3). Most cases of pulmonary stenosis are first diagnosed beyond the neonatal period probably due to the difficulty of the prenatal and neonatal diagnosis of this lesion and its progressive nature in some cases (2). Recurrence of pulmonary stenosis is estimated at 2% for one affected sibling and 6% when two siblings are affected (4).
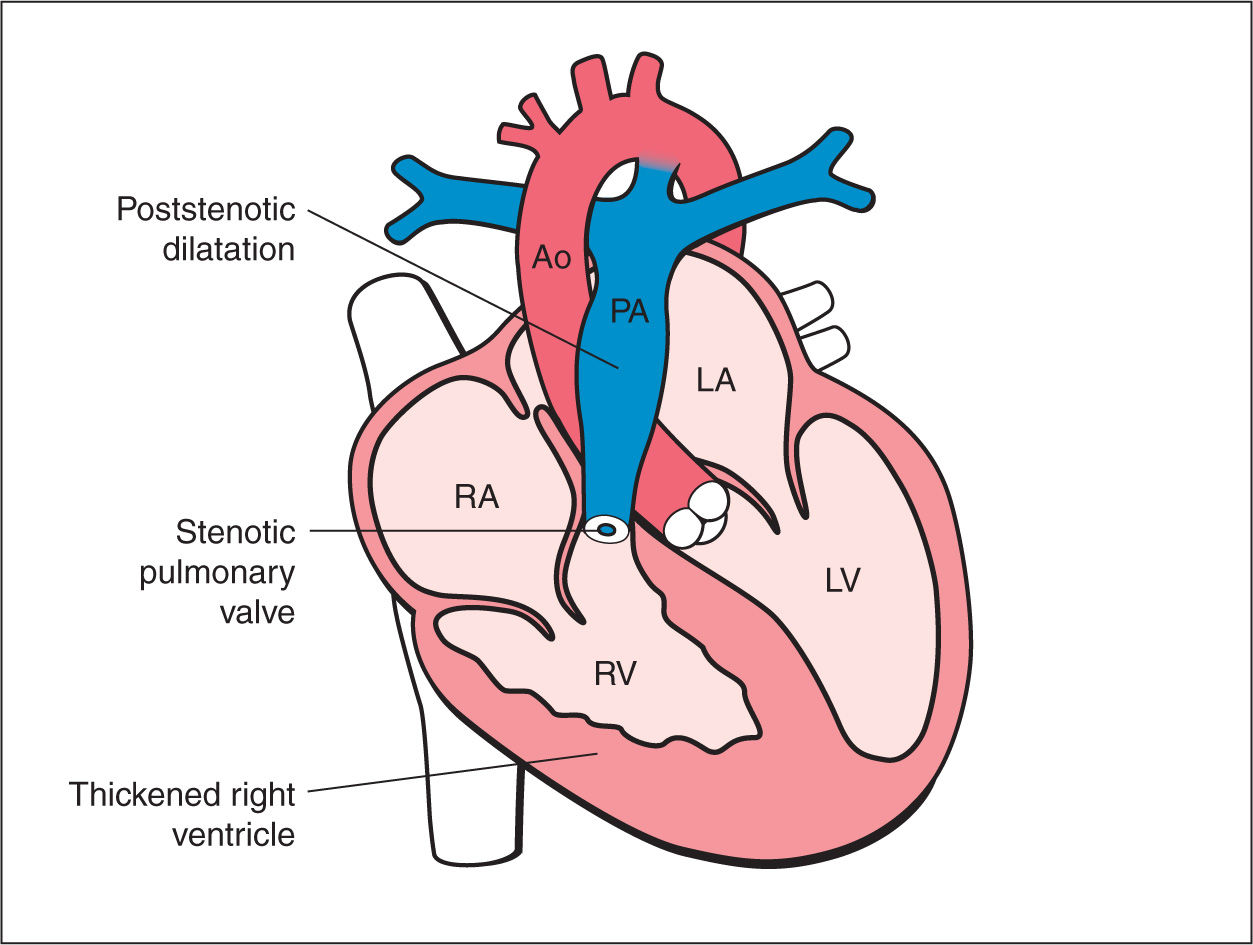
Figure 24.1: Schematic drawing of pulmonary stenosis. See text for details. LV, left ventricle; LA, left atrium; RA, right atrium; RV, right ventricle; Ao, aorta; PA, pulmonary artery.
• Tetralogy of Fallot • Absent pulmonary valve syndrome • Double outlet right ventricle • Tricuspid atresia with ventricular septal defect • Ebstein anomaly, tricuspid dysplasia • D-transposition of the great arteries • Corrected transposition of the great arteries • Heterotaxy with cardiac anomaly (right isomerism) |
Ultrasound Findings
Gray Scale
Most cases of pulmonary stenosis are not clearly demonstrated on the second trimester ultrasound. When pulmonary stenosis is suspected prenatally, the four-chamber view typically shows right ventricular hypertrophy with bulging of the interventricular septum into the left ventricle (Fig. 24.2). The right ventricular lumen may appear small due to the hypertrophied myocardium, and the tricuspid valve shows normal leaflet excursion with occasional tricuspid regurgitation on color Doppler, which may lead to right atrial dilation. Right ventricular wall hypertrophy is more commonly seen in the third trimester of pregnancy. Confirming the diagnosis of pulmonary stenosis is best performed by direct visualization of the pulmonary valve in the right ventricular outflow view, which shows abnormal excursion, thickening, and doming of valve leaflets during systole (Figs. 24.3 to 24.5). Valve leaflets are visible within the pulmonary artery throughout the cardiac cycle in pulmonary stenosis as opposed to a normal pulmonary valve, where the leaflets abut the arterial walls during systole. The three-vessel view and the three-vessel-trachea view will also show poststenotic dilation of the pulmonary artery in many cases (Figs. 24.3 to 24.5). In mild to moderate pulmonary stenosis, ultrasound diagnostic signs are subtle and primary detection is best achieved by the additional use of color and pulsed Doppler.
