Fig. 22.1
Anteroposterior (a), lateral (b), and inferior-superior (c) views of pelvis and hip joints. These illustrations serve to simulate and recreate the proximal femoral deformity seen with severe CFD on the right hip. The model is based on a normal proximal femur with a 130° neck-shaft angle. First, flex the proximal femur 90° relative to the pelvis. Next, abduct the flexed proximal femur 45° relative to the pelvis. Now reconnect the distal femur to the proximal femur. The distal femur should be placed in 45° of external rotation relative to the pelvis. The resulting deformity is the CFD femur deformity typically seen in most type 1b and some type 1a cases. Note that the femoral neck appears to be retroverted due to the 90° hip flexion of a 130° neck-shaft angle femur. Just the flexion makes it appear to have 50° of retroversion. Since the distal femur is fixed to the proximal femur in external rotation this retroversion is increased even more. Also note that the varus deformity is caused by the abduction of the proximal femur with the hip flexed. The hip flexion places the greater trochanter facing posteriorly. The proximity of the greater trochanter to the ilium and to the sacrum in this position explains why the gluteus medius and minimus, and piriformis muscles, are short
The ratio of growth in length of the short limb compared to the long limb remains relatively unchanged throughout growth [21–23]. This enables the final discrepancy in leg length to be predicted from the initial radiographs [24]. On this basis the Paley multiplier method is able to accurately calculate the predicted limb length discrepancy (LLD) at skeletal maturity [25, 26].
Ligamentous Structures
Anterior-posterior instability of the knee is common in CFD, but its severity is variable. Multiple modern era studies utilizing MRI have evaluated the ligamentous structures at the knee in patients with various degrees of CFD. The results support the conclusion that like the hip, the distal femur and proximal tibia require interaction of bony and soft tissues to develop normally.
Manner et al. [27] performed a radiographic study of 34 knees associated with CFD with radiographs and MRI. The anterior cruciate ligament (ACL) was affected in all knees studied, with 15 % hypoplastic and 85 % absent. The PCL was hypoplastic in 21 % and absent in 24 %. The most common type (14 knees, 41 %) was aplasia of the ACL and a normal PCL. The cruciate ligament dysplasias were differentiated into three groups (Fig. 22.2). Type I had a hypoplastic or absent ACL and a normal PCL. The intercondylar notch width and height were decreased compared to the normal side, and the lateral tibial spine was hypoplastic. Type II knees had aplasia of the ACL and hypoplasia of the PCL. They had even narrower and shorter intercondylar notches, and both tibial spines were hypoplastic. Two cases had lateral femoral osteochondral lesions. Type III knees had aplasia of both cruciate ligaments. The intercondylar notch was essentially absent and covered with hyaline cartilage, and both tibial spines were aplastic. The distal femoral joint surface is concave, matching the convex tibial plateau like a ball and socket. Three of these cases had discoid meniscus.
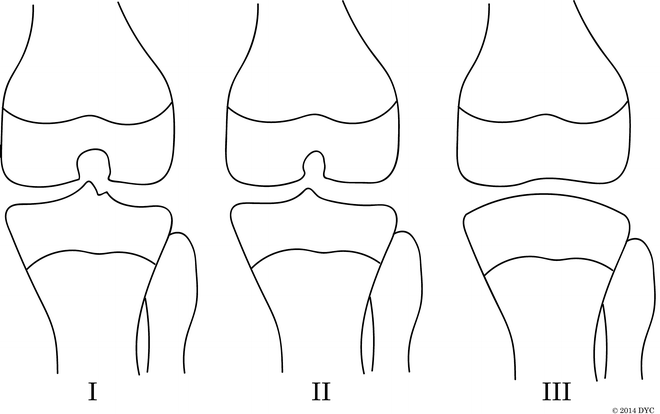

Fig. 22.2
Manner et al. [27] classification of intercondylar notch using the tunnel view correlates to deficiency of the cruciate ligaments. Type I: The intercondylar notch width and height are decreased compared to the normal side. The lateral tibial spine is hypoplastic. This corresponds to a hypoplastic or absent ACL and a normal PCL. Type II: Narrower and shorter intercondylar notch with hypoplasia of both tibial spines. This corresponds to aplasia of the ACL and hypoplasia of the PCL. Type III: The intercondylar notch is absent and covered with hyaline cartilage, and both tibial spines are aplastic. The distal femoral joint surface is concave, matching the convex tibial plateau. This corresponds to aplasia of both cruciate ligaments
Overall, the authors found that on the tunnel-view radiographs, narrowing or absence of the femoral notch and flattening of tibial eminences corresponded to a hypoplasia or deficiency of the cruciate ligaments. Thus, the shape of the distal femur and proximal tibia can be used to predict, with relative accuracy, the presence or absence of the ACL and PCL ligaments.
These radiographic studies have been validated with the use of arthroscopy to directly confirm the presence or absence of the ACL and PCL. Johansson and Aparisi [28] published a case series of six patients with cruciate ligament dysplasia. Three patients had both an anterior and posterior drawer sign, and arthroscopy confirmed aplasia of both the ACL and PCL. Three patients had an isolated anterior drawer, and had ACL aplasia on arthroscopic examination. All knees were found to have hypoplastic tibial spines on radiographs.
Chugiak [29] looked at a larger series of 21 patients with clinical and arthroscopic examinations. All patients were found to have an anterior drawer sign, and nine patients (43 %) had a posterior drawer sign. Four patients (19 %) had medial instability, including one patient who also had lateral instability. The instrumented and clinic drawer tests were not found to be reliable enough, leading the authors to recommend imaging of the cruciate ligaments prior to lengthening to avoid knee dislocation. The majority of patients (38 %) were found to be completely deficient in both the ACL and PCL. The ACL was completely deficient in 16 (71 %) and hypoplastic in 3 (14 %) patients. The PCL was completely deficient in 10 (48 %) and hypoplastic in 3 (14 %) patients. Only one patient had an intact ACL and PCL.
In the majority of patients, both menisci were intact, with only three (14 %) hypoplastic and unrelated to the cruciate ligaments. Changes in the intercondylar notch and tibial eminences were noted in some patients but it was not specifically studied, as the authors felt that they were not as appreciable in patients under 6 years of age. They did note that the femoral intercondylar notch developed in some patients that had aplasia of both cruciate ligaments.
Muscle Pathoanatomy
Despite the wide spectrum of CFD, the underlying muscular anatomical differences appear relatively consistent. Pirani et al. showed that in patients with Aitken types A through D, the muscles were all present but altered in their size, structure, and location [30]. The study used MRI to qualitatively assess the musculature. The majority of muscles were found to be smaller: gluteus maximus, gluteus medius, gluteus minimus, quadriceps, adductor magnus, adductor longus, adductor brevis, pectineus, semimembranosus, semitendinosus, and biceps femoris. The exception is the sartorius muscle that was found to be hypertrophied, to which Pirani et al. and Panting et al. attributed as the underlying cause of the deformities of the proximal femur, given the sartorius’ orientation [31]. The obturator externus was found to be elongated and the muscle belly extended almost entirely to its insertion. The short external rotators had a larger cross-sectional diameter and were found to insert on the posteromedial greater trochanter. Overall, the course of the muscles was more perpendicular and proximal than normal, inserting onto the proximally migrated femur.
We have also found this to be true in our surgical experience: the musculature is present, the muscle bellies extend nearly to their insertion, and the course of the muscles is proximal. Panting and William had previously noted similar findings, including the hypertrophied sartorius muscle, the relatively normal gluteal muscles, and a hypoplastic quadriceps muscle [31]. Biko et al. also used MRI in seven patients to primarily evaluate the osseous structures, and reported a qualitative decrease in size of the musculature in general. At the level of the triradiate cartilage, the cross-sectional size of the gluteus muscles was significantly smaller in comparison to the uninvolved contralateral limb [32].
Pirani et al. postulated that the muscles became the primary stabilizers of the deformed hip joint, since the osseous structures did not impart inherent stability. However, in our experience the majority of untreated patients with CFD do not report ipsilateral hip pain, even after knee fusion and with prosthetic use and weight bearing.
Vascular Pathoanatomy
The normal embryological development of the arteries of the lower extremity parallels the formation of limb buds, occurring between the 4th and 8th weeks of gestation. The limb begins with a single axial dorsal artery that continues as the ischiadic artery and on to the popliteal artery. The external iliac artery arises at 5 weeks of gestation and bifurcates into the inferior epigastric artery and the femoral artery, which then bifurcates at 6 weeks gestation into the lateral and medial branches. The medial branch becomes the deep femoral artery and gives rise to the medial and lateral circumflex arteries and connects to the ischiadic artery, while the lateral branch develops into the femoral artery. By 7.5 weeks the femoral artery caliber is larger than the ischiadic artery and becomes the major blood supply to the popliteal artery and leg. After full development, the dorsal axial artery remains as the inferior gluteal artery while the ischiadic artery remains as the arteria comitans nervi ischiadici, running as the vasa vasorum of the sciatic nerve [33].
The vascular anatomy is also usually abnormal in an extremity affected by CFD. Chomiak et al. reported the results of 21 patients with various degrees of CFD studied with computed tomography (CT) angiograms to identify vascular abnormalities [34]. The authors found that more severe cases of CFD had a smaller diameter and a shorter length of the femoral artery; however, the severity of osseous abnormalities did not directly correlate with the topographical vascular anatomy abnormalities. All patients, at the least, had differences compared to the contralateral unaffected extremity: mainly with smaller vessel caliber, decreased number of vessels to the thigh, and more proximal bifurcation of the external iliac into femoral artery and deep femoral artery. Despite this, 19 of the 21 patients had the blood supply to the femur and pseudoarthrosis from branches of the deep femoral artery, which originated from the external iliac artery.
Notably, Chomiak found 2 of the 21 patients with a persistent ischiadic artery as the dominant vascular supply to the leg and a diminutive femoral artery that supplied solely the medial thigh [34]. This is an extremely unusual finding in otherwise normal humans, but is seen in lower mammals [35].
It is critical to identify these vascular anomalies preoperatively when reconstruction using rotationplasty is considered. The senior author has identified several cases with a dominant ischiadic artery and absent superficial femoral artery while performing rotationplasty. In our opinion, an MR angiogram (MRA) is indicated in all such cases prior to a rotationplasty.
Box 22.1. CFD Pathology
The incidence of CFD is approximately 1 in 50,000.
Limb bud formation is affected by a complex interplay of signaling, including AER, ZPA, and Wnt.
CFD is likely caused by a somatic mutation during the development of the limb bud, but some cases may be caused by an inherited germ cell mutation.
CFD usually presents with proximal coxa vara and distal femoral valgus.
Cruciate ligament deficiency is common and can often be identified on radiographs.
Generally, most muscles around the pelvis are present and hypoplastic, though the sartorius may be hypertrophied.
An ischiadic artery may be present and more dominant than the femoral artery in some patients with CFD.
Evaluating the Child with Unilateral CFD
History
Most children born with unilateral CFD have no family history of this or other congenital anomalies. Nevertheless, inquiry should be made into family history, exposure to drugs, medications, radiation, or infectious diseases during the first trimester. Many cases of CFD are now identified with prenatal ultrasound early in the pregnancy by measuring the lengths of the two femurs. In such cases the predicted leg length discrepancy at birth and maturity can be calculated using the multiplier method [36].
Physical Exam
There is an obvious leg length discrepancy. Associated fibular hemimelia (FH) and ray deficiency may be present. The hips, knees, and ankles should be examined for range of motion and flexion contractures. Neonates and young infants normally have such contractures for the first 3–6 months. At the hip, patients may have external rotation and fixed flexion deformity of the hip, as well as limited abduction when coxa vara is present. At the knee, patients may have flexion contractures, hypoplastic or subluxation-dislocation of the patella, AP, or rotary instability of the knee.
Finally, muscle length tests are recorded to identify pre-existing limits to muscle lengthening. These tests include the Ely test for rectus femoris tightness, popliteal angle measurement for hamstring tightness, and Ober sign for fascia lata or iliotibial band tightness (Fig. 22.3).
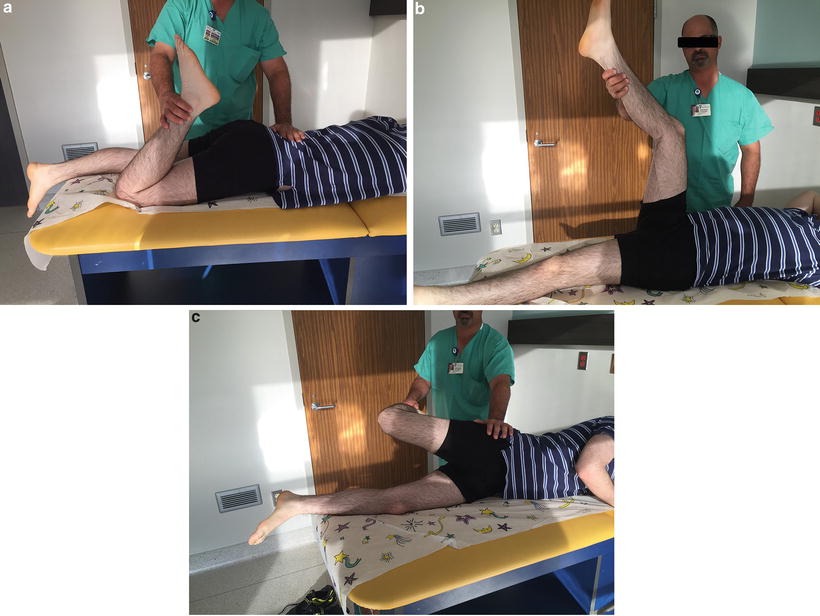

Fig. 22.3
Muscle lengthening tests: (a) The Ely test checks for rectus femoris tightness, demonstrating pelvic flexion with prone knee bend; (b) the popliteal angle is the angle between the vertical and the line of the tibia with the hip at 90° flexion and the knee maximally extended; (c) the Ober test checks for fascia lata tightness; a positive sign is the thigh going into an abduction position when the hip is hyperextended and the knee flexed to 90°
Box 22.2. Physical Examination Findings Often Seen in Patients with CFD
Hip: External rotation (ER) deformity or increased ER versus internal rotation (IR); fixed flexion deformity (FFD) of hip; limitation of abduction (when coxa vara is present).
Knee: Fixed flexion deformity of knee; no limitation of knee flexion; hypoplastic patella; lateral tracking or subluxated or dislocated patella; anteroposterior instability of knee; rotary instability of knee; anterior dislocation of tibia on femur with knee extension followed by reduction of knee with attempted flexion; hypermobile meniscal clunks; temporary locking of the knee during flexion.
Ankle: Limitations of ankle dorsiflexion; obligatory eversion with dorsiflexion; hypermobility of ankle with increased eversion; lateral malleolus more proximal than medial malleolus.
Imaging
Radiographic Examination
Radiographs should include a full-length anteroposterior (AP) standing legs with the patellas pointing forward. In children who are unable to stand, a pull-down X-ray may be performed. It allows measurement of the length of the femurs and tibia, though it does not include foot height. Long lateral leg radiographs in maximum extension allow for evaluation of knee flexion contractures and more accurate length measurement. A supine AP pelvis allows more accurate measurement of the center-edge angle (CEA) of both hips to assess for hip dysplasia. It is also a better quality X-ray to assess for ossification of the femoral neck. It is important that the pelvis be level for more accurate measurement.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is useful to assess the integrity of the proximal femur. It can help determine whether the femoral head is joined to the shaft of the femur via a cartilaginous femoral neck. In case of a femoral neck pseudoarthrosis, the MRI can help determine if the cartilage of the femoral head is fused to the acetabular cartilage. For optimal imaging, the cuts of the proximal femur should be reformatted in an oblique plane to see the entire proximal femur as a single image. MRI can also help outline the intra-articular pathology of the knee, identifying deficiency of the cruciate ligaments and outlining the shape of the joint surfaces in the frontal and sagittal plane.
Computerized Tomography (CT)
CT is only useful at an older age when the acetabulum and proximal femur are nearly fully ossified. Three-dimensional CT reconstruction is useful to compare the normal acetabulum with the dysplastic side. In older children 3D CT can show the pathologic anatomy.
Box 22.3. Evaluating the Child with CFD
CFD can be bilateral; check the contralateral side as well.
The hip, knee, and ankle should all undergo thorough physical examinations.
Fibular hemimelia is commonly associated with CFD.
Radiographic imaging should include a full-length AP legs (standing or pull-down), long leg laterals in maximum extension, and a supine AP pelvis.
MRI is useful to evaluate soft tissue, ligaments, and a cartilaginous femoral neck.
Classification Systems
Multiple classification systems have been described over the years in attempts to categorize the pathology and direct surgical treatment (Figs. 22.4, 22.5, and 22.6). Most older classification schemes were based on plain radiographs. Newer classification systems incorporate modern imaging modalities, specifically MRI, which allows detailed evaluation of osseous, cartilaginous, and soft-tissue structures, including the non-ossified femoral head, non-ossified acetabulum, labrum, pseudoarthrosis, and musculature [32]. The ability to perform MRI under anesthesia for infants allows for interpretation of these images with better reliability. As a result, MRI dramatically improves the ability to correctly categorize patients over conventional radiographs alone [37]. Table 22.1 shows a comparison of several of the classification schemes further elucidated below.
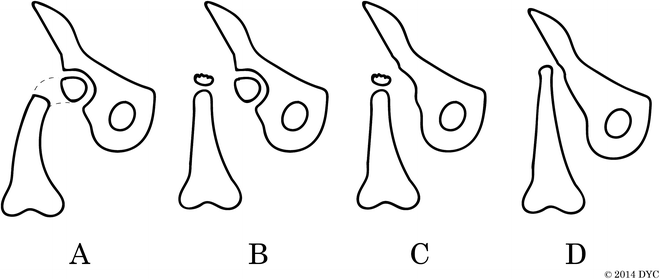
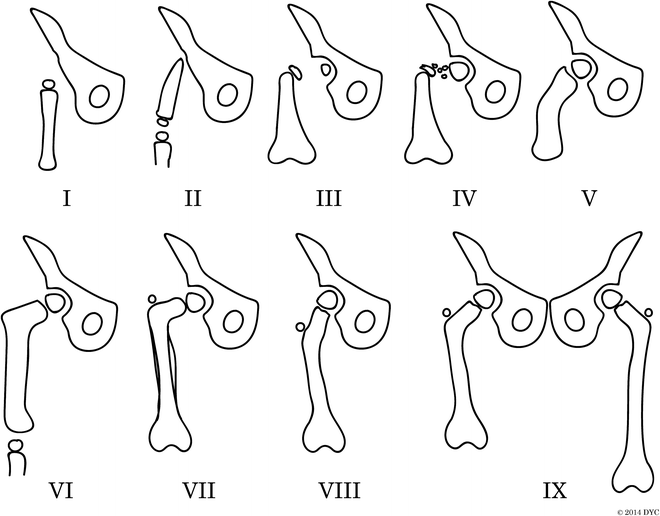
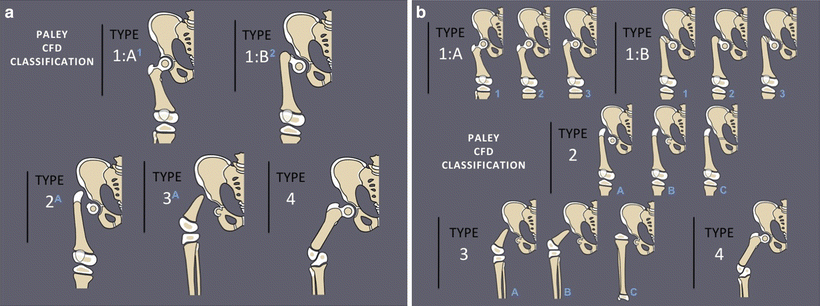

Fig. 22.4
Aitken classification of congenital femoral deficiency. The Aitken classification is based on the relationship of the acetabulum and the femoral head: (a) Adequate acetabulum, femoral head is present and attached to shaft; (b) adequate acetabulum, femoral head not connected to shaft, which instead has a proximal ossified tuft; (c) dysplastic acetabulum, minimal or absent femoral head, disconnected femoral shaft with tuft; and (d) no acetabulum, no femoral head, shortened proximal femoral shaft with no tuft

Fig. 22.5
Pappas classification of congenital femoral deficiency. The Pappas classification is divided into nine classes: (I) congenital absence, (II) proximal femoral and pelvic deficiency, (III) proximal femoral deficiency with no osseous connection between femoral shaft and head, (IV) proximal femoral deficiency with disorganized fibrosseous disconnection between femoral shaft and head, (V) midfemoral deficiency with hypoplastic proximal and distal development, (VI) distal femoral deficiency, (VII) hypoplastic femur with coxa vara and sclerosed diaphysis, (VIII) hypoplastic femur with coxa valga, and (IX) hypoplastic femur with normal proportions

Fig. 22.6
(a) Paley classification of congenital femoral deficiency. There are four types of congenital femoral deficiency: (1) intact femur normal ossification (a) or delayed ossification (b); (2) mobile pseudoarthrosis; (3) diaphyseal deficiency; (4) distal deficiency. (b) Each Paley type has several subtypes: 1a1: Normal-shaped short femur with mild genu valgum; 1a2: normal-shaped short femur with mild genu valgum and retroversion; 1a3: coxa vara, retroversion, and mild genu valgum; 1b1: subtrochanteric type; 1b2: neck type; 1b3: combined neck and subtrochanteric type; 2a: mobile femoral head; 2b: partially fused femoral head; 2c: completely fused or absent femoral head; 3a: mobile knee joint with greater than 45° of motion; 3b: stiff knee with less than 45° of motion; 3c: absent femur or fused knee joint
Table 22.1
Comparison of different classification schemes
Paley [42] | Pappas [38] | Gillespie and Torode [39] | Hamanishi [15] | Aitken [17] | |
|---|---|---|---|---|---|
Normal anatomy/minimal deformity/hypoplasia | 1A | 7,8,9 | Group 1 | Type 1, type 2 | Class A |
Subtrochanteric delayed ossification | 1B subtrochanteric | 4, 5 | Group 1 | Type 3a | Class A |
Femoral neck delayed ossification | 1B femoral neck | 3 | Group 1 | Type 3b | Class A |
Combined delayed ossification | 1B combined | 3 | Type 4a | ||
True pseudoarthrosis with no cartilaginous or osseous femoral neck | 2a, 2b | 3 | Group 2 | Type 4b | Class B |
Dysplastic acetabulum, no femoral head | 2b, 3 | 2 | Group 2 | Type 4c | Class C |
No proximal femur, short distal femur only | 3a,b | 2 | Group 2 | Type 4c | Class D |
Femoral absence | 3c | 1 | Type 5 | ||
Distal femoral deficiency only | 4 | 6 |
Aitken, 1959
In 1959 Aitken proposed a four-class schema for PFFD (see Fig. 22.4) [17]. This became the most commonly utilized classification system for PFFD. Aitken focused the classification system on the development of the femoral head, neck, and acetabulum, with progressive dysplasia from one class to the next. The Aitken classification does not include the congenital short femur group discussed earlier.