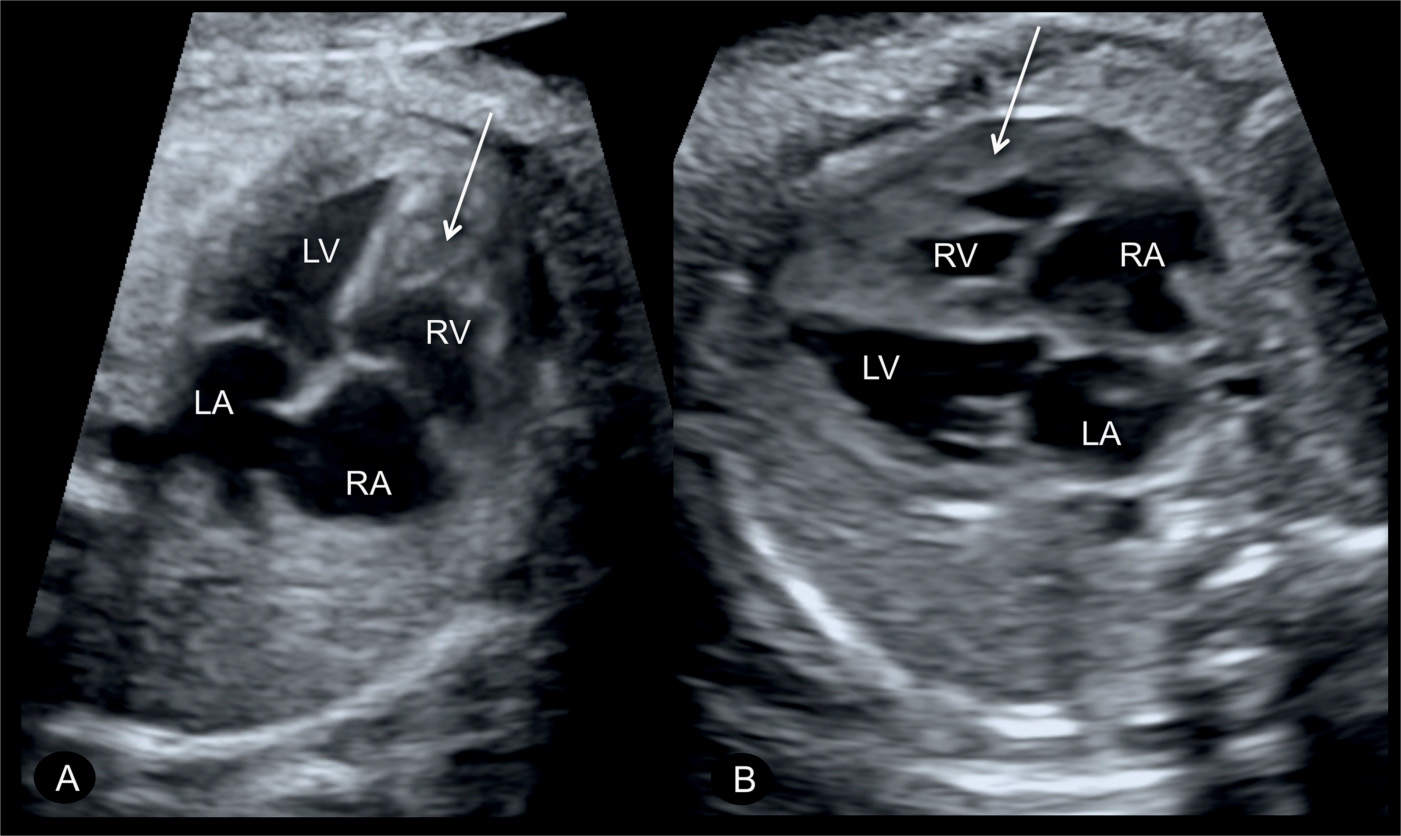
Figure 24.2: Apical (A) and transverse (B) four-chamber views in two fetuses with pulmonary stenosis, showing hypertrophy of the right ventricular wall (arrows). LA, left atrium; RA, right atrium; RV, right ventricle; LV, left ventricle.
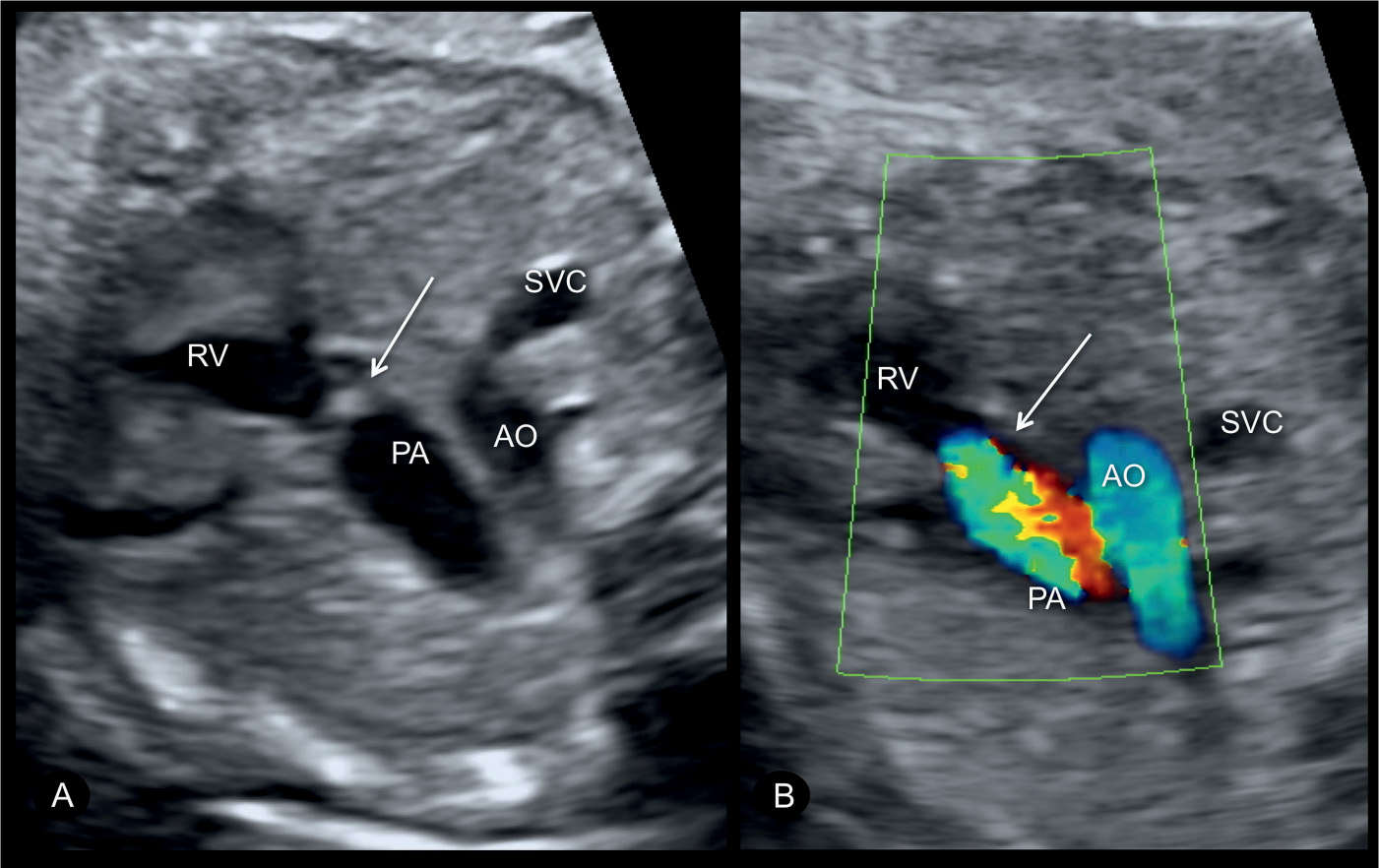
Figure 24.3: Transverse views of the upper chest (three-vessel-trachea) in gray scale (A) and color Doppler (B) in a fetus with pulmonary stenosis. In gray scale (A), note the thickened and echogenic pulmonary valve (arrow) with a dilation of a pulmonary root (see also Fig. 24.4). In color Doppler (B), color aliasing across the pulmonary valve is noted. AO, aorta; PA, pulmonary artery; SVC, superior vena cava; RV, right ventricle.