Aitken initially proposed that Class A patients were not technically PFFD, as all the structures were present, albeit deformed. These patients had a femoral head, a cartilaginous femoral neck, an adequate acetabulum, and a very short femoral segment. Severe subtrochanteric femoral varus and femoral shortening were appreciated. A pseudoarthrosis of the proximal femur was appreciated between the femoral shaft and head, either between shaft and trochanteric component (subtrochanteric pseudoarthrosis) or between trochanteric component and head (femoral neck pseudoarthrosis). Aitken noted that the pseudoarthrosis did not spontaneously resolve in all patients by skeletal maturity, but did eventually heal in some patients.
The hallmark of the Aitken Class B PFFD was a lack of connection, either bony or cartilaginous, between the femoral shaft and head that persists into skeletal maturity. These patients have a femoral head within an adequate acetabulum and a shortened, deformed femoral shaft. The proximal portion of the shaft often has an ossified tuft. The femoral head and shaft do not move as a unit.
Aitken Class C is defined by a severely dysplastic acetabulum. The femoral head does not ossify and shaft of the femur is short with an ossified tuft at the proximal end of the shaft. There is no cartilaginous or osseous femoral neck or trochanteric component.
Aitken Class D is the most dysplastic form of PFFD. This class is characterized by the absence of the acetabulum and femoral head and a deformed, shortened femoral shaft without tufting on the proximal shaft of the femur.
Pappas, 1983
The Pappas classification (see Fig. 22.5), published in 1983 [38], was based on the anatomy of 139 cases evaluated in Massachusetts. Pappas describes nine classes based on the femoral deficiency and associated deformity, with diminishing severity as the class number increased. This was the first classification scheme to separate middle and distal femoral deficiencies into their own class. Recommendations were given for treatment for each class; however, treatment was dependent on the patient’s joint stability and function, which are not part of the classification system. Therefore treatment could not be based directly on the classification schema of Pappas.
Gillespie and Torode, 1983
In 1983, Gillespie and Torode proposed a straightforward division of CFD into two categories: congenital short femur and true PFFD [39, 40]. Each of the two groups had both radiographic and clinical criteria, but were ultimately defined by the function of the hip and knee joints, rather than the type of surgery each would necessitate.
Group 1 was defined as those patients who could have good hip and knee function with or without surgical correction. Radiographically, group 1 patients had coxa vara, lateral femoral bowing, and hypoplasia of the knee with valgus but the predicted leg length discrepancy was no greater than 30 % of the normal femur and femoral deficiency was 40–60 % of the contralateral femur. Clinically this group had laxity in the sagittal plane and mobile joints without flexion deformities at hip or knee.
Group 2 patients had hip and knee joints that were not adequate to support the patient without pain. The appropriate treatment recommendation for this group of patients was amputation and/or rotationplasty of the lower limb. These patients had markedly short femurs. Radiographically, these patients had less than 40 % of the femoral length of the normal side and deficiency or absence of the femoral head and/or neck. The authors defined deficiency of the femoral head as a tenuous cartilaginous connection between femoral shaft and head. Clinically, these patients had fixed flexion deformity at hip and/or knee.
The overall goal of the Gillespie and Torode classification was to simplify the decision making. The authors rationalized for families and surgeons a “life plan” regarding treatment of the affected limb based on each of the two groups. This classification aims to aid families and physicians communicate more effectively and correlate early clinical signs with long-term treatment plans. The value of this classification schema is its simplicity and its guidance in surgical treatment. The drawbacks to this classification scheme are its inability to adapt to improved surgical techniques based on a better understanding of pathoanatomy, as well as advances in biology and technology.
Hamanishi, 1980
Hamanishi [15] published a five-tiered classification system for congenitally short femur. This schema involved a scoring system that included the entire lower limb and upper limb involvement, as opposed to other classification systems, which focus only on the femur. However, the schema does not include specifically the acetabulum, as the author believed that this component is directly related to the femoral head. It was intended to differentiate those patients with the teratologic sequelae of thalidomide exposure from those patients with idiopathic CFD. The diagnosis was based on a series of 60 total patients, 14 of which were known to have had in utero thalidomide exposure. It is an observational classification system and does not aim to guide treatment or prognosis. The study was quite comprehensive as the authors included socioeconomic class, an extensive family history, parental age, birth history, and in utero history in their review. The age of the study subjects at the time of evaluation varied from 6 months to 50 years.
Hamanishi identified several differences between the two groups: overall, the thalidomide exposure group had femora that were more severely affected than the idiopathic group; the thalidomide exposure group more often had concomitant abnormalities of the tibia and radius as opposed to the fibula and ulna in the idiopathic CFD group; the thalidomide group had lower extremity preaxial (tibial) polydactyly, whereas the idiopathic group had lower extremity postaxial (fibular) deficiency. The author defined five groups with subgroups in some utilizing radiographic measurements of the affected femur to quantify the degree of deformity.
Group 1 was defined by hypoplasia of the femur, either without deformity type 1a or with minimal angulation and cortical thickening, type 1b. The type 1a was associated with ipsilateral below the knee deformities, while the type 1b was associated with severe, symmetric bilateral below the knee deformities. The second group was defined by hypoplasia and femoral varus angulation. Type 2a had a normal neck-shaft angle but type 2b had a decreased neck-shaft angle. A transverse subtrochanteric ossification defect was noted in this type as well, but all ossified later in life.
The type 3 group had marked coxa vara from delayed and inconsistent ossification of the femoral neck and subtrochanteric region. This group also has marked retroversion of the femoral neck and distal femur external rotation. Type 3a with a straight femoral shaft was considered “stable” as the proximal femoral varus would not progress, while the “unstable” type 3b with an increased epiphyseal head angle >60° was reported to progress with time.
The type 4 femurs had absent or stunted and tapered proximal femoral shafts with the femoral head attached directly to the shaft, when present. The femoral head was connected to the shaft by a pseudoarthrosis and the entire shaft was proximally migrated. The type 4a had a pseudoarthrosis of the femoral neck and subtrochanteric region. The type 4b had an absent proximal femur with the femoral head connected directly to a tapered proximal shaft. The type 4c had a complete absence of the proximal femur.
Type 5 femurs indicated a rudimentary femur, which ossified later, or complete absence.
One major flaw in this classification system is that it divides the groups based on measurements with arbitrarily chosen cutoffs, such as the neck-shaft angle and femoral varus which change with time. Thus, young patients could move from one group to another without intervention and the treatment plan would also therefore be variable. Additionally, this classification scheme does not include the stability of the hip or knee, and the presence of knee ligaments, and does not guide surgical or nonsurgical treatment.
Kalamachi, 1985
The Kalamachi classification (1985) [41] attempted to relate the femoral deficiency directly to the limb function. Type I patients had congenital shortening of the femur with a normal hip and knee morphology and function. Type II patients had a congenitally short femur with coxa vara, with some irregularity of the growth plate, a located hip, and a well-developed acetabulum. Type III patients had a developed acetabulum but a deficient proximal femur. With time, they were separated into two groups. In the IIIA group, the radiographically apparent proximal femoral defect ossifies into a bony bridge, often in varus. In the IIIB group, the defect does not ossify, and develops into a pseudoarthrosis. Type IV patients had no acetabulum and no connection between the pelvis and the distal femoral fragment, which often was a spike proximally. Type V patients had no hip joint and no femur. For all five classification types, the most common treatment was a Boyd or Syme amputation. Knee fusions were used to correct deformity as well as provide a large lever arm for a severely shortened femoral segment. Femoral osteotomies were used mostly in type II and IIIA patients to correct coxa vara. Limb equalization procedures were done in nine patients (four epiphysiodesis, five lengthening) of type I and II. The rest were treated non-operatively or with orthotic and prosthetic fitting.
Paley, 1998
Paley proposed a classification system for CFD (first presented at a symposium in Dallas in 1996 documented in a book published from that symposium [42]) based on the degree of development and integrity of the femur (see Fig. 22.6). Unlike the previous classifications, Paley wanted to create a classification where the type would not change with the degree of ossification of the femur (age dependent). A longitudinal follow-up of different classification systems by Sanpera and Sparks [43] showed that the existing classification systems were inaccurate in predicting the final femoral morphology based on the initial radiographs. Paley also wanted a classification that was oriented towards reconstruction and not amputation. As such, the classification types and subtypes each have a separate surgical reconstructive prescription.
Box 22.4. Femur Deficiency Classification (Paley, 1998) [42]
Type 1: Intact femur with mobile hip and knee:
(a)
Normal ossification proximal femur
(b)
Delayed ossification proximal femur (neck, subtrochanteric, or combined neck subtrochanteric types)
Type 2: Mobile pseudoarthrosis (greater trochanteric apophysis present), knee usually mobile:
(a)
Femoral head mobile in acetabulum
(b)
Femoral head partially fused to acetabulum
(c)
Femoral head and acetabulum completely fused or absent
Type 3: Diaphyseal deficiency of femur (greater trochanteric apophysis absent):
(a)
Distal physis present; knee motion ≥45°
(b)
Distal physis present; knee motion <45°
(c)
Complete deficiency of distal femur or fusion of distal femoral remnant to tibia (distal physis absent)
Type 4: Distal deficiency of femur (proximal end normal)
Treatment Options for Congenital Femoral Deficiency
Rotationplasty
Rotationplasty has been a commonly accepted treatment for patients with CFD with significant proximal deficiency, which is deemed “unreconstructable” [44–48]. The most commonly employed surgical technique is the Van Nes rotationplasty and variations of this technique. In the Van Nes rotationplasty, the lower limb is rotated 180° to use the ankle and foot as a functional knee joint with a prosthesis. The rotationplasty can be combined with a knee fusion when the knee is unstable, or as in the Brown modification the knee is maintained after 180° rotation and the distal femur is fused to the pelvis to substitute for a deficient hip allowing flexion and extension [44]. This will be discussed in more detail in a later section.