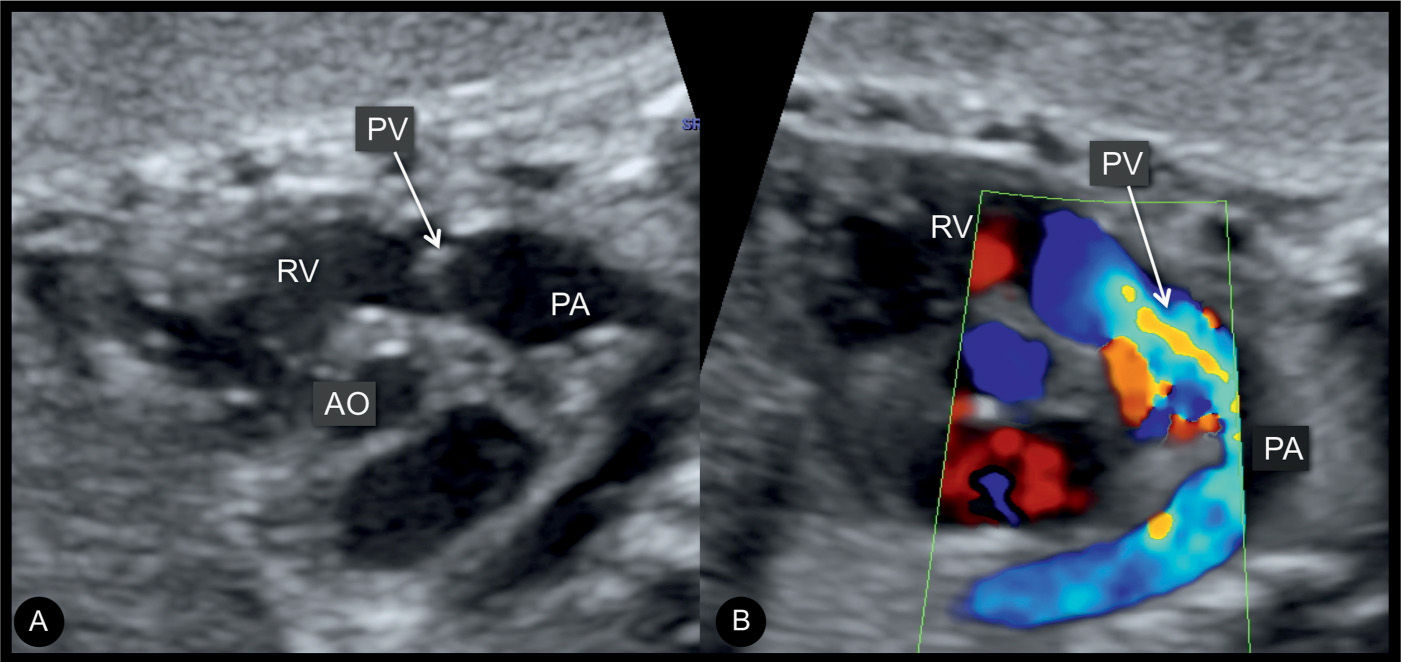
Figure 24.4: Right ventricular outflow tract views in gray scale (A) and color Doppler (B) in a fetus at 27 weeks’ gestation with pulmonary stenosis. Note in A, the thickened pulmonary valve (PV) and postvalvular dilation of the pulmonary artery (PA). B demonstrates color aliasing across the pulmonary valve. AO, aorta; RV, right ventricle.
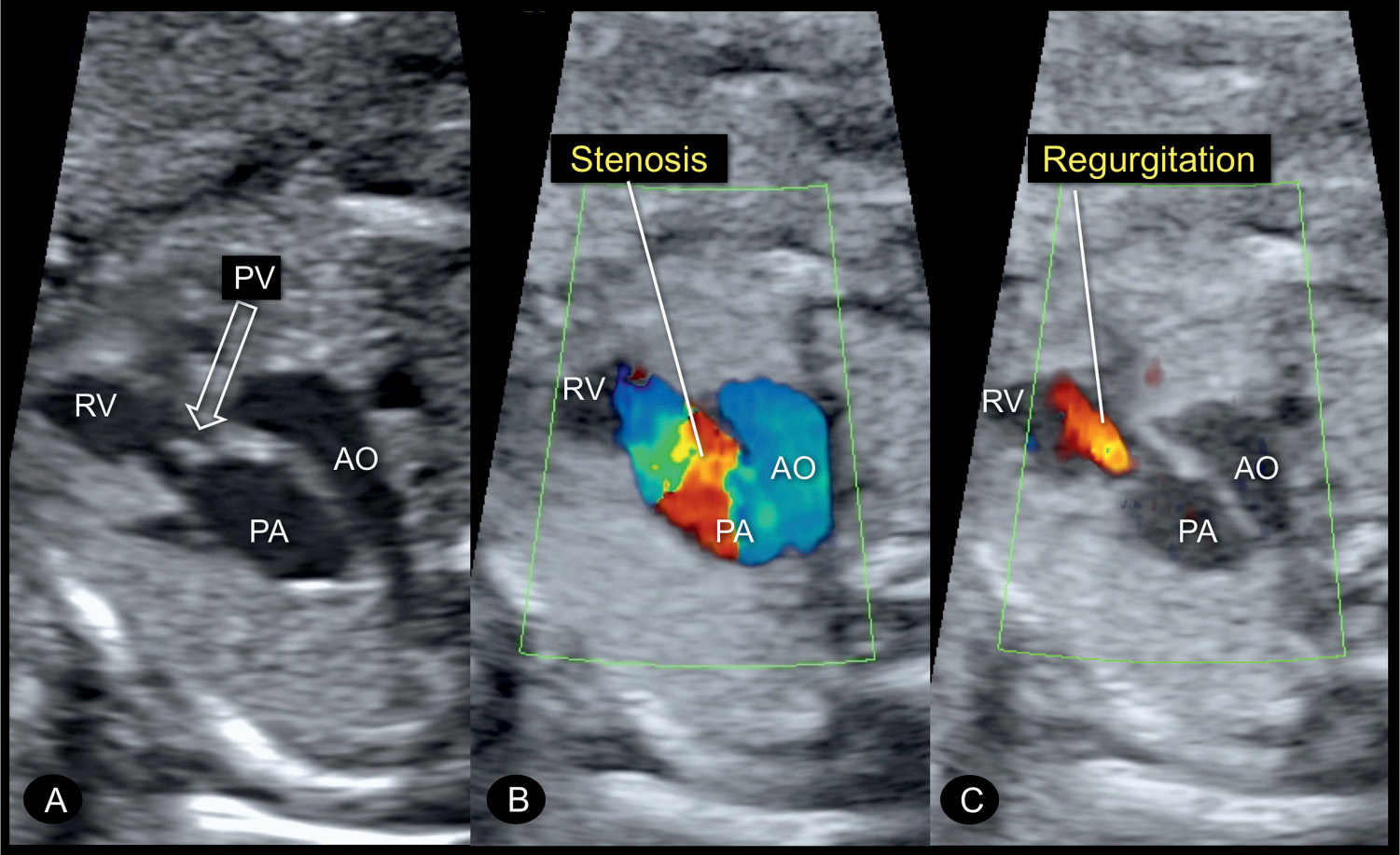
Figure 24.5: Three-vessel-trachea view in gray scale (A) and color Doppler in systole (B) and diastole (C) in a fetus at 22 weeks’ gestation with a pulmonary valve (PV) stenosis. In A, postvalvular dilation of the pulmonary artery (PA) is seen in addition to the thickened echogenic PV (open arrow). B reveals high velocities during systole across the stenotic PV recognized by color aliasing. C shows PV regurgitation, which is occasionally seen due to the dysplastic nature of the stenotic PV. RV, right ventricle; AO, aorta.
Color Doppler
As in stenosis of other valves, color and pulsed Doppler are essential in confirming the diagnosis and in assessing the severity of pulmonary stenosis. Color Doppler of the pulmonary valve in the three-vessel or short-axis view typically shows turbulent antegrade flow with color aliasing (Figs. 24.3 to 24.5). Pulsed Doppler across the pulmonary valve demonstrates high-flow velocities, exceeding 200 cm/s (Figs. 24.6 and 24.7). Ductus arteriosus (DA) flow is antegrade in the majority of cases of pulmonary stenosis. The presence of retrograde flow across the DA, however, is a worsening sign associated with the development of pulmonary atresia and poor outcome (5). When the pulmonary valve leaflets are dysplastic, valve regurgitation can also be found and demonstrated on color Doppler (Fig. 24.5C) and flow across the DA may be bidirectional in these conditions. Tricuspid regurgitation, which may be absent in mild pulmonary stenosis and holosystolic in moderate or severe cases (Fig. 24.8), can be demonstrated using color Doppler in the four-chamber view. Color and pulsed Doppler of the ductus venosus may show reverse flow during the atrial contraction phase of diastole (6).
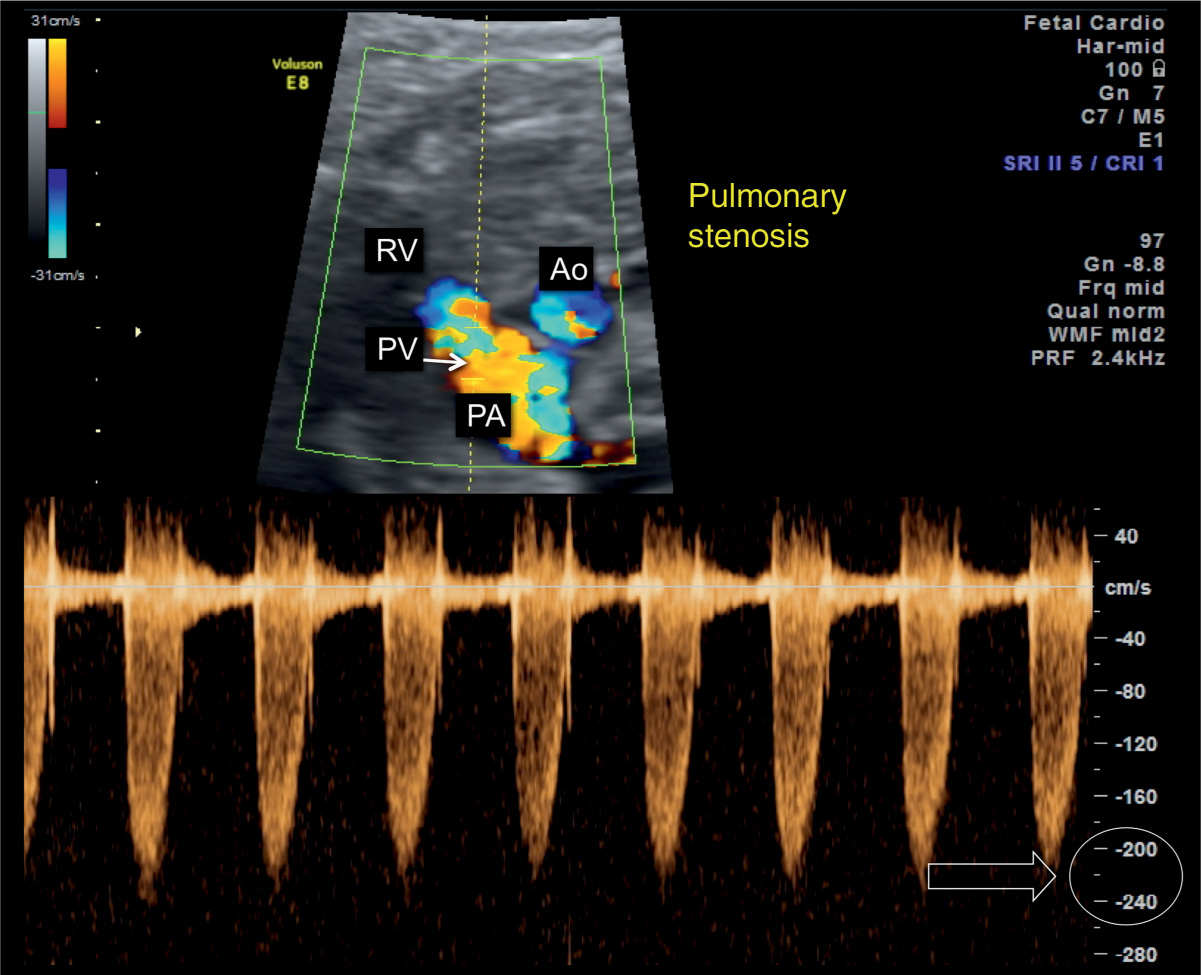
Figure 24.6: Ductal arch view in color and pulsed Doppler in a fetus with pulmonary valve (PV) stenosis. Note the presence of color aliasing in the pulmonary artery (PA). Pulsed Doppler shows elevated peak velocities across the PV, reaching 220 cm/s (open arrow) in this case. AO, aorta; RV, right ventricle.
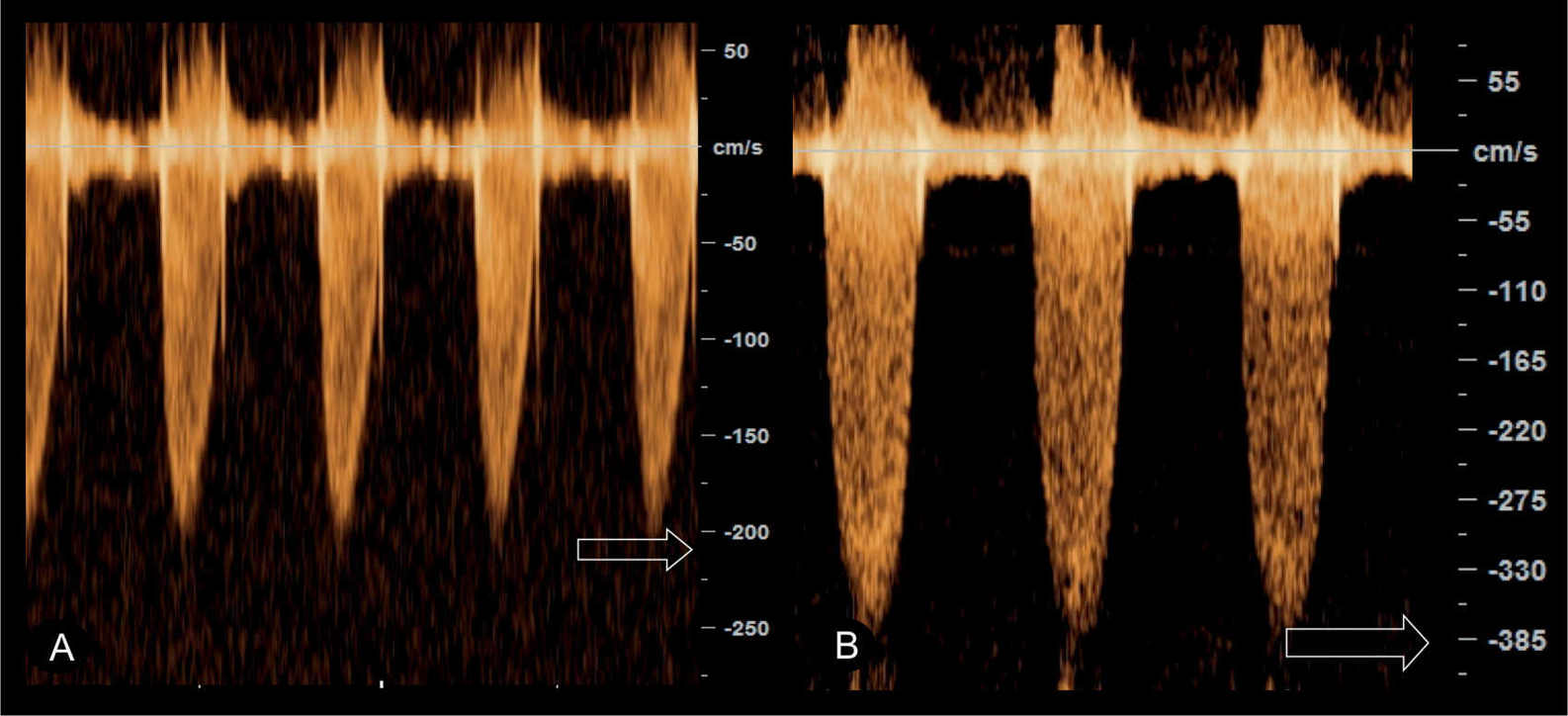
Figure 24.7: Pulsed Doppler of the pulmonary artery in two fetuses with pulmonary stenosis with varying severity. Peak velocity in A is 210 cm/s whereas in B it reaches 385 cm/s.