Gillespie and Torode reported their surgical recommendations along with their classification schema in 1983 [39, 40]. Prior to the advent of the Wagner device in 1971, patients classified as Group 1 with congenital short femur underwent rotationplasty. After the Wagner technique demonstrated the feasibility of lengthening up to 20 cm, femoral lengthening became the standard recommendation for Group 1, but to a maximum of 20 % of the femur length because the risk of hip and knee subluxation or dislocation was considered too high. Of the five Group 1 patients that underwent rotationplasty, three had good function but two required a Syme amputation and fitting with an above-the-knee prosthesis. Patients classified by Gillespie and Torode as Group 2 had true PFFD and received rotationplasty, Syme amputation for prosthesis fitting, and/or knee fusion. Of 43 Group 2 patients with instability of the hip and knee, 21 underwent rotationplasty, and of those, 8 required de-rotational surgery and 2 required a Syme amputation. They strongly advocated knee fusion for Group 2 patients to correct the valgus and flexion deformities at the knee and tibial rotationplasty to restore “knee flexion” with the ankle dorsiflexion.
Brown reported on three patients with severe CFD who underwent a modification of the Van Nes rotationplasty with mean of 6-year follow-up who had satisfactory results and range of motion of both the hip and knee to 90° of flexion [44]. The Brown modification includes resecting the deficient proximal femur, externally rotating the limb 180°, fusing the femoral remnant to the pelvis, and leaving the muscles distal to the knee undisturbed. Using this technique there was no derotation as previously recurred with the Van Nes rotationplasty which incorporated a midtibial osteotomy for rotation.
Ackman et al. reviewed 12 patients who underwent Van Nes rotationplasty for CFD to determine the long-term outcome [48]. At a mean of 21.5 years (11–45) after their rotationplasty, a total of 12 prosthetic patients were compared with 12 normal age- and gender-matched controls. The authors found no differences between the groups in overall health and well-being on the SF-36, but significant differences were seen in gait parameters in the CFD group. Patients who had undergone Van Nes rotationplasty had a high level of function and quality of life at long-term follow-up, but presented with significant differences in gait and posture compared with the control group.
Syme Amputation
Alman et al. reviewed the results of treatment of 16 patients who had had an isolated unilateral PFFD; 9 were managed with a rotationplasty and 7 with a Syme amputation combined with an arthrodesis of the knee [49]. The perceived physical appearance, gross motor function, and metabolic energy expended in walking were assessed. The mean duration of follow-up was 9.9 years (range, 4–14 years). The mean age of the patients at the time of the study was 13.9 years (range, 8–18.4 years) in the rotationplasty group and 14.8 years (range, 9.5–19.9 years) in the Syme-amputation group. There were three female patients in each group. Roentgenograms showed that the femoral head was in the acetabulum (Aitken class A or B) in four of the seven patients in the Syme-amputation group and in five of the nine patients in the rotationplasty group; the remaining patients did not have this finding (Aitken class C or D). There was no difference in gross motor function or perceived physical appearance between the groups. Rotationplasty was associated with a more energy-efficient gait than was Syme amputation.
Fowler et al. measured lower limb kinematics and kinetics during preferred and fast speeds of walking in persons with PFFD to compare outcomes after Syme amputation (nine subjects) with those after Van Nes rotationplasty (ten subjects) [50]. Subjects with a Van Nes rotationplasty and full tibial rotation (seven subjects) demonstrated prosthetic knee function during stance as they were able to support a flexed-knee posture at both speeds and produced greater knee-extensor moments at preferred speeds as compared with the Syme group. Non-prosthetic limb compensatory mechanics were significantly exacerbated in subjects with a Syme amputation compared with the Van Nes group: (1) stance-phase vaulting, resulting in greater inappropriate ankle-power generation at both walking speeds; (2) excessive hip-extensor moments at fast speeds; (3) excessive hip-power absorption and generation at both speeds; and (4) excessive knee-joint power generation at both speeds. The improved gait after Van Nes rotational osteotomy is one factor that should be considered when making clinical decisions for children with PFFD.
Other studies have found some potential issues with Syme amputation in the long term: heel pad migration, skin sloughs, and problems with prosthetic fitting. Anderson et al. reviewed 69 Syme amputations performed in 62 children with the major indication of leg length discrepancy, due to either paraxial fibula hemimelia (33 cases) or proximal focal femoral deficiency (19 cases) [51]. The average age at amputation was 5.6 years, with an average follow-up of 10.5 years (range 1–25 years). Although the results were assessed by a combination of chart review, patient recall examinations, and questionnaires, satisfaction in adulthood was found to be high. Early complications included three skin sloughs and one infection. Late complications included 2 retained os calcis apophyses, 1 exostosis, and 16 cases of heel pad migration. Only one of the heel pad groups required revision; prosthetic adjustment resolved symptoms in the remaining patients. However, prosthetic knees were often too low because of failure to limit the length of the stump appropriately, though this finding was not as problematic in the CFD group as the residual limb was significantly shorter.
Limb Lengthening
Aston et al. published a series of 27 patients with Paley type 1 CFD (Pappas grades VII, VIII, and IX) who underwent a total of 30 lengthening procedures, with 3 patients undergoing a second femoral lengthening [52]. All patients underwent femoral lengthening with a multiplanar Ilizarov-type external fixator. The mean increase in length was 5.8 cm (3.3–10.4 cm) and 18.65 % of the total length of the femur (9.7–48.8 %). The mean time in the frame was 223 days (75–363) with a mean distraction index of 1.28 months per cm. The authors initially performed the osteotomy distal, but after 17 distal osteotomies changed to a proximal osteotomy for lengthening, noticing a significant increase in mean range of knee motion from 98.1° to 124.2° (p = 0.041) and a trend towards a reduced requirement for quadricepsplasty, although this was not statistically significant (p = 0.07). The overall incidence of regenerate deformation or fracture requiring open reduction and internal fixation was similar in the distal and proximal osteotomy groups (56.7 % and 53.8 %, respectively). However, in the proximal osteotomy group, preplacement of a Rush nail reduced this rate from 100 % without a nail to 0 % with a nail (p < 0.001). When comparing a distal osteotomy with a proximal one over a Rush nail for lengthening, there was a significant decrease in fracture rate from 58.8 % to 0 % (p = 0.043). This supports the premise of Paley and Herzenberg that CFD patients should be lengthened over a Rush rod or have a Rush rod inserted after frame removal to reduce the risk of regenerate bending or fracture [53].
Aston et al. concluded that lengthening of the femur with an Ilizarov construct should be carried out through a proximal osteotomy over a Rush nail and lengthening to a maximum of 6 cm during one treatment, or 20 % of the original length of the femur, to reduce the risk of complications [52]. Paley and Standard have published on a distal osteotomy for CFD to avoid lengthening through the abnormal, sclerotic bone often seen in the proximal femoral shaft as much as possible with good success [54, 55].
Saghieh and Paley studied a group of 95 CFD patients who had undergone lengthening between the years 1988 and 2000 (Table 22.2). These patients did not undergo the Superhip or Superknee procedures that are described later in this chapter. All femoral lengthenings were with the Ilizarov device with extension of the fixator across the knee to the tibia with hinges. The postoperative result based on knee and hip range of motion score, gait score, lengthening goal score, and alignment score, pain score, and activity level score was excellent and good in over 93 % in all groups, with a mean overall lengthening of 6.0 cm (range, 2.2–12.5). Complications included femur fracture, pin site problems requiring surgery, premature consolidation, nerve irritation/palsy, delayed union, knee subluxation, and hip subluxation. The fracture rate in the older age group was significantly lower than the other groups, but it included several patients that were undergoing lengthening over nails. There was no significant difference in unplanned surgery rate between groups 1 and 2 (see Table 22.2). The difference with group 3 was related to refractures. Prophylactic rodding was not performed at the time of fixator removal in this series of patients, but is now part of the standard treatment algorithm, given the high rate of fractures [56].
Table 22.2
Limb lengthening results and complications in 95 patients
Group 1 (<6 years) | Group 2 (6–13 years) | Group 3 (>13 years) | |
|---|---|---|---|
Number of patients (n) | 30 | 40 | 25 |
Amount of length achieved | |||
Length (cm) | 5.4 (2.8–8.5) | 6.2 (2.5–11) | 6.3 (2.2–12.5) |
Relative (%) | 39 (12–71) | 24 (7–54) | 20 (5–58) |
Lengthening index (month/cm) | 1.0 (0.5–1.8) | 1.1 (0.5–2.2) | 1.1 (0.5–2.2) |
Good/excellent outcome score | 28/30 (93 %) | 37/40 (93 %) | 24/25 (96 %) |
Problems, obstacles, and complications | |||
Femur fracture | 13 | 14 | 4 |
Pin-site problems requiring surgery | 4 | 3 | 1 |
Nerve irritation/palsy | 3 | 3 | 1 |
Premature consolidation | 0 | 3 | 3 |
Delayed union | 2 | 4 | 3 |
Knee subluxation | 5 | 5 | 3 |
Hip subluxation | 3 | 3 | 1 |
Total unplanned surgery | 21 (70 %) | 22 (55 %) | 9 (36 %) |
The authors have previously presented their results on femoral lengthening after a hip stabilization procedure [57]. A retrospective review was performed of 35 patients with CFD, Paley types 1a and 1b, who underwent femoral lengthening after a pelvic osteotomy, proximal femoral osteotomy, or a combination. Patients underwent a hip stabilization procedure at a mean age of 2.4 years (2–5.5). The mean age at first femoral lengthening was 3.7 years (3–10.7). The mean overall limb length difference prior to femoral lengthening was 64.8 mm (47–100), with a mean postoperative difference of 8.1 mm (−14 to 32). The average amount of time in the external fixator was 186.1 days (107–311), or 1.1 months/cm. Preoperatively, average knee ROM was 2°–131° and hip ROM was −2° extension to 115° flexion. Follow-up at a mean of 14.4 months (5.6–45.2) showed return to baseline ROM in knee of 2°–119° and hip from −1° extension to 91° flexion. The overall rate of obstacles and complications was 37.1 % and 32.4 %, respectively [58].
Treatment of CFD has always been divided between amputation and lengthening. In the past, the indications for amputation were much more liberal. Currently, the dividing line can be based more on pathoanatomy. Treatment varies, depending on the severity of the underlying deficiency. The less involved patients, such as Paley type 1a, usually only require leg length equalization surgery, consisting of limb lengthening and/or contralateral epiphysiodesis at the appropriate time. The current treatment of more severely affected patients, such as Paley type 1b and type 2, consists of complex surgical interventions consisting of reconstruction of the hip and knee joints, correction of femoral deformity and length, ossification of the pseudoarthrosis, and reconstruction of lax or absent ligaments. This is discussed in the subsequent sections. The most severely affected patients, Paley type 3, usually undergo surgery to improve prosthetic use, as regaining satisfactory function with limb lengthening and reconstruction is often not feasible.
Box 22.5. Classification and Treatment
Multiple classification schemes exist, mostly based on their anatomic deformity, though sometimes they are age dependent or have arbitrary cutoffs.
The Paley classification separates into types and subtypes based on their surgical treatment for reconstruction.
Van Nes rotationplasty has been used historically on severely shortened femurs with good results.
Patients undergoing Syme amputation with knee arthrodesis often have more altered gait mechanics with greater energy expenditure compared to those undergoing a rotationplasty.
In the properly selected patient, limb lengthening often has good outcomes, but can have significant complications. However, these untoward events can be lessened with prophylactic joint stabilization and intramedullary rodding techniques.