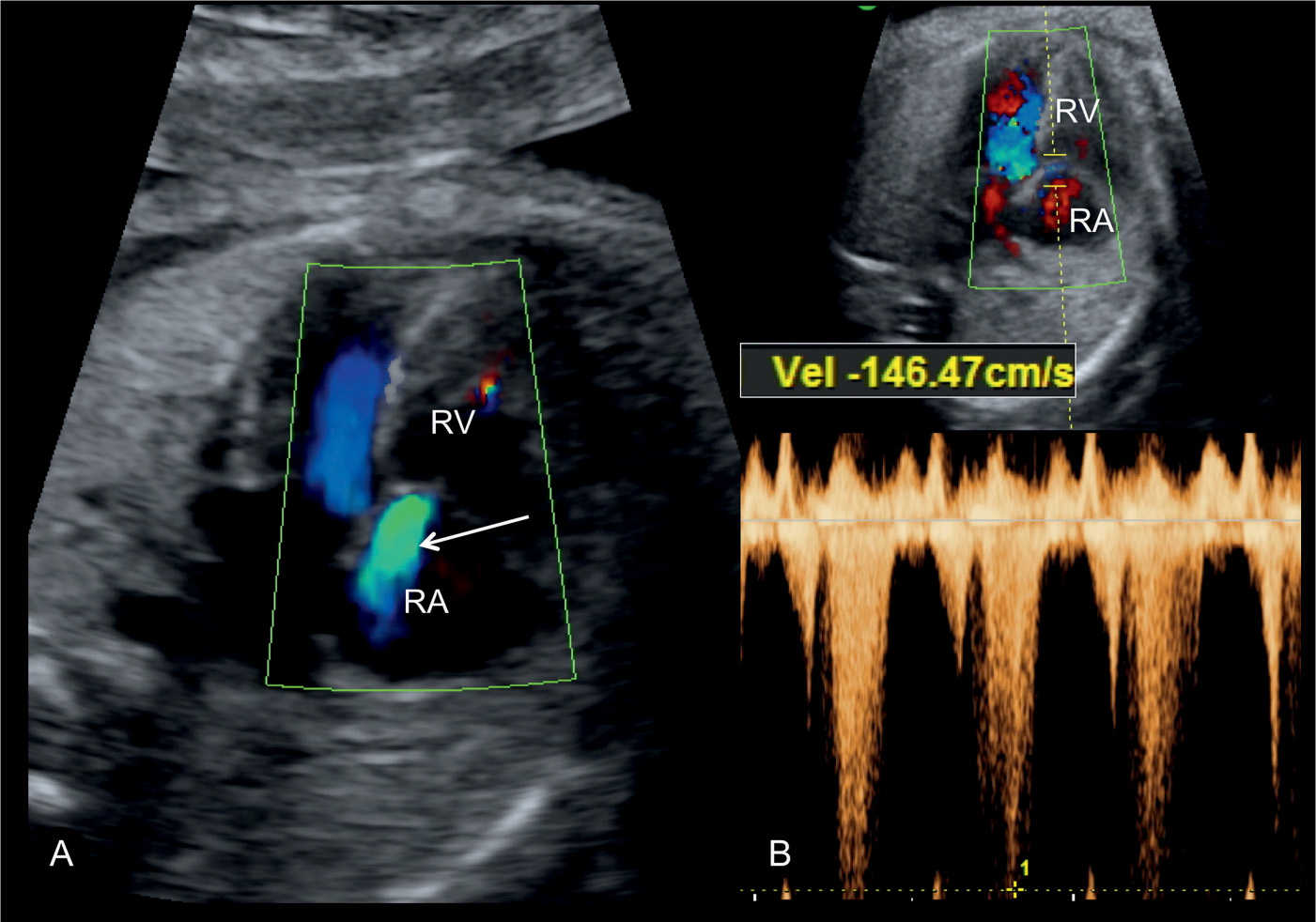
Figure 24.8: Apical four-chamber view in color (A) and pulsed (B) Doppler in a fetus with pulmonary stenosis, showing the presence of tricuspid regurgitation. Note the regurgitant jet on color Doppler (arrow in A). Pulsed Doppler in B shows peak velocities of the tricuspid regurgitant jet at 146 cm/s (B). RA, right atrium; RV, right ventricle.
Early Gestation
Pulmonary stenosis is difficult to diagnose in early gestation as the ultrasound findings may be subtle or absent. A case of pulmonary stenosis is show in Fig. 24.9. The presence of tricuspid regurgitation in early gestation should result in targeted examination of the fetal heart, including color and pulsed Doppler of the pulmonary artery. The presence of pulmonary stenosis with a thickened nuchal translucency or a cystic hygroma should alert for the association with Noonan syndrome.
Three-Dimensional Ultrasound
Three-dimensional (3D) ultrasound with tomographic imaging in gray scale and color Doppler can demonstrate in one view various ultrasound findings in pulmonary stenosis, such as the hypertrophied right ventricle, the dilated pulmonary trunk, the tricuspid regurgitation, and color aliasing across the pulmonary valve during systole (Fig. 24.10). Surface rendering of a 3D volume may show the stenotic valve with lack of complete excursion in systole (Fig. 24.11). Future applications of ventricular volume measurements may be of help in assessing fetuses at risk of developing critical stenosis and right ventricular dysfunction.
Associated Cardiac and Extracardiac Findings
Associated cardiac lesions include right ventricular hypertrophy and tricuspid regurgitation, which are resultant from the hemodynamic changes of pulmonary stenosis. Other associated cardiac abnormalities include atrial septal defect, aortic or tricuspid stenosis, and total anomalous pulmonary venous connection. Table 24.1 lists typical cardiac malformations that are associated with pulmonary stenosis.