Recommended Surgical Reconstructive Strategy for Paley Type 1 CFD
Outlining a “Life Plan” for the Family
At the initial consultation, a surgical reconstructive strategy or “life plan” projected to skeletal maturity should be outlined for the child and the family. It is helpful to write this out on the back of a printed radiograph for the family to put in the child’s scrapbook and to refer to in the ensuing years. The strategy is based on the type of CFD, the projected LLD at maturity, and the reconstructive potential of the hip and knee. In cases with combined CFD and FH, the strategy for FH must be combined with that of the CFD [18].
Step 1: Preparatory Surgery for the Hip and Knee
Prior to lengthening one must determine whether the hip and knee joints are stable and/or deformed and whether surgical procedures for these joints are required before initiating limb lengthening. At the hip, if the acetabulum has an acetabular index with comparable slope to the normal contralateral side, a center edge angle (CEA) ≥ 20°, and a neck-shaft angle (NSA) ≥ 110°, no separate hip surgery is required before the first lengthening [59]. If the acetabulum shows signs of dysplasia then a pelvic osteotomy should be performed prior to lengthening. Increased slope of the sourcil (acetabular roof) or acetabular index compared to the other side is a subtle but sensitive sign of acetabular dysplasia. Coxa vara should be corrected prior to lengthening if the NSA is less than 110°. Similarly, external rotation deformity of the hip is a factor to consider for correction at the same time as the acetabular dysplasia. If a Dega-type osteotomy is chosen, then there is usually a gain of about 1 cm in leg length. Associated hip deformities of retroversion, hip flexion contracture, and hip abduction contracture should be simultaneously addressed. The flexion contracture of the hip is treated by recession of the psoas tendon and release of the rectus femoris tendon. A bony flexion deformity may be treated by a proximal femoral extension osteotomy. The abduction contracture is treated by lengthening or resection of the fascia lata and, if necessary, an abductor muscle slide at the iliac crest. When all of these deformities are present together and especially with higher degrees of angulation, the reconstructive procedure is called the Superhip procedure [60].
Other factors should be examined prior to lengthening. The proximal femur should be normally ossified for the patient’s age. Often, severe cases of CFD have delayed ossification of the femoral neck or subtrochanteric region, and bone morphogenic protein (BMP) may be added to the femoral neck to promote ossification [14]. The fascia lata is a thin but very tough limiting membrane that resists lengthening and applies pressure across the knee joint and the distal femoral growth plate. Thus, it should always be removed or released before lengthening. It can also be used to reconstruct absent knee cruciate ligaments if procedures are done concurrently. A hemiephysiodesis may also be performed to correct frontal plane deformities. The ideal age for the preparatory procedure is between ages 2 and 3 years.
Step 2: Serial Lengthenings of the Femur and/or Tibia
A prediction of total leg length discrepancy at skeletal maturity helps determine the approximate number of lengthening surgeries required. This can also be done using the Paley multiplier method [26]. The majority of CFD cases will require at least two lengthenings. The goal of each lengthening depends on the total discrepancy at maturity. The safe range of lengthening is from 5 to 8 cm, as long as a good physical therapy program is available. This amount seems to be independent of the initial length of the femur and age of the child, and can be performed safely even in a toddler. In most cases, the maximum of 8 cm is attempted because of a large total discrepancy, as long as the patient maintains adequate knee range of motion.
The first lengthening of the femur can be performed 12 months after the preparatory surgeries, assuming that the femoral neck has ossified. If the preparatory surgery is performed between the ages of 2 and 3 years, then the first lengthening can follow between the ages of 3 and 4 years. However, if the femur is excessively short (total femoral length <75 mm) for an external fixator or if the femoral neck fails to ossify, then it would be beneficial to wait for a year or two for more growth. By beginning lengthening at a young age, the level of prosthetic or orthotic need is reduced earlier in a child’s life. In the senior author’s experience, the complication rate for limb lengthening is no different for the younger age group [61]. Lengthening of the femur in children younger than 6 years may be associated with sustained growth stimulation [62].
Between 4.5 and 7 years, children may have more difficulty psychologically dealing with limb lengthening. This is related to the normal cognitive stages of children at this age. Children at this age group seem to understand too little and too much at the same time. They are beginning to be more independent and may appear to be mature enough to handle the process. They understand that they have one short leg. They do not connect the recognition of a short leg with the solution of limb lengthening. Their cognitive level is insufficient to understand why their parents allowed someone to do this to them. The younger children do much better because their cognitive level accepts everything their parents decide without questioning. Beyond the age of 6 or 7 years, the child enters the age of reason and begins to understand that there is a problem with a solution. Their cooperation is voluntary rather than coerced. One way to explain this to children is to focus on the size of their shoe lift. It is easier for them to understand that they can wear a normal shoe after the operation instead of understanding that their leg lengths will be equal.
The frequency of lengthening should be spread out to no less than every 3 years and preferably 4 years or greater. The rule of 4 is a good guiding strategy: one lengthening every 4 years starting by 4 years. Assuming that a preparatory surgery is done between ages 2 and 3 years, the first lengthening can be done between ages 3 and 4 years. The second lengthening would occur around age 8 years and a third lengthening around age 12 years. For psychosocial reasons, it is preferable to complete all the lengthenings by age 14 when the child starts high school education. If a fourth lengthening is required it can be done around age 16 years. Patients may also have lengthenings performed later in life as an adult. One severe case treated by the author equalized a 25-cm discrepancy with two lengthenings over a nail. If the tibia is also short, a combined femoral and tibial lengthening can allow for greater total lengthening amount in a shorter time frame. However, a combined lengthening may not be able to achieve as much total length as two separate lengthenings, and growth inhibition in the tibia has been reported [62]. In addition, with the advent of smaller and more reliable implantable nails, in the author’s experience, internal lengthening can be started as early as 7 years of age.
Step 3: Hemiepiphysiodesis and Epiphysiodesis
Contralateral epiphysiodesis around the knee is used as an adjuvant method to equalize LLD. It should be calculated into the total strategy of equalization surgeries, and can be used for up to 5 cm of LLD equalization. Judicious use in some cases may avoid the need for one lengthening. For example, a predicted discrepancy of 12 cm may instead be treated with only one 7-cm lengthening and a 5-cm epiphysiodesis around puberty. Calculation of the timing of epiphysiodesis can be achieved quickly and accurately using the multiplier method [26].
Ipsilateral hemiepiphysiodesis is very useful to correct the valgus deformity of the knee from distal femoral or proximal tibial origins. The 8-plate, developed by Peter Stevens, or a similar device, is a simple way to temporarily arrest the one side of physeal growth [63, 64]. Correction of the valgus deformity of the femur permits future implantable lengthening of the femur since there is no angular deformity.
Box 22.6. Planning Surgery for the CFD Patient
Determine the classification and total discrepancy of the deficiency to plan out a timeline of surgeries.
Have a low threshold to perform preparatory surgeries of the hip and knee to prevent subluxation or dislocation, which can be disastrous.
Do not lengthen until the femoral neck has ossified. BMP can be used to promote ossification.
The fascia lata must be released or resected before lengthening.
The safe range of each lengthening is 5–8 cm.
The Rule of 4: First lengthening at 4 years of age and then every 4 years thereafter as needed.
Contralateral epiphysiodesis can be used to avoid an extra lengthening.
The Superhip Procedure
Is the proximal femur bony deformity (see Fig. 22.1) caused by the hip joint contractures, or vice versa? The net effect in the frontal plane is that the insertion of the abductor muscles (gluteus medius and minimus) on the greater trochanter is abnormally close to the pelvis. This leads to several problems, including impingement of the trochanter on the iliac bone and contracture of the gluteal muscles since the distance between their origin and insertion is short. The gluteus maximus and fascia lata, with its iliotibial band extension to the tibia, are the most lateral of the soft-tissue structures and therefore contribute the greatest to the abduction contracture of the hip. They also contribute significantly to flexion contracture of the hip. The abduction contracture is not obvious, since hip adduction is preserved due to the femoral bony varus deformity, and hip abduction is limited by ilio-trochanteric impingement. If the bony coxa vara is corrected by osteotomy, without soft tissue releases, the abduction contracture will be uncovered and will prevent the hip from coming back to a neutral position relative to the pelvis, producing a fixed pelvic tilt. An abduction pelvic tilt on the short leg makes the LLD appear less than before surgery. In the face of an open growth plate or a non-ossified neck or subtrochanteric segment, as in type 1b cases, the abduction contracture leads to recurrence of the coxa vara after osteotomy. The mechanism for this recurrence may be differential growth of the physis, bending at the non-ossified tissues, or slipped capital femoral epiphysis.
To correct these deformities, a three-dimensional osteotomy combined with a series of selective soft tissue releases was developed by Paley in 1997. It is now known as the SUPER hip procedure because of its complexity.
Box 22.7. The Evolution of the Superhip Procedure
Dror Paley, MD
The Superhip name originally arose as a billing code to avoid writing down the multiple CPT codes that comprised this multistep conglomerate procedure. While there was no intention to call this operation the Superhip procedure, the name stuck. To avoid the name being misperceived the SUPER prefix was then made into an acronym: Systematic Utilitarian Procedure for Extremity Reconstruction. Other SUPER joint reconstructive procedures for congenital knee and ankle deformities developed by Paley were subsequently renamed the SUPER knee and SUPER ankle procedures. John Birch of Dallas has expressed an aversion to the SUPER prefix and refers to theseBox 22.7. (continued)procedures as Paley hip, knee, and ankle procedures. As I learned from my mentor Dr. Robert Salter from his “no name no fame” comment regarding the innominate osteotomy now called the Salter osteotomy, it is not for a surgeon to name this procedure after themselves. I have given this procedure a generic name. If others choose to name it after me, then so be it.
My understanding of CFD began with my experience at the Hospital for Sick Children, Toronto, in 1984. I worked with Drs. Robert Gillespie and Ivan Krajbich, who treated the severe CFD cases by rotationplasty [49]. In 1986 and 1987, I witnessed reconstructive options for these deformities in Kurgan, USSR. Drs. Popkov and Maltzev showed me how to do pelvic support osteotomy to bypass the tethered and deformed hip. The message of these experts was that the CFD deformed hip did not lend itself to anatomic reconstruction.
After beginning my practice in 1987 at the University of Maryland, I tried to find alternative reconstructive solutions to the CFD hip. I performed many valgus, extension, and internal rotation osteotomies on young children with this deformity. Initially, they had excellent bony correction with reduction of the leg length difference due to a fixed pelvic obliquity that developed after surgery. Gradually, I watched the deformity recur, presumably through their proximal femoral physis or unossified femoral neck. I realized that the reason for this recurrence was the hip flexion and abduction contractures that existed prior to the surgery. In 1996, I recognized that in order to succeed, I needed to untether the proximal femur from the hip abductors and flexors and then realign the proximal femur to a neutral position at the hip joint. This was a radical concept, and I was left with the challenge of how to safely release the hip abductors. Borrowing from the concept of the Hardinge approach to the hip, which took down the anterior third of the hip abductors as a sleeve with the quadriceps muscle, I decided to take down the entire hip abductors with the vastus lateralis as one sleeve. In 1997, I performed the first such procedure.