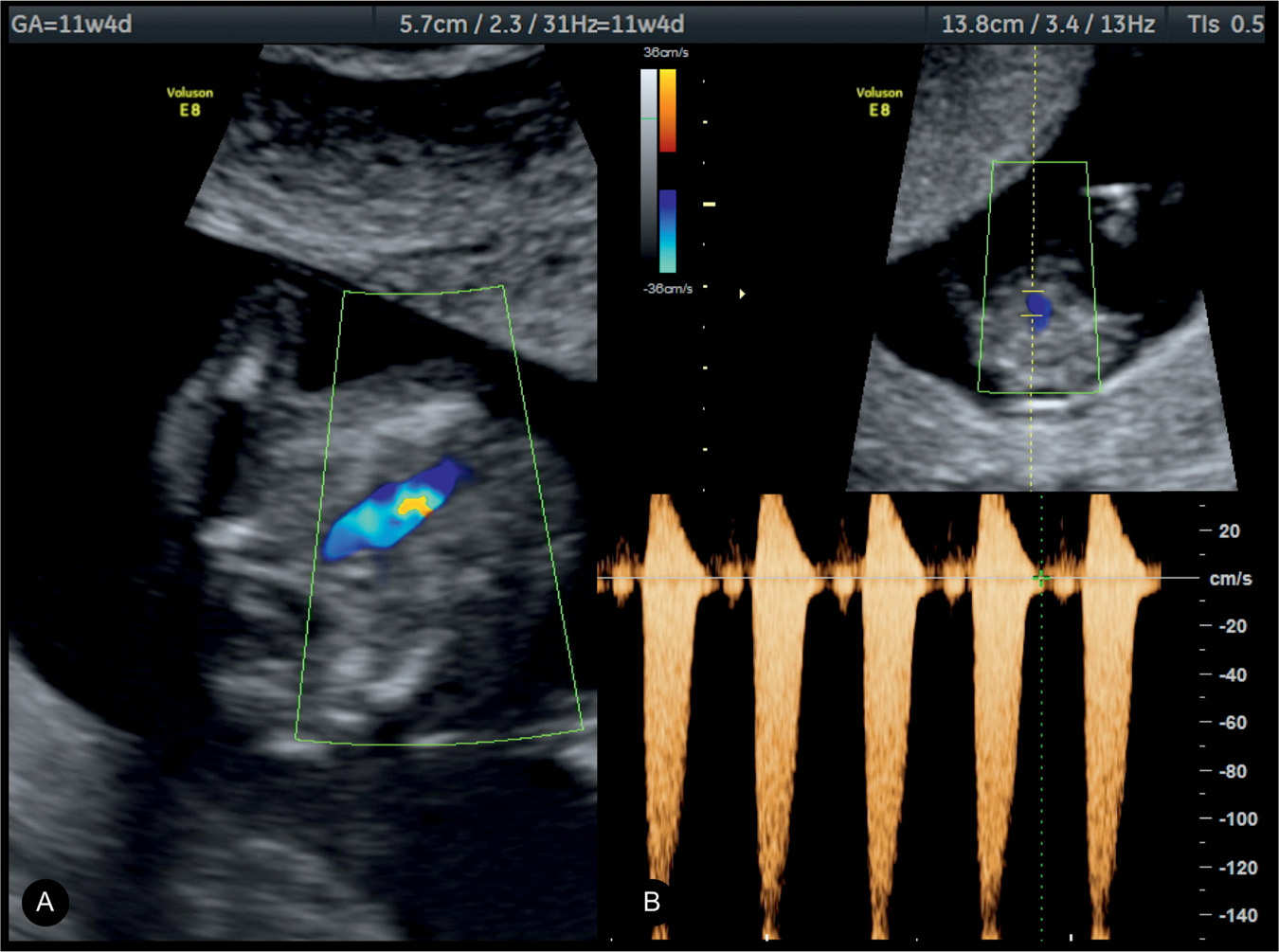
Figure 24.9: Right ventricular outflow view in color (A) and pulsed (B) Doppler in a fetus with pulmonary stenosis at 11 weeks’ gestation visualized by transvaginal ultrasound. Note the presence of color aliasing in A and peak velocities on pulsed Doppler in B at 150 cm/s.
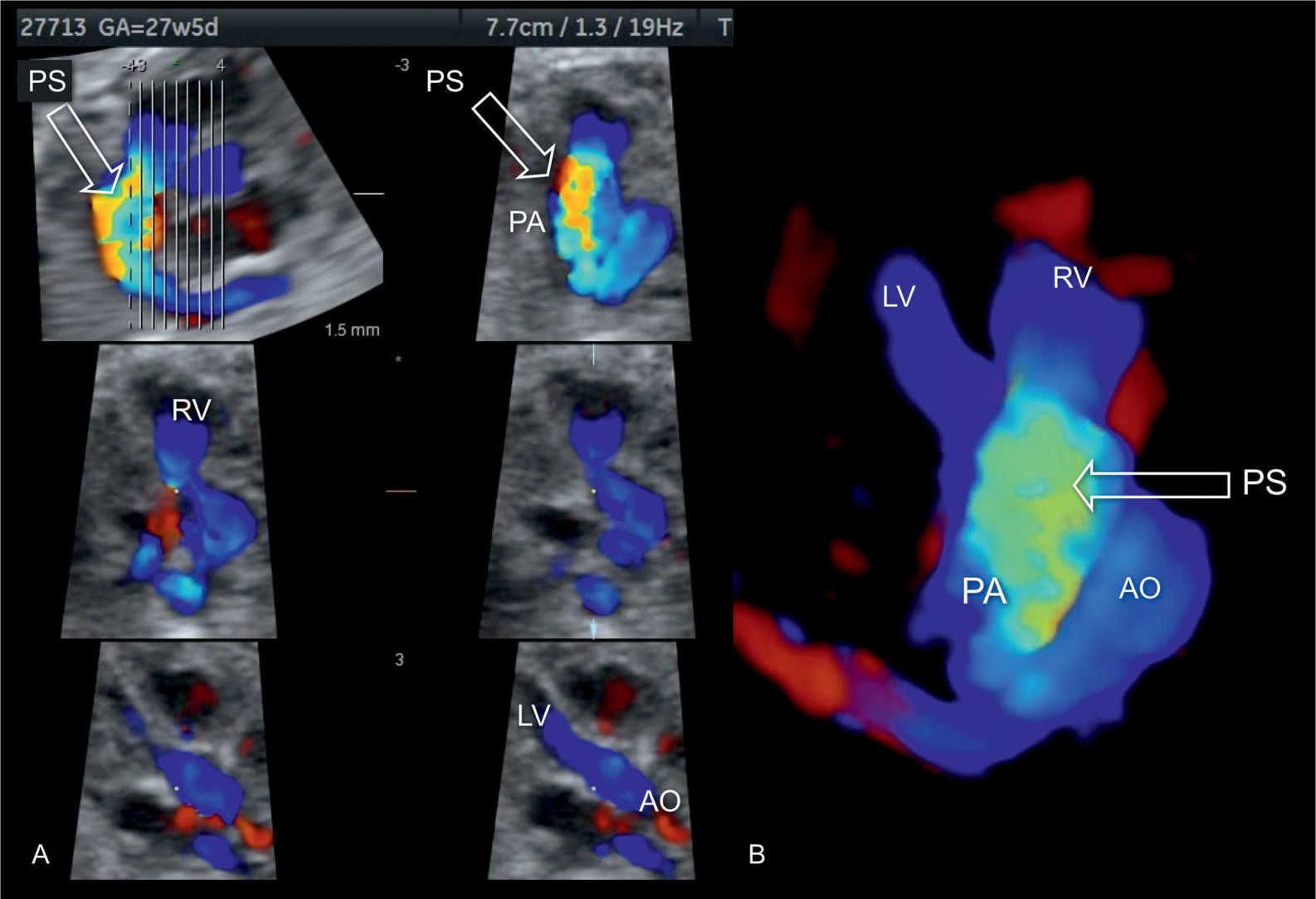
Figure 24.10: 3D volume in spatiotemporal image correlation (STIC) and color Doppler in a fetus with pulmonary stenosis (PS) displayed in systole in tomographic mode (A) and in 3D color display mode (B). Note the presence of color aliasing across the pulmonary artery (PA) (open arrow). In comparison, note that flow across the aorta (AO) shows normal laminar flow (shown in A and B). LV, left ventricle; RV, right ventricle.