While the psoas, rectus, and TFL were all lengthened as they are today, treating the hip abductors by releasing the gluteus and vastus tendons off of the greater trochanter together turned out to be a bad idea. While this freed up the proximal femur to rotate into neutral by extension, adduction, and internal rotation of the hip joint, it also changed the muscle-tendon length ratio, thus permanently weakening the hip abductors.Box 22.7. (continued)This is discussed in more detail below. I initially fixed the femur using a Rush rod and a tension band wire. There was no fixation up the femoral neck. When this procedure was performed for a subtrochanteric type Paley 1b or for a Paley 1a, no recurrence of the deformity occurred since the delayed ossification part was resected. When it was performed for a neck type Paley 1b, the varus usually recurred and the neck did not ossify.
In 2001, I switched to using a fixed-angle plate. I used the 130° sliding hip screw (Smith and Nephew, Memphis, TN). Some of the cases ossified, but most did not. Some of the plates broke and the deformity recurred, while in others the plate began to cut through the head as the varus recurred. I decided that a blade plate would be the best implant to avoid cutout as well as to control flexion and extension forces. With the engineers at Smith and Nephew, I designed an infant and pediatric 130°-angle cannulated blade plate, to correspond to the normal neck-shaft angle. Since 2004, I have been using this new plate. This minimized recurrent deformities, but incomplete ossification of the femoral neck was the usual result.
It was apparent that while we could correct this complex deformity, we could not get the delayed ossification of the cartilage to ossify. To get some of these failed cases to ossify, I resorted to insertion of BMP up the femoral neck into the non-ossified cartilage. The result was dramatic: the recalcitrant-delayed ossification cases ossified. Since BMP-2 (Infuse, Medtronic, Memphis, TN) was not FDA approved to use in children, we were initially hesitant and reserved in its application. We only used it to salvage previously failed Superhips. With the unexpected success that we saw from such application, I decided to apply it to new cases in 2006. The results were equally remarkable. All of the necks ossified and there were almost no recurrent deformities. Clearly, we needed to combine a mechanical with a biologic solution to solve the CFD deformity puzzle.
The soft tissue part of the operation also went through an evolution to its present state. In the first 5 years, we not only employed release of the conjoint tendon off of the greater trochanter to treat hip abduction contracture, but we also released the hip capsule off of the greater trochanter. This was done in an extra-articular fashion so that the joint was never exposed. No initial consequence of this was observed. However, when we started to lengthen femurs that had previously had a Superhip procedure, we encountered two new complications: hip dislocation and slipped capitalBox 22.7. (continued)femoral epiphysis (SCFE). In retrospect, we realize that both were related to the release of the superior capsular ligament from the pelvis to the greater trochanter. Our selective release of the superior capsular ligament demonstrated the importance of this band. This superior capsular pelvic-trochanteric band is essential to prevent the femoral head from moving laterally relative to the acetabulum. After recognizing this, we stopped releasing this band and we no longer experienced dislocations or slips following a Superhip.
After 10 years of performing the Superhip, we had solved the recurrent varus deformity problem, the delayed ossification problem, and the dislocation/SCFE problem. We noticed, however, that the children walked with a marked lurch or Trendelenburg gait. We attributed this to the abductor tendon release. By detaching the hip abductors from the greater trochanter, we had changed the muscle-tendon length ratio of the gluteus medius and minimus muscles, which was difficult to recover. Essentially, we had added tendon length to the glutei due to the quadriceps tendon moving proximally. To solve this problem in 2008, I began to perform the abductor muscle slide off of the ilium instead of the distal tendon lengthening. This preserved the muscle-to-tendon length ratio, keeping the muscle tendon length and tension constant after surgery. The slide is achieved by shortening the height of the iliac crest. As an added benefit, the glutei are a peculiar group of muscles since they have a growth plate connected to them that grows in a direction that lengthens the muscles. After the repair of the abductor slide, the iliac apophysis can increase the tension and re-elevate the origin of this muscle. Since adding the abductor slide, patients do not have the previously noted lurch or Trendelenburg gait.
Superhip Surgical Technique (Figs. 22.7, 22.8, 22.9, 22.10, and 22.11)
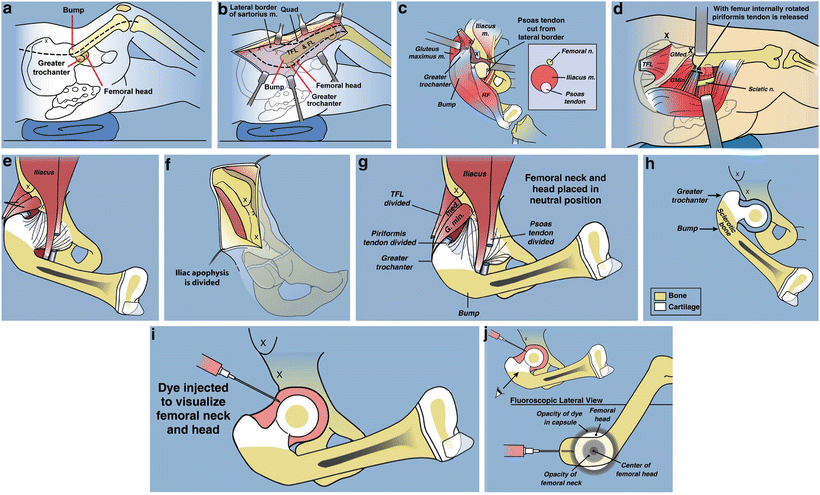
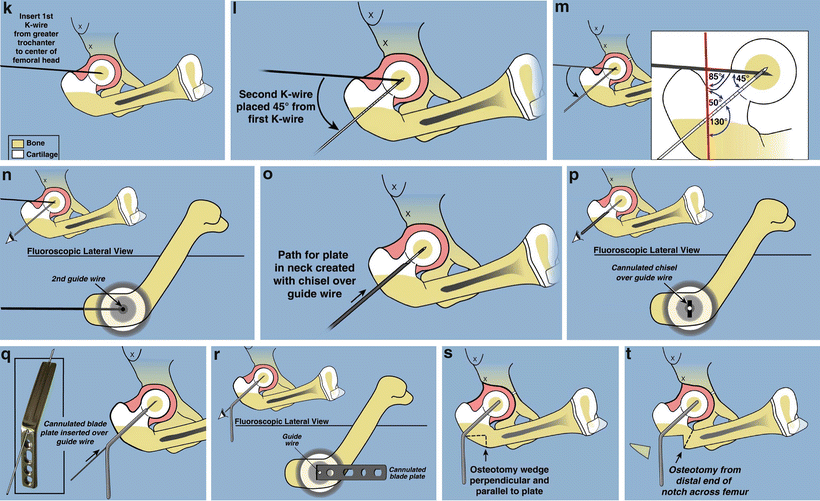
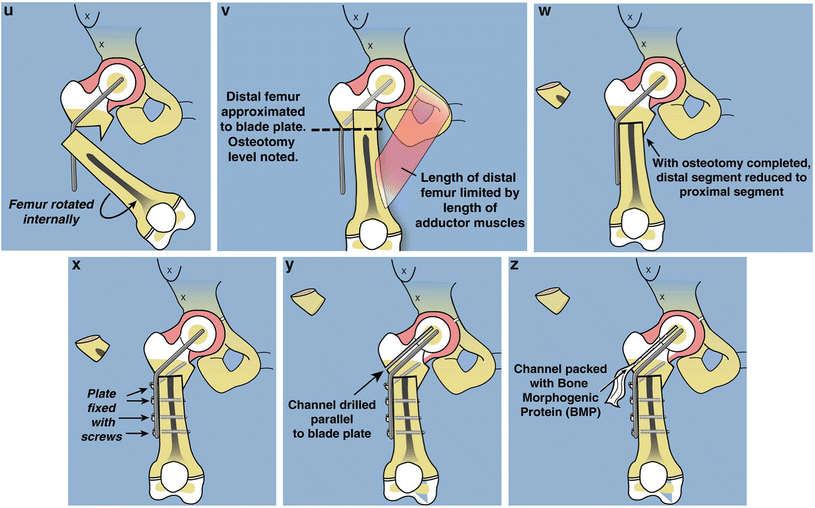
Fig. 22.7
Superhip surgical technique illustrations. (a) Straight midlateral incision from top of iliac crest to below tuberosity of knee. (b) Reflect fascia lata-iliotibial band distally and leave attached at Gerdy’s tubercle. (c) Release rectus femoris tendon, decompress femoral nerve, and recess iliopsoas tendon. The TFL muscle remains attached to the ASIS. (d) Decompress sciatic nerve and release piriformis tendon at greater trochanter. (e) Adduction is limited by hip abductor muscles. (f) Split apophysis and do abductor muscle slide. (g) All extra-articular tethers released. Hip can be placed in neutral position. (h) The quadriceps muscle is elevated to expose the lateral and anterior femur. (i) Hip arthrogram to visualize femoral head. (j) Lateral view shows concentric rings of ossific nucleus, femoral neck, and femoral head, which should be positioned in a bull’s-eye pattern. (k) Insert first guide wire from tip of greater trochanter to center of femoral head. (l) Insert second guide wire at 45° to first. (m) Geometric rationale behind 45° angle. Neck-shaft angle is 130° and medial proximal femoral angle is 85°. Therefore the angle between these