Extracardiac-associated findings are rare with the exception of syndromes such as Noonan, Beckwith–Wiedemann, Alagille, and Williams–Beuren, among others. A full listing of genetic associations with cardiac disease is presented in Chapter 4. In Noonan syndrome, approximately 50% of affected infants have congenital heart disease, primarily pulmonary stenosis (1). In twin–twin transfusion syndrome, a higher rate of pulmonary stenosis is reported in the recipient twin, possibly related to chronic in utero hemodynamic impairment. Volume overload with chronic tricuspid regurgitation may be associated with reduced antegrade flow across the pulmonary valve and a lack of growth of the valve annulus (7, 8). Chromosomal abnormalities are rare associations with cases of pulmonary stenosis.
Differential Diagnosis
Differentiating pulmonary stenosis from pulmonary atresia may be difficult. A false-positive diagnosis of pulmonary stenosis may also occur if color Doppler presets are not adjusted to cardiac flow velocities. Adjusting the velocity scale of the ultrasound machine and confirming the suspicion on pulsed Doppler help in the correct diagnosis.
Prognosis and Outcome
The course of pulmonary stenosis in utero is uneventful except in severe cases where progression may lead to an increase in the severity of tricuspid regurgitation with subsequent dilation of the right atrium, cardiomegaly, and heart failure. Progression may also lead to the development of critical stenosis with progression to pulmonary atresia with intact ventricular septum (PA-IVS) (5, 9). Progression of pulmonary stenosis to atresia, which may alter postnatal surgical management from a biventricular to a univentricular repair, has prompted in utero treatment of critical pulmonary stenosis with balloon valvuloplasty (10). Similar to other cardiac abnormalities, prenatal diagnosis of pulmonary stenosis has a worse outcome than postnatal diagnosis. When diagnosis of pulmonary stenosis was performed before 24 weeks’ gestation, a 67% survival rate was reported in one series (5).
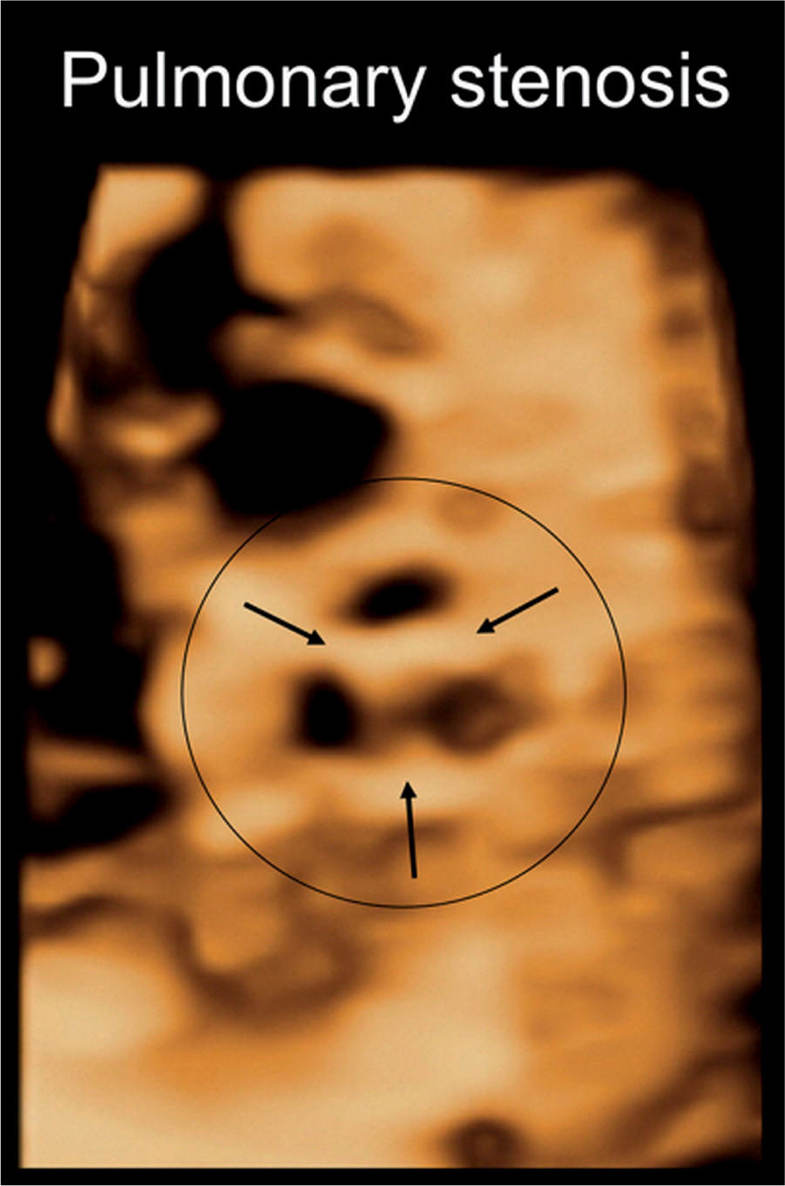
Figure 24.11: 3D volume of the heart in a fetus with pulmonary stenosis, displayed in surface mode in an en face view of the stenotic pulmonary valve. Note the three thickened pulmonary artery valve leaflets (arrows) with reduced valve aperture.
We recommend follow-up ultrasound examinations every 2 to 4 weeks on fetuses with pulmonary stenosis in order to assess peak velocities across the stenotic pulmonary valve, the severity of tricuspid regurgitation when present, antegrade or retrograde flow in the DA, and changes in tricuspid valve and right ventricular size. Occurrence of retrograde flow in the DA and a reduction of the right ventricular cavity are signs of worsening disease with poor prognosis. Reverse flow in the ductus venosus is a common finding in right heart obstruction and does not correlate with the prognostic outcome (6).
Prognosis of mild to moderate pulmonary stenosis, if isolated, is excellent, and neonatal management generally depends on the severity of the stenosis assessed postnatally. Mild forms may only need clinical follow-up with no intervention, whereas moderate to severe forms require treatment primarily with balloon valvuloplasty with excellent results. Children with dysplastic pulmonary valves may require surgical repair.
KEY POINTS  Pulmonary Stenosis
Pulmonary Stenosis
 Pulmonary stenosis is an obstruction to right ventricular outflow primarily due to pulmonary valve abnormalities.
Pulmonary stenosis is an obstruction to right ventricular outflow primarily due to pulmonary valve abnormalities.