must be 45°. (n) The second guide wire is centered in the femoral head and neck on both views. (o) Insert a cannulated chisel up the femoral neck. (p) The chisel should be perpendicular to the posterior aspect of the greater trochanter.Fig. 22.7 (continued) (q) Replace the chisel with a 130° blade plate. (r) The blade plate is in place. Note the flexion deformity of the femur shaft relative to the plate. (s) Cut perpendicular and parallel to the plate. (t) Perform a second osteotomy oblique to the femur. More recently this osteotomy starts inside the notch that was removed eliminating the medial lip. (u) Rotate the distal femur internally and abduct it to realign it with the proximal femur. (v) Overlap the bone ends and mark the level of overlap. The femur cannot be reduced due to the tethering medial and posterior soft tissues. (w) Perform a shortening osteotomy of the femur and reduce the femur to the plate. (x) Fix it with three diaphyseal screws and one interfragmentary neck screw. (y) Drill a channel parallel to the blade. (z) Insert BMP-2 collagen sponges up the femoral neck
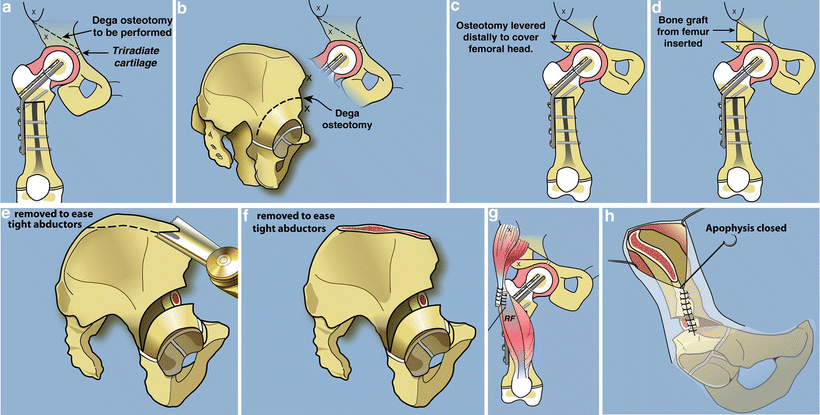
Fig. 22.8
Paley modification of Dega osteotomy. (a) The cut should start 2–3 cm proximal to the lateral acetabulum converging on the triradiate cartilage medially. (b) The cut should be parallel to the acetabulum circumferentially stopping at the triradiate between the ilium and the ischium. (c) The roof of the acetabulum is levered down to cover the femoral head such that the sourcil is horizontal. (d) Fashion the bone resected from the femur to fit into the opening wedge space. (e) Resect the top of the iliac wing to reduce the tension on the hip abductors so that the apophysis can be sutured closed. This is part of the abductor slide. (f) After the resection. (g) Transfer the rectus femoris to the TFL. (h) Close the apophysis
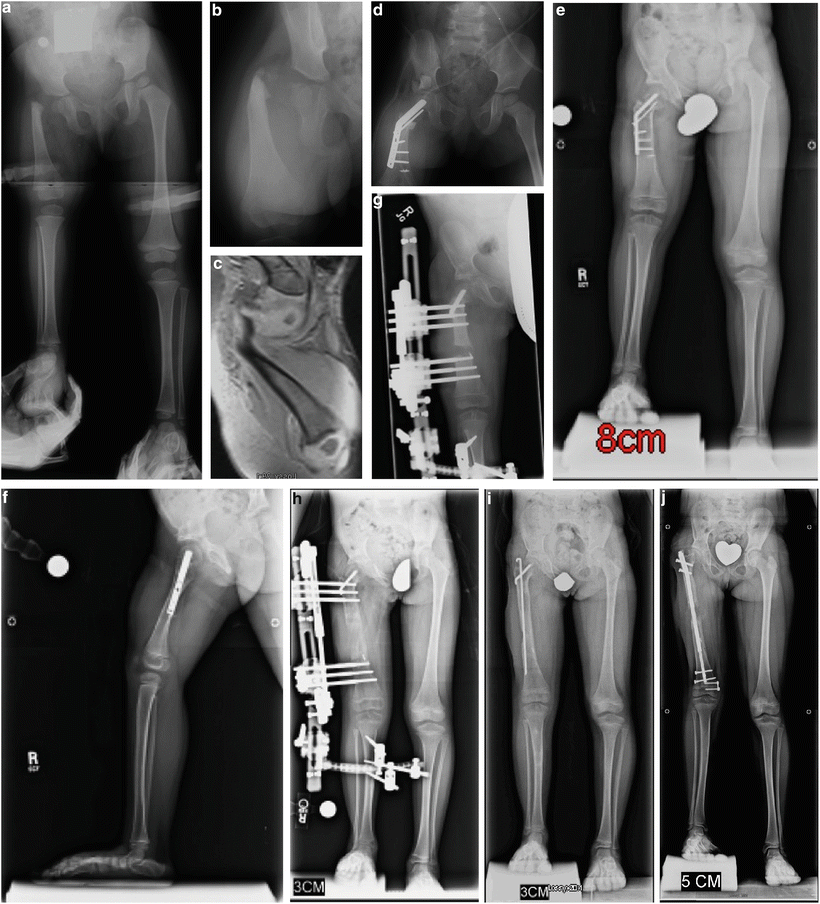
Fig. 22.9
CFD Paley type 1b with delayed ossification of femoral neck (a, b). MRI showing the cartilaginous femoral neck that remains unossified at 2 years of age (c). Superhip with Superknee procedure at 2 years of age, including insertion of BMP in femoral neck (d). The neck is fully ossified by 3 years of age. Note the significant growth that has occurred since the year before (e, f). First lengthening performed at age 4 years with Smith & Nephew Modular Rail System (Memphis, TN) external fixator with articulation across the knee joint (g). Eight centimeters of lengthening achieved (h). Removal of external fixator with Rush rodding of bone to prevent fracture (i). At the age of 8 years she underwent a second lengthening, this time using the PRECICE (Ellipse Technology, Irvine CA) 8.5-mm-diameter lengthening nail (j)
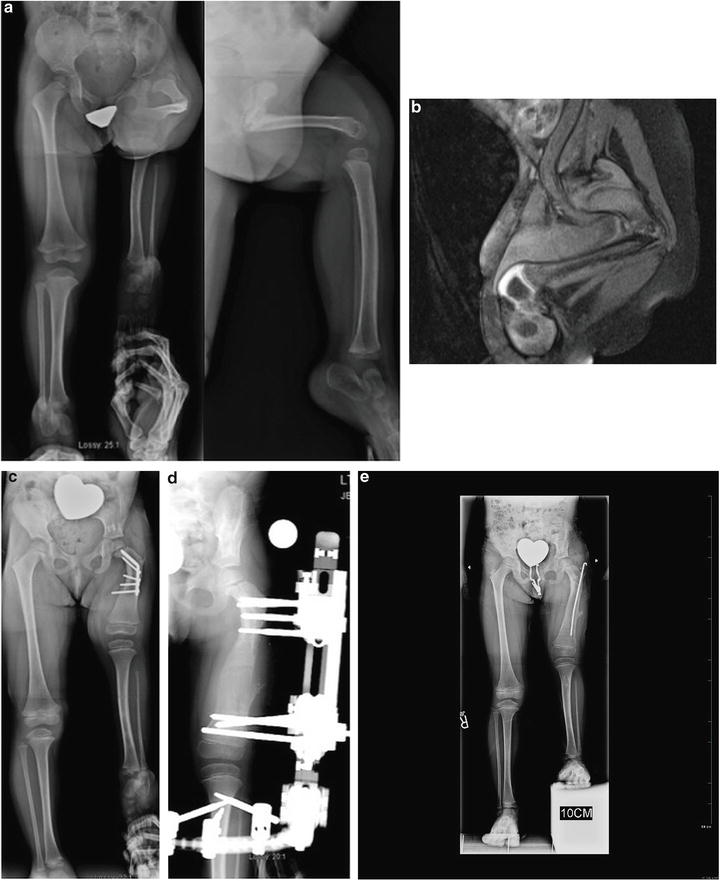
Fig. 22.10
Two-year-old girl with CFD Paley type 1b with delayed ossification and severe angulation of the subtrochanteric level of the left femur (a). MRI showing the delayed ossification of subtrochanteric region. The neck is normally ossified (b). The deformity is fully corrected and the femur is healed after the Superhip surgery. The knee has full extension following posterior capsulotomy and Superknee stabilization. This X-ray is 1 year after the Superhip and Superknee showing significant growth and remodeling which is why the screws are protruding medially (c). Lengthening of the femur was performed at age 4 years (d). X-rays after lengthening of the femur 6 cm and insertion of Rush rod at the time of removal surgery (e)
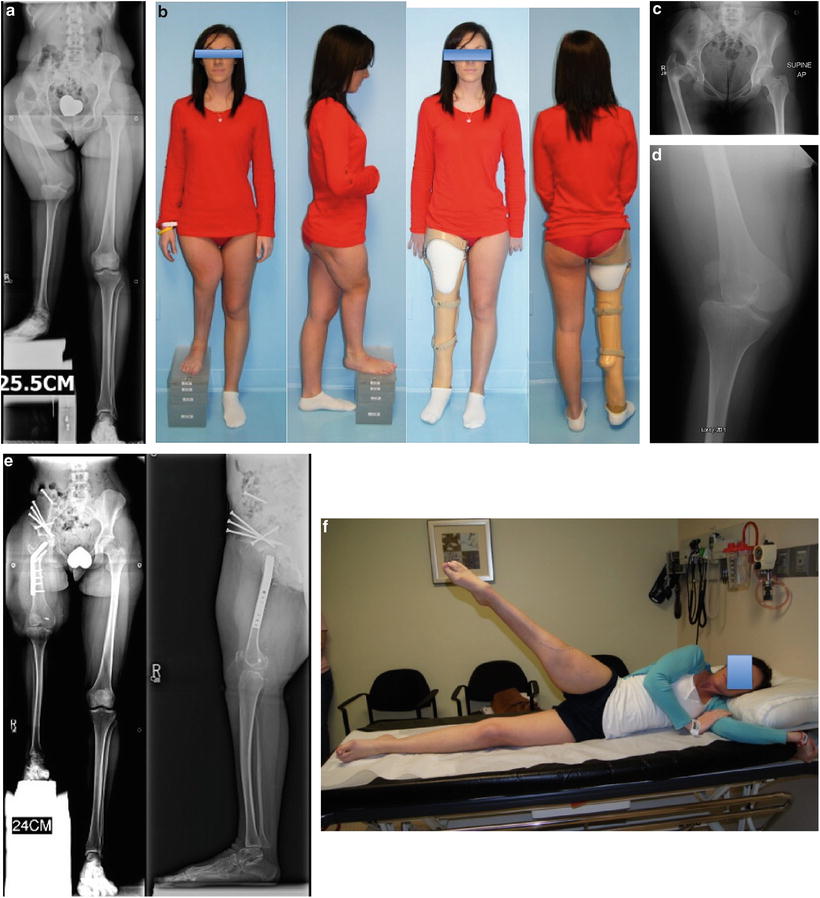
Fig. 22.11
(a) Erect leg radiographs of a 22-year-old woman with previously untreated CFD and 25-cm discrepancy. She has marked coxa vara and acetabular dysplasia as well as rotatory subluxation of the knee joint and dislocation of the patella. (b) Clinical picture before any surgery with and without her prosthesis. (c) AP pelvis X-ray showing the hip deformities. (d) AP knee X-ray showing the rotatory subluxation of the knee. (e) AP and lateral radiographs after Superhip procedure with Ganz periacetabular osteotomy (PAO) and Superknee procedure for reduction and stabilization of the knee and patella. (f) Active hip abduction after recovery. (g) Active hip flexion and knee extension after recovery. (h) Ganz PAO modified for Superhip procedure. Start by osteotomizing the iliac crest and peeling off the abductor muscles to do an abductor muscle slide. (i) The five bone cuts of the Ganz seen from the lateral side. (j) The acetabulum is internally rotated to gain posterior coverage. (k) The acetabulum is then abducted to gain lateral coverage. (l) The iliac wing is shortened, and then the crest is reattached using the same screws that are used to fix the PAO, 3 antegrade and one retrograde. (m) Radiograph after first lengthening of femur and tibia (6-cm femur and 5-cm tibia). The femur and tibia were both rodded using Rush rods at the time of fixator removal to prevent fracture
1.
Positioning, prepping, and draping. An epidural is placed by the anesthesia service with a catheter running up the back on the non-operative side. A Foley catheter is placed and also routed to the non-operative side. The patient should be moved to the edge and foot of the radiolucent table in a supine position. The ipsilateral arm should be appropriately padded and placed across the patient’s chest. A radiolucent bump (usually a folded towel or sheet) is placed beneath the ipsilateral ischium to roll the pelvis 45° towards the opposite side. The bump should not be beneath the iliac crest or lower back. The entire side should be prepped and draped free from the nipple to the toes. The drapes should extend from the crack of the buttocks to the fold between the scrotum or labia and the thigh. The lower limb should be completely free of the drapes. This is called a forequarter prep.
2.
Incision. With the leg fully extended, a long mid-lateral incision is made from the top of the iliac crest to the tibial tuberosity. The incision is kept as straight as possible, passing over the proximal femoral “bump” and continuing longitudinally towards Gerdy’s tubercle before curving slightly anteriorly towards the tibial tubercle. The incision is carried down to the depth of the underlying fascia lata and iliotibial band.
3.
Flap elevation. The subcutaneous tissues and skin are elevated as one large flap anteriorly and posteriorly off the fascia of the thigh and pelvic region. The fat is adherent to the fascia and should be dissected preferably with an electrocautery. The electrocautery should be held flat, parallel to the plane of dissection, and can be quite technically difficult. It is important not to incise or damage the fascia if it is being used for knee ligament reconstruction. Dissection may also be carried out with scissors. Anteriorly, the flap is extended medial to the Smith-Peterson interval (tensor fascia lata (TFL) and sartorius) proximally. Posteriorly, the subcutaneous flap is elevated to just posterior to the intermuscular septum. Distally, reflect the flap to the patella if no ligament reconstruction is to be done and all the way to the medial side if ligament reconstruction is to be done. The fascia lata is now fully exposed from the patella to a couple of centimeters posterior to the intermuscular septum distally and from the anterior edge of the TFL to the mid-gluteus maximus proximally.
4.
Fascia lata release. The fascia is incised at the TFL-sartorius interval making sure to stay on the TFL side in order to avoid injury to the lateral femoral cutaneous nerve. The fascial incision is extended distally to the lateral border of the patella ending at the tibia. The posterior incision of the fascia lata starts distally and posterior at the intermuscular septum and extends proximally to overlie the gluteus maximus in line with the incision. The gluteus maximus (GMax) should be separated from the overlying fascia anterior to the posterior fascial incision. The fascia should be retracted anteriorly and away from the underlying muscle, while the GMax should be dissected off of the fascia and the intermuscular septum that separates it from the TFL. The GMax should not be split in line with the fascial incision to avoid denervating the muscle anterior to the split. It can now be reflected posteriorly to allow exposure of the greater trochanter, piriformis muscle, and sciatic nerve.
If knee ligamentous reconstruction is planned, the fascia lata is cut proximally and anteriorly at the musculotendinous junction. The fascial cut should be a step cut or sloped posteriorly and proximally to include a longer fascia segment posteriorly from the fascia that was dissected off of the GMax. The fascia lata is reflected distally to Gerdy’s tubercle. The TFL can be left in place without further dissection. It does not have to be separated from the underlying gluteus medius (GMed). The two muscles are often adherent to each other, and it may be difficult to differentiate the muscle fibers. The distinguishing feature is that the GMed fibers insert on the greater trochanter while the TFL does not. The distal fascia lata becomes the iliotibial band and blends with the underlying lateral knee capsule, which may be partially reflected with the iliotibial band. The fascia should be mobilized all the way until Gerdy’s tubercle. The fascia can then be divided into two halves using a straight pair of scissors. It should be kept moist while the rest of the surgery proceeds. The two limbs of the fascia are ready for later use in the Superknee procedure.
5.
Hip flexion contracture releases. The dissection is carried beneath the sartorius to find the rectus femoris tendon. The rectus femoris tendon insertion is identified at the anterior inferior iliac spine. The constant ascending branch of the lateral femoral circumflex artery and vein is cauterized prior to cutting the tendon. The conjoint rectus femoris tendon (distal to the split into reflected and direct heads) is cut and allowed to retract distally. Care should be taken not to go too distal on the rectus femoris to avoid injury to its innervating branch of the femoral nerve. Just medial to the rectus is the iliopsoas muscle. The iliocapsularis muscle (capsular origin head of iliopsoas muscles) can also be seen here. The femoral nerve lies on the anteromedial surface of the iliopsoas muscle. Before looking for the psoas tendon, the femoral nerve should be identified and decompressed below the inguinal ligament. The posterior aspect of the iliopsoas muscle belly is now elevated from lateral to medial. The psoas tendon is located on the posteromedial surface in the substance of the muscle. The tendon is exposed and cut. Any remaining flexion contracture of the hip is due to the gluteus medius and minimus (the part of these muscles originating anterior to the center of rotation of the femoral head in the sagittal plane), and the anterior fascia of thigh. The release of the gluteus medius and minimus muscles is accomplished by the abductor muscle slide technique (see step 7). If the anterior thigh or sartorius fascia is still tight, it can also be released, taking care not to injure the lateral femoral cutaneous nerve, which should be identified and decompressed. It runs inside the fascia covering the sartorius muscle just medial to the anterior superior iliac spine.
6.
External rotation contracture release. The piriformis tendon is contracted and prevents internal rotation of the hip. It should be released off of the greater trochanter. The greater trochanter should be identified by palpation. The gluteus medius muscle posterior border is very distinct and proceeds down to the greater trochanter where it inserts. Deep to the medius is the gluteus minimus, and just distal to the minimus is the piriformis muscle. Its tendon can be palpated through its muscle. It may be difficult to identify the piriformis from the minimus. Care should be taken to avoid dissection at the distal border of the piriformis tendon. This is where the medial femoral circumflex branch anastomoses with the inferior gluteal artery branch. The entire piriformis is transected about 1 cm from its insertion onto the trochanter. The sciatic nerve can be identified and if necessary decompressed. It is more posterior to the trochanter and runs deep to the piriformis.
7.
Abductor muscle slide. The abductors may not appear to be tight on first inspection because of the coxa vara. Adduction into a true AP of the hip, with the neck oriented normally in the acetabulum, is now restricted by the gluteus medius and minimus since the fascia lata has already been cut. Furthermore, the Dega osteotomy, which lengthens the height of the ilium, makes the abductors even tighter. As noted previously, the abductors should be detached at their origin and not their insertion. This avoids changing the muscle-tendon length ratio and avoids weakening the hip abductors, avoiding a lurch or Trendelenburg gait.
The subcutaneous tissue flaps should be elevated to provide adequate exposure to the iliac crest apophysis. There is a tendency to have inadequate posterior exposure, and the flaps should be elevated just beyond the highest lateral point of the apophysis. The anterior extent is the anterior superior and inferior iliac spines, which has been exposed for the release of the rectus femoris tendon. The abdominal external oblique muscles are partially released off of the entire length of the apophysis. Once the apophysis is bare, it can be split from the ASIS to the AIIS, and then split from anterior to posterior along the iliac crest. This should be done with a #15 blade. To know where to split, pinch the apophysis between thumb and index finger of the hand not holding the knife. Stay in the middle of the apophysis along its entire length, pushing down hard with the knife blade until one feels bone. Using a periosteal elevator, pop off the apophysis from the ilium. This should be done at multiple sites to get the entire apophysis to peel back as a unit from the ilium. The apophysis and lateral periosteum are reflected distally, thus relaxing the abductor muscles. The medial half of the apophysis is reflected medially with the iliacus muscle. Since some of the abductors act as flexors of the hip, the abductor slide helps eliminate any remaining flexion deformity of the hip. Furthermore, the iliacus muscle slide also relaxes any residual tension in this muscle.
8.
Elevation of quadriceps. The quadriceps is now elevated off of the femur in a subperiosteal fashion. Since the femur is so short, the exposure may extend as far as the distal femoral physis. The perforator vessels need to be cauterized as the quadriceps is detached from the linea aspera. Proximally, the vastus lateralis should be elevated off of part of the cartilage of the greater trochanteric apophysis by sharp dissection.
9.
Arthrogram. A hip arthrogram is now performed using a 20-gauge spinal needle. With the trocar inside, the needle is placed into the hip joint from the anterolateral side. Traction of the femur may facilitate placement. Once the needle appears to be in the joint on the image intensifier, the trocar is removed and normal saline is injected. If the needle is in the joint the saline should go in with little pressure, and when the syringe is removed from the needle, the saline should drip back out. These signs confirm that the needle is in the joint space. The arthrographic dye can now be injected into the hip joint to outline the femoral head, acetabulum, and femoral neck.
10.
Guide wire insertion. Since the abduction, flexion, and rotation contractures have all been released, the femoral head and neck can now be placed in a neutral orientation to the pelvis by extending and maximally adducting the lower limb across the opposite leg. A guide wire should now be drilled up the center of the femoral neck to guide the insertion of a fixed-angle fixation device. Since the femoral neck is unossified and short, it is very difficult to drill a guide wire at the correct angle up the femoral neck. The goal is to create a 130° neck-shaft angle and a medial proximal femoral angle (MPFA) of 85°. In the normal femur, the angle between the neck-shaft line and the tip of the greater trochanter to center of femoral head line is 45°.
The first guide wire is inserted from the tip of the greater trochanter to the center of femoral head. Since the tip of the trochanter is cartilaginous in young children, it cannot be seen radiographically and the tip of the trochanter is located by palpation using the wire tip. From this point, the wire is then drilled towards the center of the femoral head as shown in the arthrogram. The image intensifier is placed into the lateral view and the leg rotated until a bull’s-eye of three concentric circles is seen. This is formed by the overlapping dye shadows: the outermost circle is the femoral head, the middle circle is the femoral neck, and the inner circle is the ossific nucleus of the femoral head. A second wire should be drilled into the center of the bull’s-eye at a 45° angle to the first wire. Using a depth gauge or another wire of the same length as the second wire, measure the amount of wire inside the femoral neck by placing it alongside the second wire and measuring the difference in length between the two wires. This will be the length of the blade of the blade plate to be used. The position of the neck wire can also be confirmed by flexing the hip to 90° and looking at a frog leg lateral of the neck.
11.
Blade plate insertion. The cannulated chisel for the blade plate should now be hammered up the femoral neck guided by the second guide wire. The chisel should be rotated until it is perpendicular to the posterior aspect of the greater trochanter. This will guide it to the correct angle in the sagittal plane. Tap the chisel out of the femur and reinsert the guide wire in its previous position. Insert the appropriate length 130° blade plate over this wire to the depth of the bend of the plate. Make sure on the image intensifier that the tip of the blade is not too deep into the femoral head. Check its position on AP and lateral planes, as well as using the approach-withdrawal technique with live fluoroscopy to ensure that the blade is not advanced too close to the articular surface of the femoral head. If the blade is suspected of being too long, then replace it with one with a shorter length. Furthermore, if plate placement is off center, there is greater risk of protrusion into the joint.
12.
Subtrochanteric osteotomy. The femur should be cut perpendicular to the shaft of the plate, starting just below the bend in the plate. This cut should be below the level of the greater trochanter cartilage. To guide this cut, drill a wire perpendicular to the plate. Keep the plane of the saw blade perpendicular to the plate in all planes. The width of the perpendicular cut surface should be as wide as the femoral diaphysis. If the deformity angle is very large, it may be parallel to the lateral cortex. A second subtrochanteric osteotomy should be made oriented less than 90° to the first osteotomy towards the base of the femoral neck to remove the bone protruding medially. The femoral head and neck can be manually tested for any residual impingement, removing any blocks to achieving 90° of hip flexion. The anteromedial corner often needs to be excised to prevent impingement.
13.




Periosteum release. After the second osteotomy, the distal femur is easily exposed and the surrounding periosteum is peeled off circumferentially. Medially, it is very thick and restricts correction of the varus and rotation deformity. Cut the periosteum transversely around the femur, carefully separating it from the surrounding muscle. Be careful to avoid injury to the profunda femoris and its perforators, which pass immediately under the periosteum. Cutting the periosteum allows the thigh to stretch longitudinally, reducing the amount of shortening required of the femur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