 Fusion of the valve commissures is the most common cause of pulmonary stenosis.
Fusion of the valve commissures is the most common cause of pulmonary stenosis.
 Pulmonary stenosis can worsen in utero resulting in critical pulmonary stenosis and atresia.
Pulmonary stenosis can worsen in utero resulting in critical pulmonary stenosis and atresia.
 The four-chamber view in pulmonary stenosis shows right ventricular hypertrophy with occasional tricuspid regurgitation, which may lead to right atrial dilation.
The four-chamber view in pulmonary stenosis shows right ventricular hypertrophy with occasional tricuspid regurgitation, which may lead to right atrial dilation.
 Direct visualization of the pulmonary valve in pulmonary stenosis shows abnormal excursion, thickening, and doming of valve leaflets during systole.
Direct visualization of the pulmonary valve in pulmonary stenosis shows abnormal excursion, thickening, and doming of valve leaflets during systole.
 Valve leaflets are visible within the pulmonary artery throughout the cardiac cycle in pulmonary stenosis.
Valve leaflets are visible within the pulmonary artery throughout the cardiac cycle in pulmonary stenosis.
 The three-vessel view shows poststenotic dilation of the pulmonary artery in many cases.
The three-vessel view shows poststenotic dilation of the pulmonary artery in many cases.
 Color and pulsed Doppler are essential in confirming the diagnosis and in assessing the severity of pulmonary stenosis.
Color and pulsed Doppler are essential in confirming the diagnosis and in assessing the severity of pulmonary stenosis.
 Color Doppler shows turbulent antegrade flow with color aliasing and pulsed Doppler demonstrates high-flow velocities in pulmonary stenosis.
Color Doppler shows turbulent antegrade flow with color aliasing and pulsed Doppler demonstrates high-flow velocities in pulmonary stenosis.
 Prognosis of isolated mild to moderate pulmonary stenosis is excellent.
Prognosis of isolated mild to moderate pulmonary stenosis is excellent.
● PULMONARY ATRESIA WITH INTACT VENTRICULAR SEPTUM
Definition, Spectrum of Disease, and Incidence
PA-IVS is a group of cardiac malformations having in common absent communication between the right ventricle and the pulmonary arterial circulation (pulmonary atresia) in combination with an intact interventricular septum (Fig. 24.12). The pulmonary atresia is usually of the membranous type with complete fusion of the valve commissures and a normally developed infundibulum. On occasion, however, the pulmonary atresia is muscular with a severely distorted right ventricular outflow tract region. The right ventricular cavity is either hypoplastic with thickened right ventricular myocardium (Fig. 24.12) or dilated with significant tricuspid valve regurgitation and a dilated right atrium (see Chapter 20). A hypoplastic right ventricle occurs in the majority of cases (5). The size of the right ventricular cavity correlates with the Z-score of the diameter of the tricuspid valve (11, 12). One of the major associated findings in hearts with PA-IVS and hypoplastic right ventricles is an anomaly of the coronary circulation, namely, ventriculocoronary arterial communication (VCAC) (Fig. 24.12B), found in about one-third of cases of PA-IVS. Systemic collateral arteries to the lungs, which are typical for pulmonary atresia with ventricular septal defect (see Chapter 25), are not found in PA-IVS.
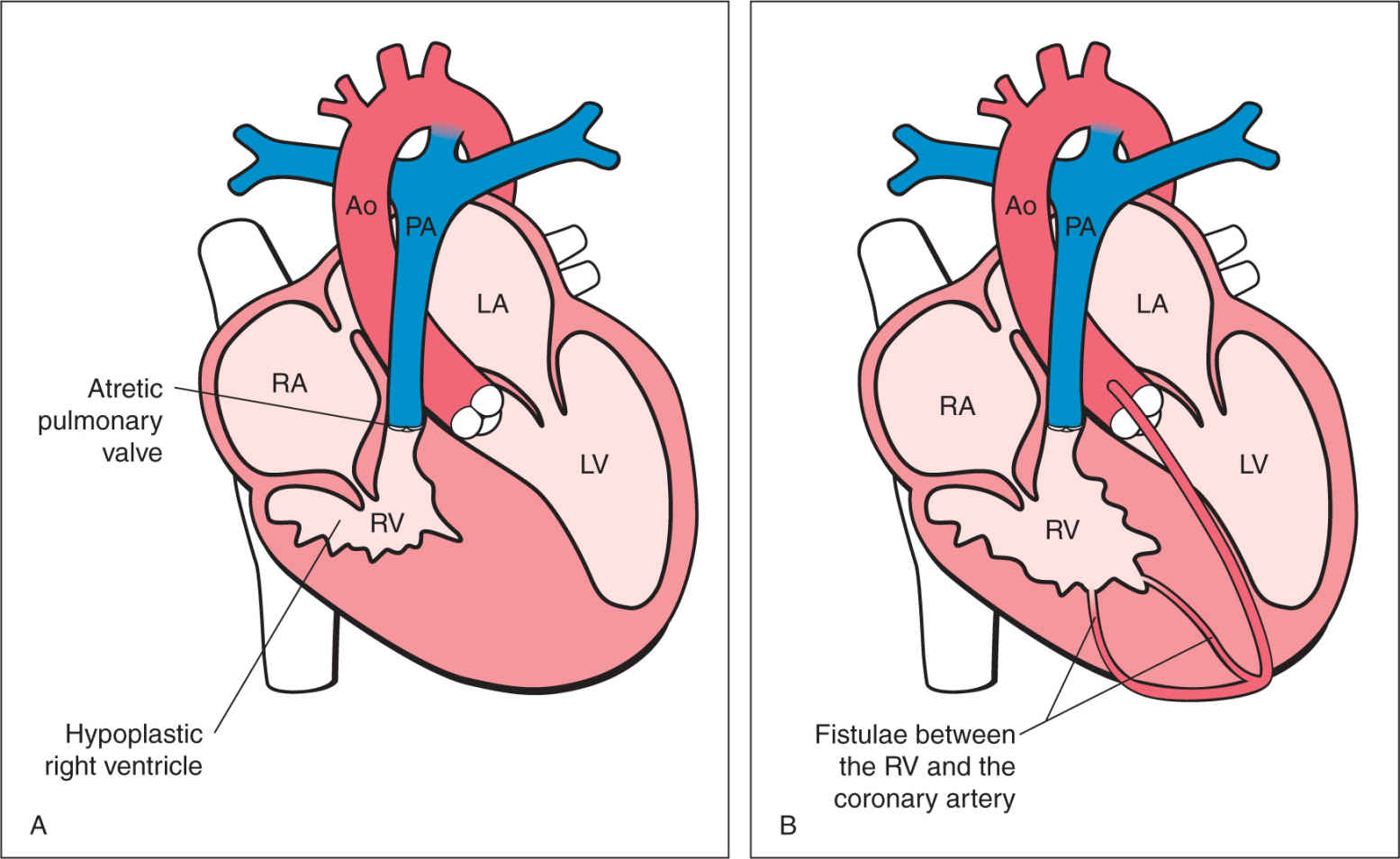
Figure 24.12: Schematic drawings of pulmonary atresia with intact ventricular septum (PA-IVS) (A) and PA-IVS with ventriculocoronary arterial communications (VCACs) (B). RA, right atrium; LV, left ventricle; LA, left atrium; Ao, aorta; PA, pulmonary artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


